The Neuropeptide S (NPS) system was discovered by a "reverse pharmacology" approach in search for the endogenous ligand of an orphan G protein-coupled receptor. Its peptide ligand and receptor are mainly found in the brain. Effects on anxiety and memory have been described for NPS, as well as genetic associations of the receptor gene with asthma and inflammatory diseases.
- transmitter
- GPCR
- brainstem
- anxiety
- memory
- genetic variation
1. Introduction
Neuropeptide S (NPS) was discovered as a ligand of a previously orphan G protein-coupled receptor (GPCR) by using the “orphan receptor strategy”, also known as “reverse pharmacology” [1]. The receptor (previously known as GPR154 or GPRA) was stably expressed in cells that served as a bait to purify the endogenous ligand from brain extracts [2]. The ligand turned out to be a peptide of 20 amino acids that contains a perfectly conserved serine (single amino acid code “S”) at the N-terminus in all species analyzed, and was thus termed accordingly [3]. NPS is encoded as a single copy peptide by a rather small precursor protein (<90 amino acids) that occurs in the genome of all tetrapods but is absent from fish [4]. The seven N-terminal residues are identical in all tetrapods, as well as the overall 20 amino acid length, while the C-terminal half shows more variation (Figure 1).
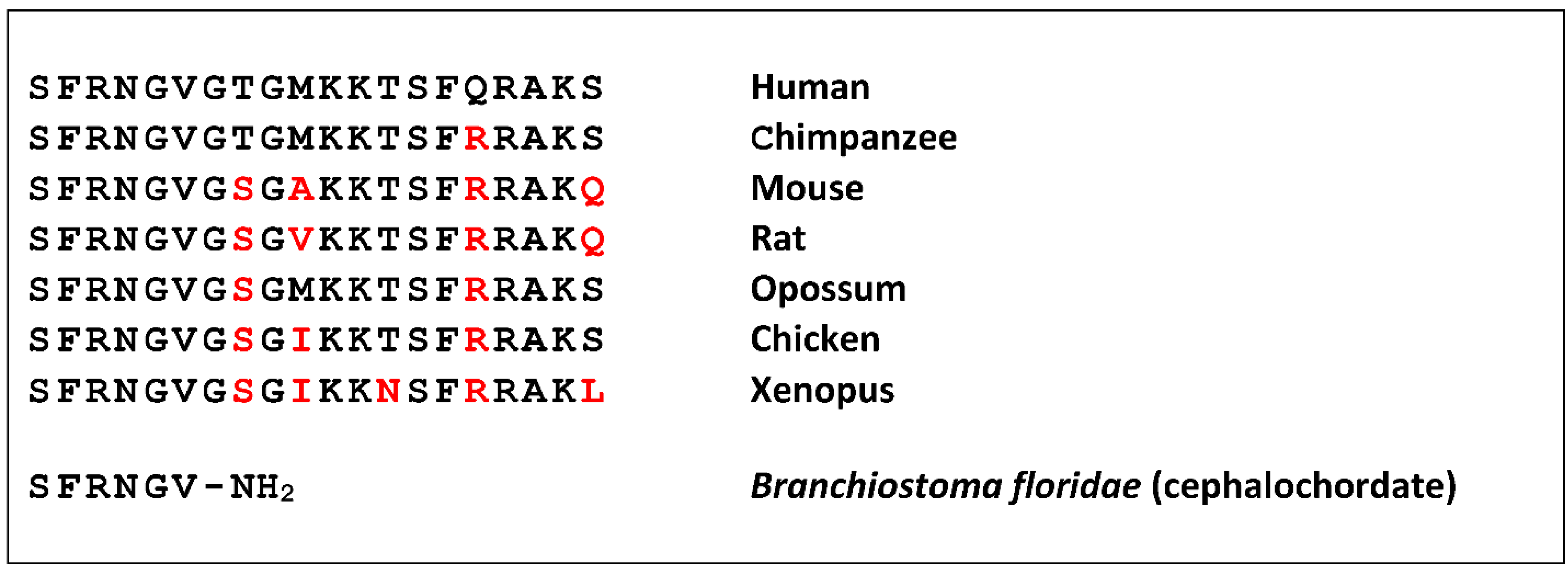
A shortened peptide has been identified in a cephalochordate that contains the conserved amino terminal residues
[5]. Together with the identification of a distantly-related GPCR from the same class of animals, this suggests a rather complex evolution of the NPS system in bilaterians, where teleost fish may have lost both genes
[6].
NPSR1 is also a single-copy gene with moderate similarity to other peptide GPCRs, the closest being vasopressin 1A and oxytocin receptors. NPSR1 is found in the genomes of all tetrapods and no convincing orthologues have been identified in fish genomes
[7].
2. General Pharmacology
NPSR1 couples via Gα
sand Gα
qto elevate intracellular cAMP and Ca
2+, thus it is an excitatory GPCR
[8]. Activation of mitogen-activated protein kinase (MAPK) pathways and opening of neuronal Ca
2+channels have also been described
[8][9][10]. NPS activates its receptor at low nanomolar concentrations and structure-activity relationship (SAR) studies have confirmed the importance of the amino terminus for agonist activity, overlapping with the high evolutionary conservation of this part of the peptide structure
[11][12][13]. Focused SAR studies performed on Gly
5lead to the identification of peptidergic NPSR1 antagonists, characterized by a D-amino acid with a short branched aliphatic side chain
[14][15]. Some well-characterized NPSR1 antagonists identified in the frame of these studies are [D-Cys(
t-Bu)
5]NPS, [D-Val
5]NPS, and [
t-Bu-D-Gly
5]NPS
[15][16][17].
