Influenza could circulate in parallel with COVID-19. In the context of COVID-19, some studies observed inverse associations between influenza vaccination and SARS-CoV-2 infection and clinical outcomes, while others did not. We conducted a meta-analysis to assess the association between influenza vaccination and SARS-CoV-2 infection and clinical outcomes, aiming to provide evidence for COVID-19 prevention and vaccination promotion. We searched four databases from inception to 10 March, 2021. Random effects and fixed effects models were used to pool odds ratios (ORs) and adjusted estimates with 95% confidence intervals (CIs).
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
The coronavirus disease (COVID-19) is an acute respiratory infectious disease that was declared a global public health emergency by the World Health Organization (WHO) in January 2020
[1]. The global pandemic has hitherto caused 119 million cases of infection and 2 million cases of death
[1], and imposed tremendous burden on global health and worldwide economics. Thus, effective cures and vaccines are imperatively needed to curtail the pandemic and decrease mortality. Seasonal influenza occurs from fall to spring annually, characterized by the circulation of influenza A or B virus
[2]. Influenza and its complications could lead to increased worldwide mortality and morbidity, which remain a public health threat.
Due to the seasonality of influenza outbreaks and the continuous prevalence of COVID-19, influenza could circulate in parallel with COVID-19, which largely increases the potential risk of co-infection. Though little is known about the epidemiology and clinical outcomes of co-infection, extant literature has found that the co-infection with influenza A virus enhances the infectivity of COVID-19 in a broad range of cell types
[3], whereas co-infected patients seem to present similar clinical symptoms and radiological images compared with patients infected with COVID-19 alone
[4,5][4][5]. In the context of the COVID-19 pandemic, the dual infection of influenza and COVID-19 could bring extra burden to health care services by utilizing limited medical resources, increasing the difficulty of treatment and the uncertainty of prognosis. Annual influenza vaccination has long been recommended by WHO to prevent influenza, especially to the high-risk populations with disproportionate infection and severe complications, such as older adults (aged > 65 years) and pregnant women
[6]. To date, no highly effective pharmaceutical treatment is available against COVID-19
[7]. Though COVID-19 vaccines remain the most effective long-term solution to combat COVID-19 pandemic
[8], the overall effectiveness and safety of the licensed COVID-19 vaccines remain to be fully evaluated based on real-world evidence.
According to a previous study by Wolff
[9], which investigated influenza vaccine-related virus interference by specific respiratory viruses (e.g., coronavirus, human bocavirus, and adenovirus), there was an increased odd of coronavirus in individuals receiving influenza vaccination. This finding raised much concerns of the possible relationship between influenza vaccination and coronavirus, especially in the COVID-19 pandemic. In addition, as COVID-19 and influenza are both respiratory infectious diseases caused by enveloped RNA viruses that share similarities in transmission routes and clinical characteristics
[10], more and more researchers began to seek for relationships between SARS-CoV-2 infection and influenza immunity. Based on the assumptions, Del Riccio et al.
[11] conducted a systematic review and found that there was overall no evidence to suggest a negative impact of influenza vaccination on SARS-CoV-2 related infections, illness, or deaths, while some of the included studies even reported significantly inverse associations. Though some of the recent studies have found that influenza vaccine uptake was negatively associated with COVID-19 incidence
[12[12][13],
13], severity
[13,14][13][14], and mortality
[13[13][15],
15], others showed no evidence of such associations
[16,17,18][16][17][18]. Therefore, a systematic review and meta-analysis of the association between influenza vaccination and SARS-CoV-2 infection and its outcomes is needed to provide conclusive evidence.
In the dual epidemics of COVID-19 and influenza, influenza vaccination has a more significant implication than ever for preventing both influenza and COVID-19. It is especially of great necessity for vulnerable populations to receive influenza vaccination. Given the limited data of COVID-19 vaccine effectiveness among vulnerable groups, as well as the necessity of influenza vaccination in the context of COVID-19, and the lack of conclusive evidence of influenza vaccination’s effect on SARS-CoV-2 infection and its clinical outcomes, there is a need to systematically assess the potential association between influenza vaccination and COVID-19.
