Pomegranate extract (PG-E) has been reported to exert a protective effect on the skin due to its antioxidant activity. Ingredients rich in phenolic compounds are unstable in extract solutions, and, therefore, the use of a suitable nanosystem to encapsulate this type of extract could be necessary in different biotechnological applications. Thus, we investigated the capacity of Brassica oleracea L. (cauliflower) inflorescence vesicles (CI-vesicles) to encapsulate PG-E and determined the stability and the antioxidant capacity of the system over time. In addition, the protective effect against UV radiation and heavy metals in HaCaT cells was also tested. The CI-vesicles had an entrapment efficiency of around 50%, and accelerated stability tests did not show significant changes in the parameters tested. The results for the HaCaT cells showed the non-cytotoxicity of the CI-vesicles containing PG-E and their protection against heavy metals (lead acetate and mercuric chloride) and UV-B radiation through a reduction of oxidative stress. The reduction of the percentage of deleted mtDNA (mtDNA4977, “common deletion”) in UV-treated HaCaT cells due to the presence of CI-vesicles containing PG-E indicated the mechanism of protection. Therefore, the effects of CI-vesicles loaded with PG-E against oxidative stress support their utilization as natural cosmeceuticals to protect skin health against external damage from environmental pollution and UV radiation.
1. Introduction
Pomegranate (
Punica granatum L.), a fruit of the
Punicaceae family, is considered a fruit with high pharmaceutical value since its bioactive compounds have been shown to have biological activities in the treatment of several human diseases
[1]. The main benefit is due to the antioxidant potential derived from the high concentrations of phenolic compounds, such as galloylglucose, punicalagin, punicalin, ellagic acid, and gallic acid
[2][3][2,3]. Besides, anthocyanins and other nutraceutical components, such as sterols, γ-tocopherol, punicic acid, and hydroxybenzoic acids, have been found in the different parts of pomegranate
[4][5][4,5]. Thus, functional products enriched with pomegranate extract (PG-E) have been reported to be useful for the treatment of certain diseases—such as diabetes mellitus, obesity, and cardiovascular and gastrointestinal diseases
[1][6][7][1,6,7]—since their antioxidant potential gives protection from inflammation because it reduces the activity of cytokines, such as tumor necrosis factor-α (TNF-α) or interleukin-6 (IL-6)
[8][9][10][8,9,10], as well as the levels of total cholesterol, low density lipoprotein (LDL), and lipid peroxidation
[11]. Further, beneficial and protective effects of PG-E in the skin are also due to antioxidant activity, as reported in different studies
[12][13][12,13]. In this regard, it is important to focus on the keratinocytes, as they comprise much of the outermost layer of skin (epidermis)
[14]. Therefore, keratinocytes suffer damage due to extrinsic stimuli (UV exposure or pollutants, such as heavy metals)
[15][16][15,16]. These stimuli trigger an excessive production of reactive oxygen species (ROS), which entails a loss of cellular functions and even cell death
[17][18][17,18]. It is well known that ROS is a threat to cellular integrity, as it causes damage to essential macromolecules, including DNA, lipids, and proteins
[19]. Regarding DNA damage, it has been observed to be more persistent in mitochondrial DNA (mtDNA) than in nuclear DNA due, among other causes, to limited repair mechanisms
[20]. An indicator of DNA damage is a large deletion of 4977 bp from mtDNA called “common deletion”, which is considered an early marker for mutations induced by high levels of ROS
[21][22][21,22]. It has been reported that PG-E can reduce the H
2O
2 overproduction as well as the cytotoxicity and the inflammatory stress induced by UV exposure
[13][23][13,23].