The prototype synthetic NPSR1 antagonist is SHA 68, belonging to the class of diphenyltetrahydro-1
H-oxazolo [3,4-α]pyrazines
[18](Figure 2). SHA 68, and the closely related SHA 66, display nanomolar affinity for NPSR1 in vitro but only limited bioavailability in vivo, due to its high lipophilicity. Using the core structure of SHA 68, several refinements have been made to improve in vivo potency. It appears that slight increases in polarity can indeed cause an increased in vivo potency, albeit with a net loss in receptor affinity
[19][20][21][22]. Additional high-throughput drug screening programs have yielded further structures with NPSR1 antagonistic profiles (Figure 2), although with lower in vitro and in vivo potency compared to SHA 68
[23][24][25]. None of these compounds has progressed beyond the preclinical stage yet.
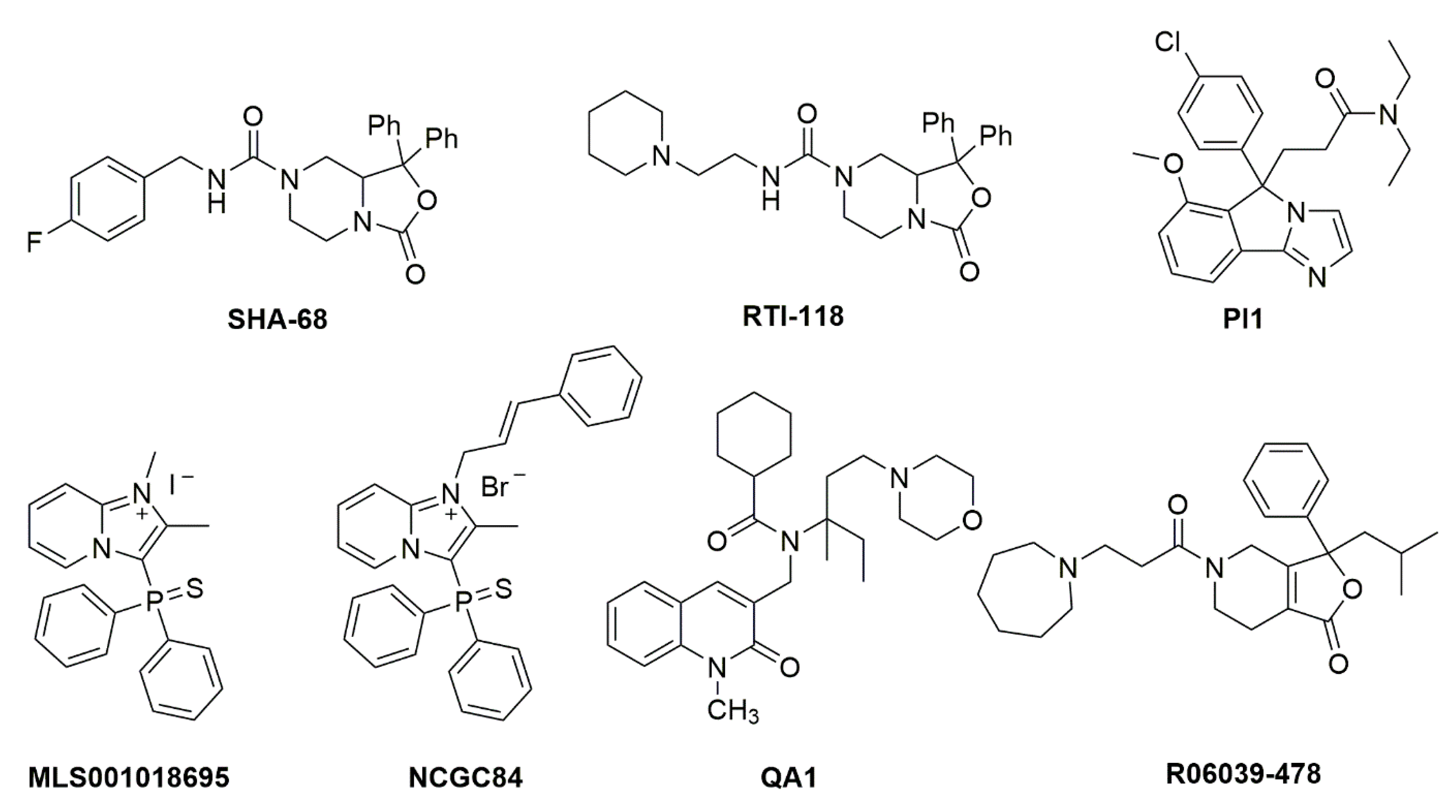
Figure 2. Chemical structures of NPSR1 antagonists. Details about synthesis and pharmacological activities can be found in the original literature. SHA 68 [18], RTI-118 [26], PI1 [27], MLS001018695 [28], NCGC84 [23], QA1 [29], R06039-478 [30].
3. Physiological and Behavioral Effects of NPS
3. Physiological and Behavioral Effects of NPS
3.1. Modulation of Animal Behavior
3.2. NPS and Immune Functions
4. Concluding Remarks
An impressive number of physiological functions have been identified for the NPS system in the relatively short time since its discovery. Together with the plethora of genetic association data for NPSR1 variants with human disease and behaviors, increased efforts to identify therapeutic applications for this interesting transmitter system appear to be promising and warranted. The articles in this Special Issue reflect the continuing progress in our knowledge about the NPS system and its therapeutic potential.
