Endosome-derived small extracellular vesicles (EVs), often referred to as exosomes, are produced by almost all, if not all, cell types, and are critical for intercellular communication. They are composed of a lipid bilayer associated with membrane proteins and contain a payload of lipids, proteins and regulatory RNAs that depends on the parental cell physiological condition. By transferring their “cargo”, exosomes can modulate the phenotype of neighboring and distant cells. Stem cells (SC) were widely studied for therapeutic applications regarding their regenerative/reparative potential as well as their immunomodulatory properties. Whether from autologous or allogeneic source, SC beneficial effects in terms of repair and regeneration are largely attributed to their paracrine signaling notably through secreted EVs. Subsequently, SC-derived EVs have been investigated for the treatment of various diseases, including inflammatory skin disorders, and are today fast-track cell-free tools for regenerative/reparative strategies.
- extracellular vesicles
- exosomes
- stem cells
- skin
- wound healing
- chronic inflammation
- regenerative medicine
1. Introduction
The last three decades have witnessed a wealth of studies to provide the proof-of-concept in stem cells (SC)-based regenerative strategies to manage diseases that cannot be treated. Nonetheless, the development of clinically viable regenerative therapies remained challenging because SC faced major hurdle from immunological barriers. For instance, the nature and magnitude of host immune response to a given cell-based therapy are governed by several factors including cell properties and the degree of class I and II human leukocytes antigen (HLA) mismatch as well as by the mode of action and clinical efficacy [1][2]. To circumvent the barrier issues, autologous SC became an obvious choice for regenerative therapies. Yet, the demanding logistics to achieve immediate availability of sufficient numbers limited the potential use of autologous cells including iPSCs [3]. The well-documented ability of allogeneic adult SC to evade and/or regulate the host immune system comforted their consideration as a pragmatic choice that offers an efficient way for the development of off-the-shelf regenerative therapies with appropriate number of cells [4][5][6][7][8]. This paradigm shift in SC allogenicity led to the concept of “allogeneic-driven-benefit” [9]. As the field evolves it become evident that both autologous or allogeneic infused SC do not persist long enough to achieve direct regenerative/reparative effects. Most of the beneficial effects of transplanted cells are rather mediated through paracrine signaling pathways that promote endogenous tissue regeneration and repair. In this context, the immunomodulatory/anti-inflammatory properties of various types of SC are highlighted today as integral to their paracrine beneficial signaling in various disorders [9][10].
Extracellular vesicles (EVs) are natural highly conserved cell-derived vesicles secreted by various cell types including SC to ensure local and remote cell-to-cell communication. EVs carry lipids, small RNAs and proteins that can be transferred from parent cells to target cells and regulate their activities [11]. Small-sized EVs or exosomes are proposed today, as the main mediators of SC paracrine beneficial effects. The in vivo visualization of small-size EVs in tissues treated with SC, such as injured mouse heart, [12][13] further supported the role of EVs as important players mediating the wholesome effects of therapeutic SC [14].
This notion raised hope for the development of novel cell-free therapies that would overcome the hurdles of using SC in regenerative medicine. By interacting with different cells, including immune cells, EVs can provoke and maintain the regenerative/reparative processes. In regard of their remarkably broad biological functions and their capacity to transport large molecules, EVs offer a unique platform for the development of novel therapeutic strategies for a variety of diseases and disorders, including wound healing in chronic inflammatory skin diseases.
2. Extracellular Vesicles/Exosomes: General Aspects
Cells release EVs of different sizes and intracellular origin which can be classified as ectosomes, exosomes, and apoptotic bodies [15]. Apoptotic bodies are a product of apoptosis and contain fragments from the dying cells, their size range from 50 to 5000 nm. Ectosomes (or microvesicles) result from protrusions of the plasma membrane that eventually detach and are shed in the extracellular space, their diameter is between 50 and 500 nm. The smallest EVs also called exosomes (Exs), category of interest referred to thereafter as EVs/Exs, have a diameter ranging between 50–150 nm. EVs/Exs arise from larger intracellular vesicles called multivesicular bodies (MVBs). They are secreted via exocytosis as a consequence of fusion between MVBs and the plasma membrane (Figure 1). Classical EVs/Exs express CD63, CD81, CD9 markers while the non-classical express CD63 and CD81 but lack CD9. EVs/Exs can also express several other proteins including heat-shock proteins (Hsp60, Hsp70, and Hsp90), programmed cell death 6 interacting protein (Alix/PDCD6IP), tumor susceptibility gene 101 (Tsg101), and clathrin h [16]. A wide range of cell types including SC, immune cells and cancer cells, produces EVs/Exs as mediators of their paracrine effects. EVs/Exs function is governed by the payload of lipids, proteins and different types of RNAs (mRNA, miRNA, lncRNA, etc.) originating from the parent cell [16].