Based on the above, this type of extract is of great interest as a natural cosmeceutical for skin health. But, one problem is that phenolic compounds are unstable in extract solutions, and, therefore, it is necessary to remove the solvents of the extracts to stabilize them. The shelf life of the phenolics could be enhanced in the dry extracts, but the stability of the formulated liquid extracts is very limited. Thus, procedures to prolong the stability of the final product, such as the addition of pectins for jelly formation, have been investigated
[24]. Similarly, microencapsulation has been reported as a suitable option to stabilize the phenolics of the PG-E
[25]. In this procedure, the phenolics are surrounded by a maltodextrin matrix in order to produce small capsules, with significant improvement of the antioxidant and α-glucosidase inhibitory activities.
Recently, new technologies of encapsulation, such as the use of membrane vesicles derived from natural sources, have been studied for different applications, such as cosmetics or therapy, such as treatment of colitis or melanoma
[26][27][28][29][30][26,27,28,29,30]. The most profitable sources may well be those of plant origin since, in many crops, by-products are produced, which can be used to obtain membrane vesicles. The latest research in our group has focused on the study of stable natural membrane vesicles from brassicas. Plasma membrane vesicles from broccoli (
Brassica oleracea L. var. italica) are characterized by their potential to stabilize the bioactive glucosinolate glucoraphanin
[31]; the stability of this type of vesicle was studied in other work
[32] and was found to be related to aquaporins. Recent work confirmed the potential of these vesicles as carriers in cosmetic or therapeutic applications
[28]. Besides, in this study, an interaction between plant and human cell membranes was shown, revealing their potential in numerous applications in nanotechnology. In addition to broccoli-derived vesicles, membrane vesicles from cauliflower inflorescence have been well characterized
[33]. The vesicles described in this study had sizes between 300 and 400 nm, appropriate for use in various biotechnological applications
[34]. Besides, the osmotic permeability (
Pf) values are related to vesicle functionality and membrane integrity, and high values of
Pf have been determined in vesicles from
Brassica oleracea L. var. botrytis inflorescences
[33]. These types of vesicles are defined by their versatility since, in addition to their use in cosmetics, applications in agriculture are being studied
[35][36][35,36]. All these findings lead us to propose these membrane vesicles as nanocarriers, whose advantages are based on their specific lipid/protein composition, their biodegradability, and their ability to carry the encapsulated substance to the target cells.
2. Physicochemical and Morphological Characterization
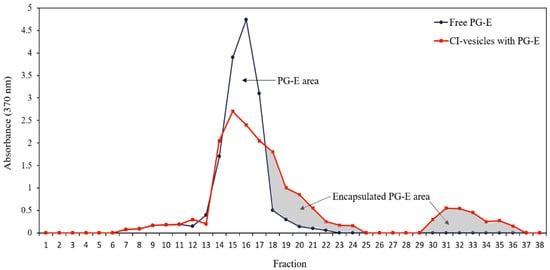
shows DLS analysis performed in a similar way to that previously reported
[37][38][39][38,39,40]. The CI-vesicles had an average hydrodynamic diameter around 620.7 nm, which increased when PG-E was encapsulated in CI-vesicles (797.5 nm). TEM picture of the shape of CI-vesicles with PG-E is shown in
Supplemental material (Figure S1). Zeta potential values of −21 mV were obtained in both CI-vesicles and CI-vesicles with PG-E, indicating adequate stability of the formulations
[40][52] and a negative electric charge on the surface of the vesicles. Regarding free PG-E, it was not possible to measure the size by DLS, and the zeta potential value was −15 mV.
Table 12. Characteristics of CI-vesicles, CI-vesicles with PG-E, and PG-E: Particle Size, Polydispersity Index, and Zeta Potential.
| |
CI-Vesicles |
CI-Vesicles with PG-E |
PG-E |
| Z-average (nm) |
620.72 ± 25.17 a |
797.50 ± 38.93 b |
- |
| Polydispersity index (0–1) |
0.70 ± 0.03 a |
0.76 ± 0.12 a |
- |
| Z-potential (mV) |
−21.56 ± 0.38 a |
−21.65 ± 0.24 a |
−15.04 ± 0.40 b |