References
- Civelli, O.; Saito, Y.; Wang, Z.; Nothacker, H.-P.; Reinscheid, R.K. Orphan GPCRs and their ligands. Pharmacol. Ther. 2006, 110, 525–532.Xu, Y.-L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; Brucher, F.A.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497.
- Sato, S.; Shintani, Y.; Miyajima, N.; Yoshimura, K. Novel G protein-coupled receptor protein and DNA thereof. World Patent WO 02/31145 A1, 18 April 2002.Rizzi, A.; Vergura, R.; Marzola, G.; Ruzza, C.; Guerrini, R.; Salvadori, S.; Regoli, D.; Calo, G. Neuropeptide S is a stimulatory anxiolytic agent: A behavioural study in mice. Br. J. Pharmacol. 2008, 154, 471–479.
- Xu, Y.-L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; Brucher, F.A.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497.Leonard, S.K.; Dwyer, J.M.; Sukoff Rizzo, S.J.; Platt, B.; Logue, S.F.; Neal, S.J.; Malberg, J.E.; Beyer, C.E.; Schechter, L.E.; Rosenzweig-Lipson, S.; et al. Pharmacology of neuropeptide S in mice: Therapeutic relevance to anxiety disorders. Psychopharmacology 2008, 197, 601–611.
- Reinscheid, R.K. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides 2007, 28, 830–837.Ionescu, I.A.; Dine, J.; Yen, Y.C.; Buell, D.R.; Herrmann, L.; Holsboer, F.; Eder, M.; Landgraf, R.; Schmidt, U. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology 2012, 37, 1323–1337.
- Elphick, M.R. NG peptides: A novel family of neurophysin-associated neuropeptides. Gene 2010, 458, 20–26.Dine, J.; Ionescu, I.A.; Avrabos, C.; Yen, Y.C.; Holsboer, F.; Landgraf, R.; Schmidt, U.; Eder, M. Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a “normal”-anxiety one. PLoS ONE 2015, 10, e0120272.
- Mirabeau, O.; Joly, J.S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. USA 2013, 110, E2028–E2037.Slattery, D.A.; Naik, R.R.; Grund, T.; Yen, Y.C.; Sartori, S.B.; Füchsl, A.; Finger, B.C.; Elfving, B.; Nordemann, U.; Guerrini, R.; et al. Selective breeding for high anxiety introduces a synonymous SNP that increases Neuropeptide S receptor activity. J. Neurosci. 2015, 35, 4599–4613.
- Pitti, T.; Manoj, N. Molecular evolution of the neuropeptide S receptor. PLoS ONE 2012, 7, e34046.Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291.
- Reinscheid, R.K.; Xu, Y.-L.; Okamura, N.; Zeng, J.; Chung, S.; Pai, R.; Wang, Z.; Civelli, O. Pharmacological characterization of human and murine neuropeptide S receptor variants. J. Pharmacol. Exp. Ther. 2005, 315, 1338–1345.Okamura, N.; Garau, C.; Duangdao, D.M.; Clark, S.D.; Jüngling, K.; Pape, H.-C.; Reinscheid, R.K. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology 2011, 36, 744–752.
- Pulkkinen, V.; Ezer, S.; Sundman, L.; Hagström, J.; Remes, S.; Söderhäll, C.; Dario, G.; Haglund, C.; Kere, J.; Arola, J. Neuropeptide S receptor 1 (NPSR1) activates cancer-related pathways and is widely expressed in neuroendocrine tumors. Virchows Arch. 2014, 465, 173–183.Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405.
- Erdmann, F.; Kügler, S.; Blaesse, P.; Lange, M.D.; Skryabin, B.V.; Pape, H.C.; Jüngling, K. Neuronal expression of the human neuropeptide S receptor NPSR1 identifies NPS-induced calcium signaling pathways. PLoS ONE 2015, 10, e0117319.Han, R.W.; Zhang, R.S.; Xu, H.J.; Chang, M.; Peng, Y.L.; Wang, R. Neuropeptide S enhances memory and mitigates memory impairment induced by MK801, scopolamine or Aβ1-42 in mice novel object and object location recognition tasks. Neuropharmacology 2013, 70, 261–267.
- Roth, A.L.; Marzola, E.; Rizzi, A.; Arduin, M.; Trapella, C.; Corti, C.; Vergura, R.; Martinelli, P.; Salvadori, S.; Regoli, D.; et al. Structure-activity studies on neuropeptide S: Identification of the amino acid residues crucial for receptor activation. J. Biol. Chem. 2006, 281, 20809–20816.Jüngling, K.; Seidenbecher, T.; Sosulina, L.; Lesting, J.; Sangha, S.; Clark, S.D.; Okamura, N.; Duangdao, D.M.; Xu, Y.-L.; Reinscheid, R.K.; et al. Neuropeptide S-Mediated Control of Fear Expression and Extinction: Role of Intercalated GABAergic Neurons in the Amygdala. Neuron 2008, 59, 298–310.
