This review articlentry aims to cover the most recent advances regarding the synthesis of linear ABC-type triblock terpolymers by RAFT polymerization, as well as their self-assembly properties in aqueous solutions. RAFT polymerization has received extensive attention, as it is a versatile technique, compatible with a great variety of functional monomers and reaction conditions, while providing exceptional and precise control over the final structure, with well-defined side-groups and post-polymerization engineering potential. Linear triblock terpolymers synthesis can lead to very interesting novel ideas, since there are countless combinations of stimuli/non-stimuli and hydrophilic/hydrophobic monomers that someone can use. One of their most interesting features is their ubiquitous ability to self-assemble in different nanostructures depending on their degree of polymerization (DP), block composition, solubilization protocol, internal and external stimuli.
- ABC triblock terpolymers
- RAFT polymerization
1. General Synthetic Strategies for ABC Triblock Copolymers by RAFT
2. Stimuli-Responsive ABC Triblock Copolymers
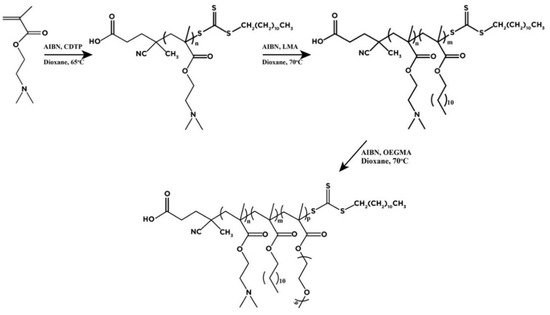
3. Triblock Terpolymers Self-Assembly Properties in Solvent Exchange Protocols
References
- Altintas, O.; Artar, M.; ter Huurne, G.; Voets, I.K.; Palmans, A.R.A.; Barner-Kowollik, C.; Meijer, E.W. Design and Synthesis of Triblock Copolymers for Creating Complex Secondary Structures by Orthogonal Self-Assembly. Macromolecules 2015, 48, 8921–8932.
- Germack, D.S.;Wooley, K.L. RAFT-Based Synthesis and Characterization of ABC versus ACB Triblock Copolymers Containingtert-Butyl Acrylate, Isoprene, and Styrene Blocks. Macromol. Chem. Phys. 2007, 208, 2481–2491.Germack, D.S.; Wooley, K.L. RAFT-Based Synthesis and Characterization of ABC versus ACB Triblock Copolymers Containingtert-Butyl Acrylate, Isoprene, and Styrene Blocks. Macromol. Chem. Phys. 2007, 208, 2481–2491.
- Ghamkhari, A. Antimicrobial activity evaluation of a novel triblock cationic copolymer (PHEMA-b-PNIPAM-b-PVEAH). J. Hum. Health Halal Metr. 2020, 1, 35–41.
- Xu, Q.; Zhang, Y.; Li, X.; He, J.; Tan, J.; Zhang, L. Enzyme catalysis-induced RAFT polymerization in water for the preparation of epoxy-functionalized triblock copolymer vesicles. Polym. Chem. 2018, 9, 4908–4916.
- Zhang, K.; Fahs, G.B.; Aiba, M.; Moore, R.B.; Long, T.E. Nucleobase-functionalized ABC triblock copolymers: Self-assembly of supramolecular architectures. Chem. Commun. 2014, 50, 9145–9148.
- Messerschmidt, M.; Komber, H.; Häußler, L.; Hanzelmann, C.; Stamm, M.; Raether, B.; da Costa e Silva, O.; Uhlmann, P. Amphiphilic ABC Triblock Copolymers Tailored via RAFT Polymerization as Textile Surface Modifiers with Dual-Action Properties. Macromolecules 2013, 46, 2616–2627. Polymers 2021, 13, 1698 22 of 23Messerschmidt, M.; Komber, H.; Häußler, L.; Hanzelmann, C.; Stamm, M.; Raether, B.; da Costa e Silva, O.; Uhlmann, P. Amphiphilic ABC Triblock Copolymers Tailored via RAFT Polymerization as Textile Surface Modifiers with Dual-Action Properties. Macromolecules 2013, 46, 2616–2627.
- Chen, S.; Alcouffe, P.; Rousseau, A.; Gérard, J.-F.; Lortie, F.; Zhu, J.; Bernard, J. Design of Semicrystalline Elastomeric Glassy Triblock Copolymers from Oligoamide-Based RAFT Agents. Macromolecules 2020, 54, 94–104.