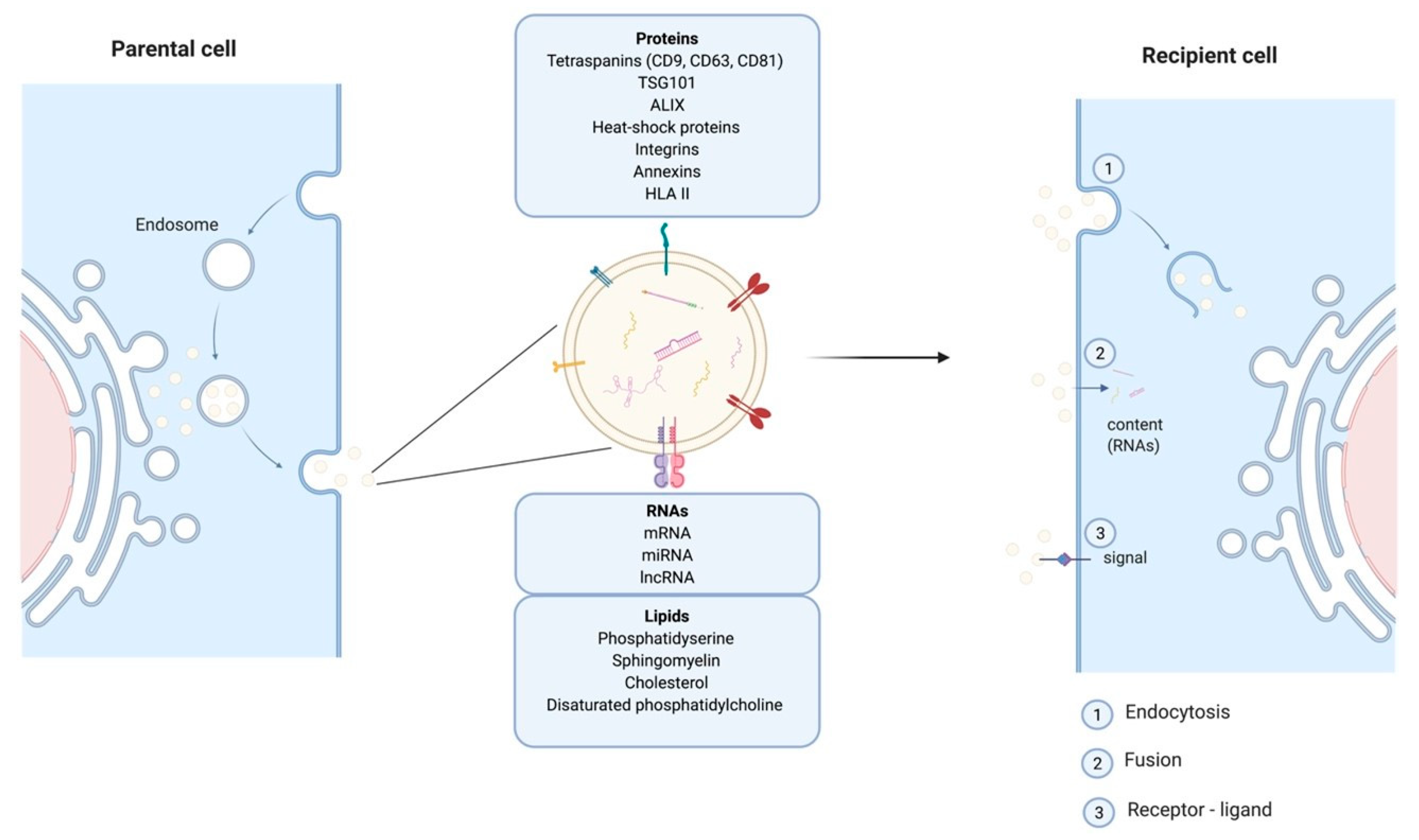
Figure 1. Schematic representation of exosomes endosomal budding detailing their different components and mode of action on recipient cells.
EVs/Exs are considered as one of the major modes of cellular communication. They exert local and long-range action to impact other tissues. Their intercellular communication can be conferred by mediators expressed at their surface, or delivery of their “cargo” into target cell lumen after internalization through fusion or endocytosis [17]. Within these properties EVs/Exs are proposed as essential actors in tumorigenesis and distant metastasis development [18]. However, EVs/Exs are also key elements of SC-mediated paracrine regulation of cells/tissues repair and regeneration [14][19].