- Camarda, V.; Trapella, C.; Caló, G.; Guerrini, R.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Synthesis and biological activity of human neuropeptide S analogues modified in position 2. J. Med. Chem. 2008, 51, 655–658.Sartori, S.B.; Maurer, V.; Murphy, C.; Schmuckermair, C.; Muigg, P.; Neumann, I.D.; Whittle, N.; Singewald, N. Combined neuropeptide S and D-cycloserine augmentation prevents the return of fear in extinction-impaired rodents: Advantage of dual versus single drug approaches. Int. J. Neuropsychopharmacol. 2016, 19, 1–11.
- Camarda, V.; Trapella, C.; Calo’, G.; Guerrini, R.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Structure-activity study at positions 3 and 4 of human neuropeptide S. Bioorganic Med. Chem. 2008, 16, 8841–8845.Okamura, N.; Reinscheid, R.K.; Ohgake, S.; Iyo, M.; Hashimoto, K. Neuropeptide S attenuates neuropathological, neurochemical and behavioral changes induced by the NMDA receptor antagonist MK-801. Neuropharmacology 2010, 58, 166–172.
- Guerrini, R.; Camarda, V.; Trapella, C.; Calò, G.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Synthesis and biological activity of human neuropeptide S analogues modified in position 5: Identification of potent and pure neuropeptide S receptor antagonists. J. Med. Chem. 2009, 52, 524–529.Smith, K.L.; Patterson, M.; Dhillo, W.S.; Patel, S.R.; Semjonous, N.M.; Gardiner, J.V.; Ghatei, M.A.; Bloom, S.R. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology 2006, 147, 3510–3518.
- Guerrini, R.; Camarda, V.; Trapella, C.; Caló, G.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Further studies at neuropeptide S position 5: Discovery of novel neuropeptide S receptor antagonists. J. Med. Chem. 2009, 52, 4068–4071.Zhu, H.; Mingler, M.K.; McBride, M.L.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Williams, M.T.; Vorhees, C.V.; Rothenberg, M.E. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 2010, 35, 1119–1132.
- Ruzza, C.; Rizzi, A.; Camarda, V.; Pulga, A.; Marzola, G.; Filaferro, M.; Novi, C.; Ruggieri, V.; Marzola, E.; Vitale, G.; et al. [tBu-D-Gly5]NPS, a pure and potent antagonist of the neuropeptide S receptor: In vitro and in vivo studies. Peptides 2012, 34, 404–411.Si, W.; Aluisio, L.; Okamura, N.; Clark, S.D.; Fraser, I.; Sutton, S.W.; Bonaventure, P.; Reinscheid, R.K. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J. Neurochem. 2010, 115, 475–482.
- Camarda, V.; Rizzi, A.; Ruzza, C.; Zucchini, S.; Marzola, G.; Marzola, E.; Guerrini, R.; Salvadori, S.; Reinscheid, R.K.; Regoli, D.; et al. In vitro and in vivo pharmacological characterization of the neuropeptide S receptor antagonist [D-Cys(tBu)5]neuropeptide S. J. Pharmacol. Exp. Ther. 2009, 328, 549–555.Li, W.; Chang, M.; Peng, Y.L.; Gao, Y.H.; Zhang, J.N.; Han, R.W.; Wang, R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul. Pept. 2009, 156, 90–95.
- Okamura, N.; Habay, S.A.; Zeng, J.; Chamberlin, A.R.; Reinscheid, R.K. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J. Pharmacol. Exp. Ther. 2008, 325, 893–901.Peng, Y.L.; Zhang, J.N.; Chang, M.; Li, W.; Han, R.W.; Wang, R. Effects of central neuropeptide S in the mouse formalin test. Peptides 2010, 31, 1878–1883.
- Ruzza, C.; Rizzi, A.; Trapella, C.; Pela’, M.; Camarda, V.; Ruggieri, V.; Filaferro, M.; Cifani, C.; Reinscheid, R.K.; Vitale, G.; et al. Further studies on the pharmacological profile of the neuropeptide S receptor antagonist SHA 68. Peptides 2010, 31, 915–925.Victor Holanda, A.D.; Asth, L.; Santos, A.R.; Guerrini, R.; Soares-Rachetti, V.D.P.; Calo’, G.; André, E.; Gavioli, E.C. Central adenosine A1 and A2A receptors mediate the antinociceptive effects of neuropeptide S in the mouse formalin test. Life Sci. 2015, 120, 8–12.
- Trapella, C.; Pela, M.; Del Zoppo, L.; Calo, G.; Camarda, V.; Ruzza, C.; Cavazzini, A.; Costa, V.; Bertolasi, V.; Reinscheid, R.K.; et al. Synthesis and separation of the enantiomers of the neuropeptide S receptor antagonist (9 R/S)-3-Oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic Acid 4-fluoro-benzylamide (SHA 68). J. Med. Chem. 2011, 54, 2738–2744.Holanda, V.A.D.; Oliveira, M.C.; Souza, L.S.; Lobão-Soares, B.; André, E.; Da Silva Junior, E.D.; Guerrini, R.; Calo, G.; Ruzza, C.; Gavioli, E.C. Dopamine D1 and D2 receptors mediate neuropeptide S-induced antinociception in the mouse formalin test. Eur. J. Pharmacol. 2019, 859, 172557.