- Mable, C.J.; Thompson, K.L.; Derry, M.J.; Mykhaylyk, O.O.; Binks, B.P.; Armes, S.P. ABC Triblock Copolymer Worms: Synthesis, Characterization, and Evaluation as Pickering Emulsifiers for Millimeter-Sized Droplets. Macromolecules 2016, 49, 7897–7907.
- Samanta, S.; Banerjee, S.L.; Ghosh, S.K.; Singha, N.K. Smart Polyacrylate Emulsion Based on a New ABC-Type Triblock Copolymer via RAFT-Mediated Surfactant-Free Miniemulsion Polymerization: Its Multifunctional Properties. ACS Appl. Mater. Interfaces 2019, 11, 44722–44734.
- Pal, S.; Kather, M.; Banerjee, S.L.; Saha, P.; Pich, A.; Singha, N.K. Dual Stimuli-Responsive Self-Assembly Behavior of a Tailor-Made ABC-Type Amphiphilic Tri-Block Copolymer. J. Polym. Sci. 2020, 58, 843–851.
- Skandalis, A.; Pispas, S. PDMAEMA-b-PLMA-b-POEGMA triblock terpolymers via RAFT polymerization and their self-assembly in aqueous solutions. Polym. Chem. 2017, 8, 4538–4547.
- Skandalis, A.; Uchman, M.; Štˇepánek, M.; Kere¨che, S.; Pispas, S. Complexation of DNA with QPDMAEMA-b-PLMA-b-POEGMA Cationic Triblock Terpolymer Micelles. Macromolecules 2020, 53, 5747–5755.Skandalis, A.; Uchman, M.; Štěpánek, M.; Kereїche, S.; Pispas, S. Complexation of DNA with QPDMAEMA-b-PLMA-b-POEGMA Cationic Triblock Terpolymer Micelles. Macromolecules 2020, 53, 5747–5755.
- Skandalis, A.; Murmiliuk, A.; Stepanek, M.; Pispas, S. Physicochemical Evaluation of Insulin Complexes with QPDMAEMA-b-PLMA-b-POEGMA Cationic Amphiphlic Triblock Terpolymer Micelles. Polymers 2020, 12, 309.
- Skandalis, A.; Pispas, S. pH- and thermo-responsive solution behavior of amphiphilic, linear triblock terpolymers. Polymer 2018, 157, 9–18.
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Keiderling, U. Thermal response of self-organization in an amphiphilic triblock polyelectrolyte and the influence of the globular protein lysozyme. Eur. Polym. J. 2018, 99, 49–57.
- Giaouzi, D.; Pispas, S. Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer. Polymers 2020, 12, 1382.
- Liu, S.; Tian, L.; Mao, H.; Ning,W.; Shang, P.;Wu, J.; Shi, X. Micellization and sol-gel transition of novel thermo- and pH-responsive ABC triblock copolymer synthesized by RAFT. J. Polym. Res. 2018, 25, 1–12.Liu, S.; Tian, L.; Mao, H.; Ning, W.; Shang, P.; Wu, J.; Shi, X. Micellization and sol-gel transition of novel thermo- and pH-responsive ABC triblock copolymer synthesized by RAFT. J. Polym. Res. 2018, 25, 1–12.
- Mahmoodzadeh, F.; Abbasian, M.; Jaymand, M.; Amirshaghaghi, A. A novel dual stimuli-responsive thiol-end-capped ABC triblock copolymer: Synthesis via reversible addition-fragmentation chain transfer technique, and investigation of its self-assembly behavior. Polym. Int. 2017, 66, 1651–1661.
- Guragain, S.; Perez-Mercader, J. Light-mediated one-pot synthesis of an ABC triblock copolymer in aqueous solution via RAFT and the effect of pH on copolymer self-assembly. Polym. Chem. 2018, 9, 4000–4006.
- Ye, Z.; Li, Y.; An, Z.; Wu, P. Exploration of Doubly Thermal Phase Transition Process of PDEGA-b-PDMA-b-PVCL in Water. Langmuir 2016, 32, 6691–6700.
- Mäkinen, L.; Varadharajan, D.; Tenhu, H.; Hietala, S. Triple Hydrophilic UCST–LCST Block Copolymers. Macromolecules 2016, 49, 986–993.Huo, F.; Li, S.; Li, Q.; Qu, Y.; Zhang, W. In-Situ Synthesis of Multicompartment Nanoparticles of Linear BAC Triblock Terpolymer by Seeded RAFT Polymerization. Macromolecules 2014, 47, 2340–2349.