Given their growing importance in modern regenerative/reparative medicine, various techniques have been adopted to facilitate the challenging isolation of EVs/Exs. Ultracentrifugation, consecutive centrifugation, ultrafiltration, immunoaffinity precipitation or size exclusion chromatography, as well as high-resolution density gradient fractionation in combination with direct immunoaffinity capture have been used with continuous ameliorations to isolate various populations of EVs [20][21]. However, each approach has different pros and cons in regard of purity and number of isolated particles. For example, ultracentrifugation is efficient to remove several contaminants, but it is time consuming and not always suitable for EVs/Exs isolation from small clinical samples. Ultrafiltration is fast and results in highly pure vesicles, but the disadvantage comes from the difficulty to remove contaminating proteins which could be problematic for clinical purposes [20][21]. These examples illustrate the current challenges of improving isolation and purification technique for EVs/Exs. It is accepted that none of the up-to-date developed techniques can permit to clearly separate different types of EVs rendering pure EVs/Exs fractions extremely hard to obtain. Most preparations could be called “exosome-enriched fractions” of EVs, explaining our choice to use the term EVs/Exs in this review.
Much of the interest in EVs/Exs was triggered by their biological properties and their function especially the delivery of their “cargo” to neighboring and distant cells. The differences in purification strategies and the heterogeneity of EVs/Exs preparations may confound their proper characterization, which is essential for their biological properties. Therefore, a combination of different methodologies is often applied to best characterize these nanovesicles. Among the most common, colorimetric dosage for protein concentration, Tunable-Resistive Pulse Sensing (TRPS), Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA) which can precisely measure particles concentration (number of particles/mL) and size distribution of EVs/EXs [22]. Atomic-Force Microscopy (AFM) or Transmission Electron Microscopy (TEM), are both for visualization and characterization of EVs structure, morphology, and size, while Western blotting, polymerase chain reaction (PCR), microarray, next-generation sequencing (NGS) and lipidomic approaches are used to determine the content of EVs. Additionally, surface markers are characterized through flow cytometry approaches [23].
3. Extracellular Vesicles/Exosomes: Immune Properties
3.1. Activation of Immune Response
Typically, specific immune response is triggered upon direct interaction of peptide-loaded class I and II major histocompatibility complex (MHC) on antigen-presenting cells (APCs) with CD8+ or CD4+ T cells, respectively. T cell receptor (TCR) recognition of these peptide loaded MHC complexes leads to the formation of the immune synapse (IS), a stable interaction between the T cell and the APC. The synaptic zone allows the transfer of information between the two cells by means of trogocytosis, tunneling nanotubes and, polarized secretion of soluble factors and EVs/Exs. As EVs/Exs are formed by reverse budding of the multivesicular body, functional proteins specifically associated with plasma membrane are exposed on their outer surface [24][25]. Accumulating evidence has demonstrated that EVs/Exs can be transferred at a distance and mediate antigen presentation [26]. EVs/Exs loaded with peptides from Epstein-Barr virus (EBV), cytomegalovirus and influenza virus could directly induce in vitro the secretion of IFNγ by human peripheral CD8+ T cells, but the magnitude of T cell activation is 10 times lower than direct stimulation with APCs [27]. Antigen presentation also occurs in an indirect manner after EVs/Exs internalization by APCs and degradation of their peptide/MHC complexes. For instance, HLA-DR4-positive EVs/Exs loaded with a serum albumin peptide were able to stimulate T cells following internalization by HLA-DR4-positive APCs [28]. This suggests that EVs/Exs are able to transfer either the preformed peptide–MHC II complex or the specific peptide to MHC II molecules on APCs promoting specific T cell activation without further antigen processing. EVs/Exs can also trigger an immune response by delivering native antigens to APCs. Tumor-derived EVs/Exs are internalized by APCs, processed and cross-presented to cytotoxic CD8+ T cells, and vaccination of mice with tumor-derived EVs/Exs induces a potent CD8+ T cell–mediated anti-tumor response against not only the parental tumor, but also other tumors expressing similar tumor antigens [29].
Triggering an immune response by EVs/Exs also occurs via mechanisms other than antigen presentation. EVs derived from bacteria-infected macrophages stimulate macrophages and neutrophils to secrete pro-inflammatory cytokines [30]. In diabetes, EVs/Exs-mediated transfer of specific miRNAs to pancreatic cells lead to T-cell death and expression of chemokine genes, which would in turn further the infiltration of activated T cells [31]. In cancer, miR-21 and miR-29a from tumor-derived EVs/Exs bind to Toll-like receptors, such as human TLR8, and lead to TLR-mediated NFκB activation and secretion of pro-metastatic inflammatory cytokines, which in turn promotes tumor growth and metastasis [32]. Overall, the capacity of some EVs/Exs to activate the immune system could be beneficial to diseases where immune response is defective but also in cancer to improve anti-tumor response.