- Hassler, C.; Zhang, Y.; Gilmour, B.; Graf, T.; Fennell, T.; Snyder, R.; Deschamps, J.R.; Reinscheid, R.K.; Garau, C.; Runyon, S.P. Identification of neuropeptide s antagonists: Structure-Activity relationship studies, x-ray crystallography, and in vivo evaluation. ACS Chem. Neurosci. 2014, 5, 731–744.Lee, M.T.; Chiu, Y.T.; Chiu, Y.C.; Hor, C.C.; Lee, H.J.; Guerrini, R.; Calo, G.; Chiou, L.C. Neuropeptide S-initiated sequential cascade mediated by OX1, NK1, mGlu5 and CB1 receptors: A pivotal role in stress-induced analgesia. J. Biomed. Sci. 2020, 27, 7.
- Albanese, V.; Ruzza, C.; Marzola, E.; Bernadi, T.; Fabbri, M.; Fantinati, A.; Trapella, C.; Reinscheid, R.K.; Ferrari, F.; Sturaro, C.; et al. Structure-Activity Relationship Studies on Oxazolo[3,4-a]pyrazine Derivatives Leading to the Discovery of a Novel Neuropeptide S Receptor Antagonist with Potent In Vivo Activity. J. Med. Chem. 2021.Jinushi, K.; Kushikata, T.; Kudo, T.; Calo, G.; Guerrini, R.; Hirota, K. Central noradrenergic activity affects analgesic effect of Neuropeptide S. J. Anesth. 2018, 32, 48–53.
- Thorsell, A.; Tapocik, J.D.; Liu, K.; Zook, M.; Bell, L.; Flanigan, M.; Patnaik, S.; Marugan, J.; Damadzic, R.; Dehdashti, S.J.; et al. A novel brain penetrant NPS receptor antagonist, NCGC00185684, blocks alcohol-induced ERK-phosphorylation in the central amygdala and decreases operant alcohol self-administration in rats. J. Neurosci. 2013, 33, 10132–10142.Peng, Y.L.; Han, R.W.; Chang, M.; Zhang, L.; Zhang, R.S.; Li, W.; Han, Y.F.; Wang, R. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides 2010, 31, 2259–2263.
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. Coumarinyl pyranopyrimidines as new neuropeptide S receptor antagonists; design, synthesis, homology and molecular docking. Bioorg. Chem. 2017, 75, 274–290.Cifani, C.; Di Bonaventura, M.V.M.; Cannella, N.; Fedeli, A.; Guerrini, R.; Calo, G.; Ciccocioppo, R.; Ubaldi, M. Effect of neuropeptide S receptor antagonists and partial agonists on palatable food consumption in the rat. Peptides 2011, 32, 44–50.
- Ruzza, C.; Calò, G.; Di Maro, S.; Pacifico, S.; Trapella, C.; Salvadori, S.; Preti, D.; Guerrini, R. Neuropeptide S receptor ligands: A patent review (2005–2016). Expert Opin. Ther. Pat. 2017, 27, 347–362.Didonet, J.J.; Cavalcante, J.C.; Souza, L.D.; Costa, M.S.; André, E.; Soares-Rachetti, V.D.; Guerrini, R.; Gavioli, E.C. Neuropeptide S counteracts 6-OHDA-induced motor deficits in mice. Behav. Brain Res. 2014, 266, 29–36.
- Schmoutz, C.D.; Zhang, Y.; Runyon, S.P.; Goeders, N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012, 103, 332–337.Cannella, N.; Economidou, D.; Kallupi, M.; Stopponi, S.; Heilig, M.; Massi, M.; Ciccocioppo, R. Persistent Increase of Alcohol-Seeking Evoked by Neuropeptide S: An Effect Mediated by the Hypothalamic Hypocretin System. Neuropsychopharmacology 2009, 34, 2125–2134.
- Trotter, B.W.; Nanda, K.K.; Manley, P.J.; Uebele, V.N.; Condra, C.L.; Gotter, A.L.; Menzel, K.; Henault, M.; Stocco, R.; Renger, J.J.; et al. Tricyclic imidazole antagonists of the Neuropeptide S Receptor. Bioorganic Med. Chem. Lett. 2010, 20, 4704–4708.Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology 2016, 233, 2915–2924.
- Patnaik, S.; Marugan, J.J.; Liu, K.; Zheng, W.; Southall, N.; Dehdashti, S.J.; Thorsell, A.; Heilig, M.; Bell, L.; Zook, M.; et al. Structure-activity relationship of imidazopyridinium analogues as antagonists of neuropeptide s receptor. J. Med. Chem. 2013, 56, 9045–9056.Pañeda, C.; Huitron-Resendiz, S.; Frago, L.M.; Chowen, J.A.; Picetti, R.; De Lecea, L.; Roberts, A.J. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. 2009, 29, 4155–4161.
- Melamed, J.Y.; Zartman, A.E.; Kett, N.R.; Gotter, A.L.; Uebele, V.N.; Reiss, D.R.; Condra, C.L.; Fandozzi, C.; Lubbers, L.S.; Rowe, B.A.; et al. Synthesis and evaluation of a new series of Neuropeptide S receptor antagonists. Bioorganic Med. Chem. Lett. 2010, 20, 4700–4703.Kallupi, M.; Cannella, N.; Economidou, D.; Ubaldi, M.; Ruggeri, B.; Weiss, F.; Massi, M.; Marugan, J.; Heilig, M.; Bonnavion, P.; et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. USA 2010, 107, 19567–19572.
- Runyon, S.; Zhang, Y.; Hassler, C.; Gilmour, B. Composition and method for Neuropeptide S receptor (NPSR) antagonists. World Patent WO/2013/086200, 13 June 2013.Kallupi, M.; De Guglielmo, G.; Cannella, N.; Li, H.W.; Caló, G.; Guerrini, R.; Ubaldi, M.; Renger, J.J.; Uebele, V.N.; Ciccocioppo, R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 2013, 226, 347–355.