2. Study Selection and Study Characteristics
A total of 2895 records were retrieved from the four databases and 1467 duplicates were excluded. After screening titles and abstracts, we excluded 1387 reviews, conference papers, animal experiments, case reports, and other studies irrelevant to the subject or published before December 2019. Among the 41 articles assessed based on full texts, 25 articles were excluded for lacking specific data or did not meet the inclusion criteria. A total of 16 studies were finally included in the review (12 studies on the association between influenza vaccination and SARS-CoV-2 infection
[16,18,21,22,23,24,25[16][18][19][20][21][22][23][24][25][26][27][28],
26,27,28,29,30], 6 on the association between influenza vaccination and COVID-19 clinical outcomes
[10[10][17][20][24][29][30],
17,22,26,31,32], 2 studies containing data on both the associations
[22,26][20][24]. Nine of the 12 studies on the association between influenza vaccination and SARS-CoV-2 infection contained adjusted estimates. One
[21][19] of the 16 studies has moderate risk of bias, while the others have low risk of bias. The primary outcome (the association between influenza vaccination and SARS-CoV-2 infection) comprised a total of 208,132 people (72,820 vaccinated and 135,112 unvaccinated). The secondary outcome (the association between influenza vaccination and clinical outcomes of SARS-CoV-2 infection) comprised a total of 82,684 COVID-19 patients and was assessed by different outcomes (mortality, intensive care, and hospitalization). The study selection procedure is shown in . The baseline characteristics of the included studies are listed in (primary outcome) and (secondary outcome). The adjusted variables of the included studies were basically age, sex, comorbidities, prescribed medications and smoking status, but were not the same across studies (see and ).
Figure 1. PRISMA flow diagram of the study selection procedure.
Table 1. Baseline characteristics of the 12 included studies that assessed the association between influenza vaccination and SARS-CoV-2 infection.
| Study |
Study Design |
Vaccination Season |
Identification of COVID-19 |
Country |
Sample Size |
Infected (n)/Vaccinated(n) |
Infected (n)/Unvaccinated (n) |
Adjusted Estimate (95%CI) |
Quality Score and Risk of Bias Assessment |
Adjusted Factors |
| Massoudi et al., (2021) [ |
| 693/14,204 |
| 0.76 (0.68–0.86) |
| 8 (low) |
| Ethnicity, race, sex, age, BMI, Elixhauser score, smoking status, and comorbidities |
Table 2. Baseline characteristics of the six included studies that assessed the association between influenza vaccination and SARS-CoV-2 outcomes.
| Study |
Study Design |
Vaccination Season |
Identification of COVID-19 |
Country |
Sample Size |
Events (n)/Vaccinated(n) |
Events (n)/Unvaccinated (n) |
Adjusted Estimate (95%CI) |
Quality Score and Risk of Bias Assessment |
Adjusted Factors |