- Davaran, S.; Ghamkhari, A.; Alizadeh, E.; Massoumi, B.; Jaymand, M. Novel dual stimuli-responsive ABC triblock copolymer: RAFT synthesis, “schizophrenic” micellization, and its performance as an anticancer drug delivery nanosystem. J. Colloid Interface Sci. 2017, 488, 282–293.Qu, Y.; Huo, F.; Li, Q.; He, X.; Li, S.; Zhang, W. In situ synthesis of thermo-responsive ABC triblock terpolymer nano-objects by seeded RAFT polymerization. Polym. Chem. 2014, 5, 5569–5577.
- Huang, Y.; Yong, P.; Chen, Y.; Gao, Y.; Xu, W.; Lv, Y.; Yang, L.; Reis, R.L.; Pirraco, R.P.; Chen, J. Micellization and gelatinization in aqueous media of pH- and thermo-responsive amphiphilic ABC (PMMA82-b-PDMAEMA150-b-PNIPAM65) triblock copolymer synthesized by consecutive RAFT polymerization. RSC Adv. 2017, 7, 28711–28722.Li, Z.; Ma, J.; Lee, N.S.; Wooley, K.L. Dynamic cylindrical assembly of triblock copolymers by a hierarchical process of covalent and supramolecular interactions. J. Am. Chem. Soc. 2011, 133, 1228–1231.
- Banerjee, R.; Dhara, D. Functional group-dependent self-assembled nanostructures from thermo-responsive triblock copolymers. Langmuir 2014, 30, 4137–4146.Muslim, A.; Malik, D.; Hojiahmat, M. RAFT polymerization of linear ABC triblock copolymer PtBA-b-PS-b-P2VP and regulation of its hierarchical self-assembly structure in solution. Chem. Pap. 2015, 69, 1512–1518.
- Koonar, I.; Zhou, C.; Hillmyer, M.A.; Lodge, T.P.; Siegel, R.A. ABC triblock terpolymers exhibiting both temperature- and pH-sensitive micellar aggregation and gelation in aqueous solution. Langmuir 2012, 28, 17785–17794.Fernandez-Alvarez, R.; Hlavatovičová, E.; Rodzeń, K.; Strachota, A.; Kereïche, S.; Matějíček, P.; Cabrera-González, J.; Núñez, R.; Uchman, M. Synthesis and self-assembly of a carborane-containing ABC triblock terpolymer: Morphology control on a dual-stimuli responsive system. Polym. Chem. 2019, 10, 2774–2780.
- Guang, N.-e.; Liu, S.-x.; Li, X.; Tian, L.; Mao, H.-g. Micellization and gelation of the double thermoresponsive ABC-type triblock copolymer synthesized by RAFT. Chin. J. Polym. Sci. 2016, 34, 965–980.
- Li, Q.; Gao, C.; Li, S.; Huo, F.; Zhang,W. Doubly thermo-responsive ABC triblock copolymer nanoparticles prepared through dispersion RAFT polymerization. Polym. Chem. 2014, 5, 2961–2972.
- Huo, F.; Li, S.; Li, Q.; Qu, Y.; Zhang, W. In-Situ Synthesis of Multicompartment Nanoparticles of Linear BAC Triblock Terpolymer by Seeded RAFT Polymerization. Macromolecules 2014, 47, 2340–2349.
- Qu, Y.; Huo, F.; Li, Q.; He, X.; Li, S.; Zhang,W. In situ synthesis of thermo-responsive ABC triblock terpolymer nano-objects by seeded RAFT polymerization. Polym. Chem. 2014, 5, 5569–5577.
- Li, Z.; Ma, J.; Lee, N.S.; Wooley, K.L. Dynamic cylindrical assembly of triblock copolymers by a hierarchical process of covalent and supramolecular interactions. J. Am. Chem. Soc. 2011, 133, 1228–1231.
- Muslim, A.; Malik, D.; Hojiahmat, M. RAFT polymerization of linear ABC triblock copolymer PtBA-b-PS-b-P2VP and regulation of its hierarchical self-assembly structure in solution. Chem. Pap. 2015, 69, 1512–1518.
- Fernandez-Alvarez, R.; Hlavatoviˇcová, E.; Rodze´ n, K.; Strachota, A.; Kereïche, S.; Matˇejíˇcek, P.; Cabrera-González, J.; Núñez, R.; Uchman, M. Synthesis and self-assembly of a carborane-containing ABC triblock terpolymer: Morphology control on a dual-stimuli responsive system. Polym. Chem. 2019, 10, 2774–2780.