- Rizzi, A.; Vergura, R.; Marzola, G.; Ruzza, C.; Guerrini, R.; Salvadori, S.; Regoli, D.; Calo, G. Neuropeptide S is a stimulatory anxiolytic agent: A behavioural study in mice. Br. J. Pharmacol. 2008, 154, 471–479.Ruzza, C.; Calò, G.; Di Maro, S.; Pacifico, S.; Trapella, C.; Salvadori, S.; Preti, D.; Guerrini, R. Neuropeptide S receptor ligands: A patent review (2005–2016). Expert Opin. Ther. Pat. 2017, 27, 347–362.
- Leonard, S.K.; Dwyer, J.M.; Sukoff Rizzo, S.J.; Platt, B.; Logue, S.F.; Neal, S.J.; Malberg, J.E.; Beyer, C.E.; Schechter, L.E.; Rosenzweig-Lipson, S.; et al. Pharmacology of neuropeptide S in mice: Therapeutic relevance to anxiety disorders. Psychopharmacology 2008, 197, 601–611.Schmoutz, C.D.; Zhang, Y.; Runyon, S.P.; Goeders, N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012, 103, 332–337.
- Ionescu, I.A.; Dine, J.; Yen, Y.C.; Buell, D.R.; Herrmann, L.; Holsboer, F.; Eder, M.; Landgraf, R.; Schmidt, U. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology 2012, 37, 1323–1337.Cannella, N.; Kallupi, M.; Ruggeri, B.; Ciccocioppo, R.; Ubaldi, M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013, 100, 48–59.
- Dine, J.; Ionescu, I.A.; Avrabos, C.; Yen, Y.C.; Holsboer, F.; Landgraf, R.; Schmidt, U.; Eder, M. Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a “normal”-anxiety one. PLoS ONE 2015, 10, e0120272.Ubaldi, M.; Giordano, A.; Severi, I.; Li, H.; Kallupi, M.; De Guglielmo, G.; Ruggeri, B.; Stopponi, S.; Ciccocioppo, R.; Cannella, N. Activation of hypocretin-1/orexin-a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol. Psychiatry 2016, 79, 452–462.
- Slattery, D.A.; Naik, R.R.; Grund, T.; Yen, Y.C.; Sartori, S.B.; Füchsl, A.; Finger, B.C.; Elfving, B.; Nordemann, U.; Guerrini, R.; et al. Selective breeding for high anxiety introduces a synonymous SNP that increases Neuropeptide S receptor activity. J. Neurosci. 2015, 35, 4599–4613.Wegener, G.; Finger, B.C.; Elfving, B.; Keller, K.; Liebenberg, N.; Fischer, C.W.; Singewald, N.; Slattery, D.A.; Neumann, I.D.; Mathé, A.A. Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: A genetic animal model of depression. Int. J. Neuropsychopharmacol. 2012, 15, 375–387.
- Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291.Shirayama, Y.; Ishima, T.; Oda, Y.; Okamura, N.; Iyo, M.; Hashimoto, K. Opposite roles for neuropeptide S in the nucleus accumbens and bed nucleus of the stria terminalis in learned helplessness rats. Behav. Brain Res. 2015, 291, 67–71.
- Okamura, N.; Garau, C.; Duangdao, D.M.; Clark, S.D.; Jüngling, K.; Pape, H.-C.; Reinscheid, R.K. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology 2011, 36, 744–752.Duangdao, D.M.; Clark, S.D.; Okamura, N.; Reinscheid, R.K. Behavioral phenotyping of Neuropeptide S receptor knockout mice. Behav. Brain Res. 2009, 205, 1–9.
- Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405.Fendt, M.; Buchi, M.; Bürki, H.; Imobersteg, S.; Ricoux, B.; Suply, T.; Sailer, A.W. Neuropeptide S receptor deficiency modulates spontaneous locomotor activity and the acoustic startle response. Behav. Brain Res. 2011, 217, 1–9.
- Han, R.W.; Zhang, R.S.; Xu, H.J.; Chang, M.; Peng, Y.L.; Wang, R. Neuropeptide S enhances memory and mitigates memory impairment induced by MK801, scopolamine or Aβ1-42 in mice novel object and object location recognition tasks. Neuropharmacology 2013, 70, 261–267.Liu, X.; Si, W.; Garau, C.; Jüngling, K.; Pape, H.-C.; Schulz, S.; Reinscheid, R.K. Neuropeptide S precursor knockout mice display memory and arousal deficits. Eur. J. Neurosci. 2017, 46, 1689–1700.
- Jüngling, K.; Seidenbecher, T.; Sosulina, L.; Lesting, J.; Sangha, S.; Clark, S.D.; Okamura, N.; Duangdao, D.M.; Xu, Y.-L.; Reinscheid, R.K.; et al. Neuropeptide S-Mediated Control of Fear Expression and Extinction: Role of Intercalated GABAergic Neurons in the Amygdala. Neuron 2008, 59, 298–310.Ruzza, C.; Pulga, A.; Rizzi, A.; Marzola, G.; Guerrini, R.; Calo’, G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 2012, 62, 1999–2009.