| 21] | Massoudi et al., (2021) [19] |
Case-control study |
2019–2020 |
pulmonologist-confirmed |
Iran |
261 |
3/90 |
77/171 |
| Intensive Care |
| - |
6(moderate) |
- |
| Kissling et al., (2021) [18] |
Case-control study |
2019–2020 |
rt-PCR |
| Pawlowski et al., (2020) [26] | Pawlowski et al., (2020) [24] | Europe |
Retrospective cohort study |
2019–2020 |
rt-PCR | a |
1701 |
68/429 |
157/1272 |
0.93 (0.66–1.32) |
America |
959 |
15/441 |
16/5188 (low) |
-Study site, time, age, sex, and chronic condition |
| 8 (low) |
- |
Ragni et al., (2020) [22] | Ragni et al., (2020) [20] |
Case-control study |
2019–2020 |
rt-PCR |
Italy |
17,608 |
1676/5427 |
3209/12,181 |
0.89 (0.80–0.99) |
9 (low) |
| de la Cruz Conty et al., (2021) | a | [17 | Age, sex, Charlson index, and time of the swab test |
| ] |
Prospective cohort study |
- | b |
rt-PCR |
Spain |
1150 |
7/438 |
15/712 |
- |
7 (low) |
- |
Belingheri et al., (2020) [23] | Belingheri et al., (2020) [21] |
Cross-sectional study |
| Fink et al., (2020) [10] |
Retrospective cohort study | 2019–2020 |
rt-PCR |
Italy |
3520 |
28/817 |
100/2703 |
0.41 (0.07–2.39) |
7 (low) |
- Age, sex, and an interaction term between age and the vaccination intake in 2019/3020 |
| b |
Clinical diagnosis | c |
Brazil |
53,752 |
- |
- |
0.93 (0.87–0.99) |
7 (low) |
Age, sex, race, educational level, treatment facility, and comorbidities |
Vila-Córcoles et al., (2020) [24] | Vila-Córcoles et al., (2020) [22] |
Retrospective cohort study |
| Yang et al., (2021) [ | 2019–2020 |
32] | rt-PCR |
Yang et al., (2021) [30Spain |
]1547 |
189/705 |
160/842 |
0.63 (0.43–0.92) | b |
8 (low) |
Age, sex, and comorbidities |
| Retrospective cohort study |
2019–2020 |
rt-PCR |
America |
2005 |
3/214 |
133/1791 |
0.30 (0.07–0.85) |
8 (low) |
Age, sex race/ethnicity, hypertension, and comorbidities |
Pawlowski et al., (2021) [26] | Pawlowski et al., (2021) [24] |
Retrospective cohort study |
2019–2020 |
rt-PCR |
America |
25,582 |
442/12,791 |
521/12,791 |
- |
8 (low) |
| Hospitalization |
| - |
| Pawlowski et al., (2020) [26] | Pawlowski et al., (2020) [24] |
Retrospective cohort study |
2019–2020 |
rt-PCR |
America |
959 |
74/441 |
78/518 |
- |
8 (low) |
- |
3. The Association between Influenza Vaccination and COVID-19 and Its Outcomes
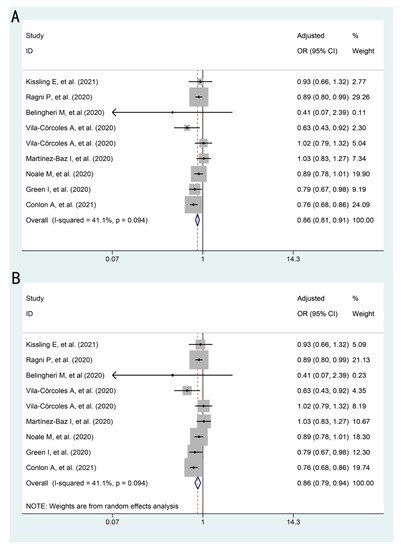
The association between influenza vaccination and SARS-CoV-2 infection is presented in . Influenza vaccination was shown to be associated with a lower risk of SARS-CoV-2 infection in both models (fixed effects model: pooled adjusted OR: 0.86, 95%CI: 0.81–0.91; random effects model: pooled adjusted OR: 0.86, 95%CI: 0.79–0.94).
Figure 2. Forest plots for the association between influenza vaccination and SARS-CoV-2 infection: (A) adjusted OR by fixed effects model (B) adjusted OR by random effects model.
Table 3. Summary of the overall association between influenza vaccination and SARS-CoV-2 infection and clinical outcomes.