- Sartori, S.B.; Maurer, V.; Murphy, C.; Schmuckermair, C.; Muigg, P.; Neumann, I.D.; Whittle, N.; Singewald, N. Combined neuropeptide S and D-cycloserine augmentation prevents the return of fear in extinction-impaired rodents: Advantage of dual versus single drug approaches. Int. J. Neuropsychopharmacol. 2016, 19, 1–11.Allen, I.C.; Pace, A.J.; Jania, L.A.; Ledford, J.G.; Latour, A.M.; Snouwaert, J.N.; Bernier, V.; Stocco, R.; Therien, A.G.; Koller, B.H. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1005–L1017.
- Okamura, N.; Reinscheid, R.K.; Ohgake, S.; Iyo, M.; Hashimoto, K. Neuropeptide S attenuates neuropathological, neurochemical and behavioral changes induced by the NMDA receptor antagonist MK-801. Neuropharmacology 2010, 58, 166–172.Zhu, H.; Perkins, C.; Mingler, M.K.; Finkelman, F.D.; Rothenberg, M.E. The role of neuropeptide S and neuropeptide S receptor 1 in regulation of respiratory function in mice. Peptides 2011, 32, 818–825.
- Smith, K.L.; Patterson, M.; Dhillo, W.S.; Patel, S.R.; Semjonous, N.M.; Gardiner, J.V.; Ghatei, M.A.; Bloom, S.R. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology 2006, 147, 3510–3518.Pulkkinen, V.; Majuri, M.L.; Wang, G.; Holopainen, P.; Obase, Y.; Vendelin, J.; Wolff, H.; Rytilä, P.; Laitinen, L.A.; Haahtela, T.; et al. Neuropeptide S and G protein-coupled receptor 154 modulate macrophage immune responses. Hum. Mol. Genet. 2006, 15, 1667–1679.
- Zhu, H.; Mingler, M.K.; McBride, M.L.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Williams, M.T.; Vorhees, C.V.; Rothenberg, M.E. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 2010, 35, 1119–1132.Yao, Y.; Su, J.; Yang, G.; Zhang, G.; Lei, Z.; Zhang, F.; Li, X.; Kou, R.; Liu, Y.; Liu, J. Effects of neuropeptide S on the proliferation of splenic lymphocytes, phagocytosis, and proinflammatory cytokine production of pulmonary alveolar macrophages in the pig. Peptides 2011, 32, 118–124.
- Si, W.; Aluisio, L.; Okamura, N.; Clark, S.D.; Fraser, I.; Sutton, S.W.; Bonaventure, P.; Reinscheid, R.K. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J. Neurochem. 2010, 115, 475–482.Yao, Y.; Su, J.; Zhang, F.; Lei, Z. Effects of central and peripheral administration of neuropeptide S on the level of serum proinflammatory cytokines in pigs. Neuroimmunomodulation 2014, 21, 45–51.
- Li, W.; Chang, M.; Peng, Y.L.; Gao, Y.H.; Zhang, J.N.; Han, R.W.; Wang, R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul. Pept. 2009, 156, 90–95.Ilmarinen, P.; James, A.; Moilanen, E.; Pulkkinen, V.; Daham, K.; Saarelainen, S.; Laitinen, T.; Dahlén, S.E.; Kere, J.; Dahlén, B.; et al. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100 IU/ml. Peptides 2014, 51, 100–109.
- Peng, Y.L.; Zhang, J.N.; Chang, M.; Li, W.; Han, R.W.; Wang, R. Effects of central neuropeptide S in the mouse formalin test. Peptides 2010, 31, 1878–1883.
- Victor Holanda, A.D.; Asth, L.; Santos, A.R.; Guerrini, R.; Soares-Rachetti, V.D.P.; Calo’, G.; André, E.; Gavioli, E.C. Central adenosine A1 and A2A receptors mediate the antinociceptive effects of neuropeptide S in the mouse formalin test. Life Sci. 2015, 120, 8–12.
- Holanda, V.A.D.; Oliveira, M.C.; Souza, L.S.; Lobão-Soares, B.; André, E.; Da Silva Junior, E.D.; Guerrini, R.; Calo, G.; Ruzza, C.; Gavioli, E.C. Dopamine D1 and D2 receptors mediate neuropeptide S-induced antinociception in the mouse formalin test. Eur. J. Pharmacol. 2019, 859, 172557.
- Lee, M.T.; Chiu, Y.T.; Chiu, Y.C.; Hor, C.C.; Lee, H.J.; Guerrini, R.; Calo, G.; Chiou, L.C. Neuropeptide S-initiated sequential cascade mediated by OX1, NK1, mGlu5 and CB1 receptors: A pivotal role in stress-induced analgesia. J. Biomed. Sci. 2020, 27, 7.
- Jinushi, K.; Kushikata, T.; Kudo, T.; Calo, G.; Guerrini, R.; Hirota, K. Central noradrenergic activity affects analgesic effect of Neuropeptide S. J. Anesth. 2018, 32, 48–53.
- Peng, Y.L.; Han, R.W.; Chang, M.; Zhang, L.; Zhang, R.S.; Li, W.; Han, Y.F.; Wang, R. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides 2010, 31, 2259–2263.
- Cifani, C.; Di Bonaventura, M.V.M.; Cannella, N.; Fedeli, A.; Guerrini, R.; Calo, G.; Ciccocioppo, R.; Ubaldi, M. Effect of neuropeptide S receptor antagonists and partial agonists on palatable food consumption in the rat. Peptides 2011, 32, 44–50.