| Outcomes |
Number of Studies |
I2 | Value (%) |
p | Value |
Adjusted Estimates | a | (95%CI) |
| Fixed Effects Model |
Random Effects Model |
| Brazil |
| 53,752 |
| - |
| SARS-CoV-2 infection |
9 |
41.1 |
0.09 |
| 0.63 (0.22–1.81) |
| - |
| 0.84 (0.77–0.91) |
| 7 (low) |
| Age, sex, race, educational level, treatment facility, and comorbidities |
| 0.86 (0.81–0.91) |
0.86 (0.79–0.94) |
| Intensive care |
2 |
68.2 |
0.08 |
0.93 (0.87–0.99) |
| Hospitalization |
3 |
87.6 |
<0.01 |
0.84 (0.75–0.93) |
0.74 (0.51–1.06) |
| Mortality |
3 |
82.5 |
<0.01 |
0.86 (0.81–0.93) |
0.89 (0.73–1.09) |
Jehi et al., (2020) [27] | Jehi et al., (2020) [25] |
Prospective cohort study |
- | c |
rt-PCR |
America |
11,672 |
384/6324 |
434/5348 |
- |
7 (low) |
- |
| Vila-Córcoles et al., (2020) | d [25] | [23] |
Retrospective cohort study |
2019–2020 |
rt-PCR |
Spain |
78,883 |
205/22,606 |
175/56,277 |
1.02 (0.79–1.32) | b |
7 (low) |
Age, sex, comorbidities, and medications use. |
| Yang et al., (2021) [32] | Yang et al., (2021) [30] |
Retrospective cohort study |
2019–2020 |
rt-PCR |
America |
2005 |
43/214 |
747/1791 |
0.41 (0.28–0.60) |
8 (low) |
Age, sex race/ethnicity, hypertension, and comorbidities |
Martínez-Baz et al., (2020) | e | [16] |
Prospective cohort study |
2019–2020 |
rt-PCR |
| Ragni et al., (2020) [22] | Spain |
Ragni et al., (2020) | 10,714 |
155/3677 |
248/7037 |
1.03 (0.83–1.27) |
7 (low) |
Age groups, sex, major chronic conditions, profession, and any ILI diagnosis in the previous five years |
| [20] |
retrospective cohort study |
2019–2020 |
rt-PCR |
Italy |
17,608 |
- |
- |
0.84 (0.83–1.29) | d |
7 (low) |
Age, sex, Charlson index, and time of the swab test |
Noale et al., (2020) [28] | Noale et al., (2020) [26] |
| Wilcox et al., (2021) | e | Cross-sectional study |
[31] | [29]2019–2020 |
rt-PCR |
Italy |
6680 |
562/2246 |
1114/4434 |
retrospective cohort study |
2019–2020 |
rt-PCR0.89 (0.78–1.01) |
8 (low) |
Age, sex, education, area of residence, self-reported comorbidities, and smoking status |
| England |
6921 |
1166/2613 |
1584/4308 |
0.85 (0.75–0.97) |
8 (low) |
Age, sex, BMI, socioeconomic status, smoking status, frailty score, comorbidities, and the number of prescribed medications |
Green et al., (2020) [29] | Green et al., (2020) [27] |
Cross-sectional study |
2019–2020 |
rt-PCR |
Israel |
22,563 |
244/4711 |
1580/17,852 |
0.79 (0.67–0.98) |
9 (low) |
Age, ethnic, smoking status, socioeconomic status, and comorbidities |
| Mortality |
|
Conlon et al., (2021) [30] | Conlon et al., (2021) [28] |
| Fink et al., (2020) [10] | Retrospective cohort study |
2019–2020 |
rt-PCR |
America |
27,201 |
Retrospective cohort study |
- | b |
Clinical diagnosis | c | 525/12,997 | Ragni et al., (2020) [22] | Ragni et al., (2020) [20] |
retrospective cohort study |
2019–2020 |
rt-PCR |
Italy |
17,608 |
- |
- |
1.14 (0.95–1.37) | d |
7 (low) |
Age, sex, Charlson index, and time of the swab test |
| Wilcox et al., (2021) [31] | Wilcox et al., (2021) [29] |
retrospective cohort study |
2019–2020 |
rt-PCR |
England |
6921 |
372/2613 |
553/4308 |
0.76 (0.64–0.90) |
8 (low) |
Age, sex, BMI, socioeconomic status, smoking status, frailty score, comorbidities, and the number of prescribed medications |
The association between influenza vaccination and COVID-19 outcomes are presented in . The association between influenza vaccination and intensive care (adjusted OR: 0.63, 95%CI: 0.22–1.81), hospitalization (adjusted OR: 0.74, 95%CI: 0.51–1.06), or mortality (adjusted OR: 0.89, 95%CI: 0.73–1.09) among COVID-19 patients was not statistically significant by random effects model, while results by fixed effects model was somehow significant. This may be due to the substantial heterogeneity between the small number of studies (2–3 studies) and participants involved in each outcome.