- Didonet, J.J.; Cavalcante, J.C.; Souza, L.D.; Costa, M.S.; André, E.; Soares-Rachetti, V.D.; Guerrini, R.; Gavioli, E.C. Neuropeptide S counteracts 6-OHDA-induced motor deficits in mice. Behav. Brain Res. 2014, 266, 29–36.
- Cannella, N.; Economidou, D.; Kallupi, M.; Stopponi, S.; Heilig, M.; Massi, M.; Ciccocioppo, R. Persistent Increase of Alcohol-Seeking Evoked by Neuropeptide S: An Effect Mediated by the Hypothalamic Hypocretin System. Neuropsychopharmacology 2009, 34, 2125–2134.
- Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology 2016, 233, 2915–2924.
- Pañeda, C.; Huitron-Resendiz, S.; Frago, L.M.; Chowen, J.A.; Picetti, R.; De Lecea, L.; Roberts, A.J. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. 2009, 29, 4155–4161.
- Kallupi, M.; Cannella, N.; Economidou, D.; Ubaldi, M.; Ruggeri, B.; Weiss, F.; Massi, M.; Marugan, J.; Heilig, M.; Bonnavion, P.; et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. USA 2010, 107, 19567–19572.
- Kallupi, M.; De Guglielmo, G.; Cannella, N.; Li, H.W.; Caló, G.; Guerrini, R.; Ubaldi, M.; Renger, J.J.; Uebele, V.N.; Ciccocioppo, R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 2013, 226, 347–355.
- Cannella, N.; Kallupi, M.; Ruggeri, B.; Ciccocioppo, R.; Ubaldi, M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013, 100, 48–59.
- Ubaldi, M.; Giordano, A.; Severi, I.; Li, H.; Kallupi, M.; De Guglielmo, G.; Ruggeri, B.; Stopponi, S.; Ciccocioppo, R.; Cannella, N. Activation of hypocretin-1/orexin-a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol. Psychiatry 2016, 79, 452–462.
- Wegener, G.; Finger, B.C.; Elfving, B.; Keller, K.; Liebenberg, N.; Fischer, C.W.; Singewald, N.; Slattery, D.A.; Neumann, I.D.; Mathé, A.A. Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: A genetic animal model of depression. Int. J. Neuropsychopharmacol. 2012, 15, 375–387.
- Shirayama, Y.; Ishima, T.; Oda, Y.; Okamura, N.; Iyo, M.; Hashimoto, K. Opposite roles for neuropeptide S in the nucleus accumbens and bed nucleus of the stria terminalis in learned helplessness rats. Behav. Brain Res. 2015, 291, 67–71.
- Duangdao, D.M.; Clark, S.D.; Okamura, N.; Reinscheid, R.K. Behavioral phenotyping of Neuropeptide S receptor knockout mice. Behav. Brain Res. 2009, 205, 1–9.
- Fendt, M.; Buchi, M.; Bürki, H.; Imobersteg, S.; Ricoux, B.; Suply, T.; Sailer, A.W. Neuropeptide S receptor deficiency modulates spontaneous locomotor activity and the acoustic startle response. Behav. Brain Res. 2011, 217, 1–9.
- Liu, X.; Si, W.; Garau, C.; Jüngling, K.; Pape, H.-C.; Schulz, S.; Reinscheid, R.K. Neuropeptide S precursor knockout mice display memory and arousal deficits. Eur. J. Neurosci. 2017, 46, 1689–1700.
- Ruzza, C.; Pulga, A.; Rizzi, A.; Marzola, G.; Guerrini, R.; Calo’, G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 2012, 62, 1999–2009.
- Allen, I.C.; Pace, A.J.; Jania, L.A.; Ledford, J.G.; Latour, A.M.; Snouwaert, J.N.; Bernier, V.; Stocco, R.; Therien, A.G.; Koller, B.H. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1005–L1017.
- Zhu, H.; Perkins, C.; Mingler, M.K.; Finkelman, F.D.; Rothenberg, M.E. The role of neuropeptide S and neuropeptide S receptor 1 in regulation of respiratory function in mice. Peptides 2011, 32, 818–825.
- Pulkkinen, V.; Majuri, M.L.; Wang, G.; Holopainen, P.; Obase, Y.; Vendelin, J.; Wolff, H.; Rytilä, P.; Laitinen, L.A.; Haahtela, T.; et al. Neuropeptide S and G protein-coupled receptor 154 modulate macrophage immune responses. Hum. Mol. Genet. 2006, 15, 1667–1679.
- Yao, Y.; Su, J.; Yang, G.; Zhang, G.; Lei, Z.; Zhang, F.; Li, X.; Kou, R.; Liu, Y.; Liu, J. Effects of neuropeptide S on the proliferation of splenic lymphocytes, phagocytosis, and proinflammatory cytokine production of pulmonary alveolar macrophages in the pig. Peptides 2011, 32, 118–124.
- Yao, Y.; Su, J.; Zhang, F.; Lei, Z. Effects of central and peripheral administration of neuropeptide S on the level of serum proinflammatory cytokines in pigs. Neuroimmunomodulation 2014, 21, 45–51.
- Ilmarinen, P.; James, A.; Moilanen, E.; Pulkkinen, V.; Daham, K.; Saarelainen, S.; Laitinen, T.; Dahlén, S.E.; Kere, J.; Dahlén, B.; et al. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100 IU/ml. Peptides 2014, 51, 100–109.