3.2. Regulation/Suppression of Immune Response
EVs/Exs activate the immune response, yet, depending on their cellular origin, they can also act as key immunoregulators/suppressers. Human regulatory T cell-secreted EVs/Exs suppress effector T cell proliferation and cytokine production in vitro, and can prevent allograft rejection in vivo [33]. Tumor-derived EVs/Exs express FasL and TRAIL membranous death ligands as well as regulatory proteins such as PD-L1 and CD40L [34][35]. These surface molecules could trigger the apoptotic death of activated T cells, inhibit effector T cells activity and promote generation of regulatory T cells. Through their “cargo” of proteins, RNAs, and lipids, tumor-derived EVs/Exs down-regulate anti-tumor immune responses, and are currently recognized as key actors of tumor-induced immunomodulation/suppression. In addition to regulating anti-tumor T cells response, tumor-derived EVs/Exs can directly suppress NK cell anti-tumor response, and can facilitate angiogenesis and wound healing [36][37][38].
EVs/Exs secreted by adult stem cells, including mesenchymal stem cells (MSCs) and cardiac stem/progenitor cells (CPCs), confer immunomodulatory/anti-inflammatory regenerative/reparative effects in animal models of diseases and tissue injury. Notably, MSC-derived EVs/Exs were more efficient than their parental cells in reducing the percentage of effector CD8+ and CD4+ T cells while increasing regulatory T cells in inflammatory arthritis mouse models [39], whereas CPCs-derived EVs/Exs were as efficient as their parental cells in myocardial infarction experimental models [40][41]. Similar to tumor-derived EVs/Exs, SC-derived EVs/Exs can down-regulate immune functions via direct interaction of surface PD-L1, or CD40L with extracellular proteins on immune cells. For instance, EVs/Exs from fetal SC express PD-L1 and mediate T cell suppression by inhibiting the CD3-zeta and JAK3 pathway [42], which is critical for T cell proliferation in response to antigen receptor cross-linking. Together these findings strongly supported the active contribution of EVs/Exs to the immunoregulatory/anti-inflammatory properties of adult and fetal SCs.
Interestingly, inflammation, often marking degenerative disorders and tissue injury, seems to reinforce the immunomodulatory/suppressive capacity of SCs-derived EVs/Exs. MSC treated with IFNγ and TNFα produce EVs/Exs with higher immunosuppressive/anti-inflammatory capacity directing the differentiation of M1 macrophages (pro-inflammatory) into an M2 (anti-inflammatory) phenotype with IL-10 production [43]. Hypoxia can also enhance the immunomodulatory and angiogenic properties of MSC-derived EVs/Exs [44][45]. Similarly, under inflammatory conditions, CPCs-derived EVs/Exs protect monocytes from spontaneous death and fine-tune their phenotypes towards anti-inflammatory/immunoregulatory profile enhancing repair and healing of injured heart [14][41].
In brief, EVs/Exs are at the center of the most exciting and growing field of SC secretomics. EVs/Exs not only display various immune relevant proteins at their surface but also carry a “cargo” of growth factors, microRNAs, long non-coding RNAs with tremendous immune regulatory activities as well as beneficial regenerative properties. As such, EVs/Exs became promising therapeutic strategies for autoimmune and inflammatory diseases but also for many degenerative diseases [46]. Indeed, as therapeutics, they might be useful in overcoming current limitations of SC-based strategies including tumor formation and other hurdles that might be caused by SC transplantation. In this context, furthering the characterization and understanding of EVs/Exs derived from various types of SCs is essential not only to elect an optimal cell source but also to develop cell-free therapeutics for various degenerative diseases and devastating injuries.
4. SC-Derived EVs/Exs and Skin Wound Healing
The skin, composed of epidermis, dermis, and hypodermis, represents 15% of the body, and is the first line of defense against external aggressions such as pathogens or UV light. Dysfunction or injury of the skin is highly prevalent and can lead to a range of diseases. It can result from trauma or surgical incision in the case of acute wounds but also from diseases such as chronic inflammatory or autoimmune skin diseases, which are marked by chronic skin wounds with serious healing defects. Keratinocytes and fibroblasts are main cellular composites of the skin and are critical for wound healing. Patients suffering from skin disorders not only face decreased quality of life, but also are often embarrassed about the appearance of their skin, which can cause an immense psychological burden. Skin wound healing is a complex process involving three major overlapping phases: inflammatory, proliferative and remodeling phases where several cell types are involved [47] (Figure 2).
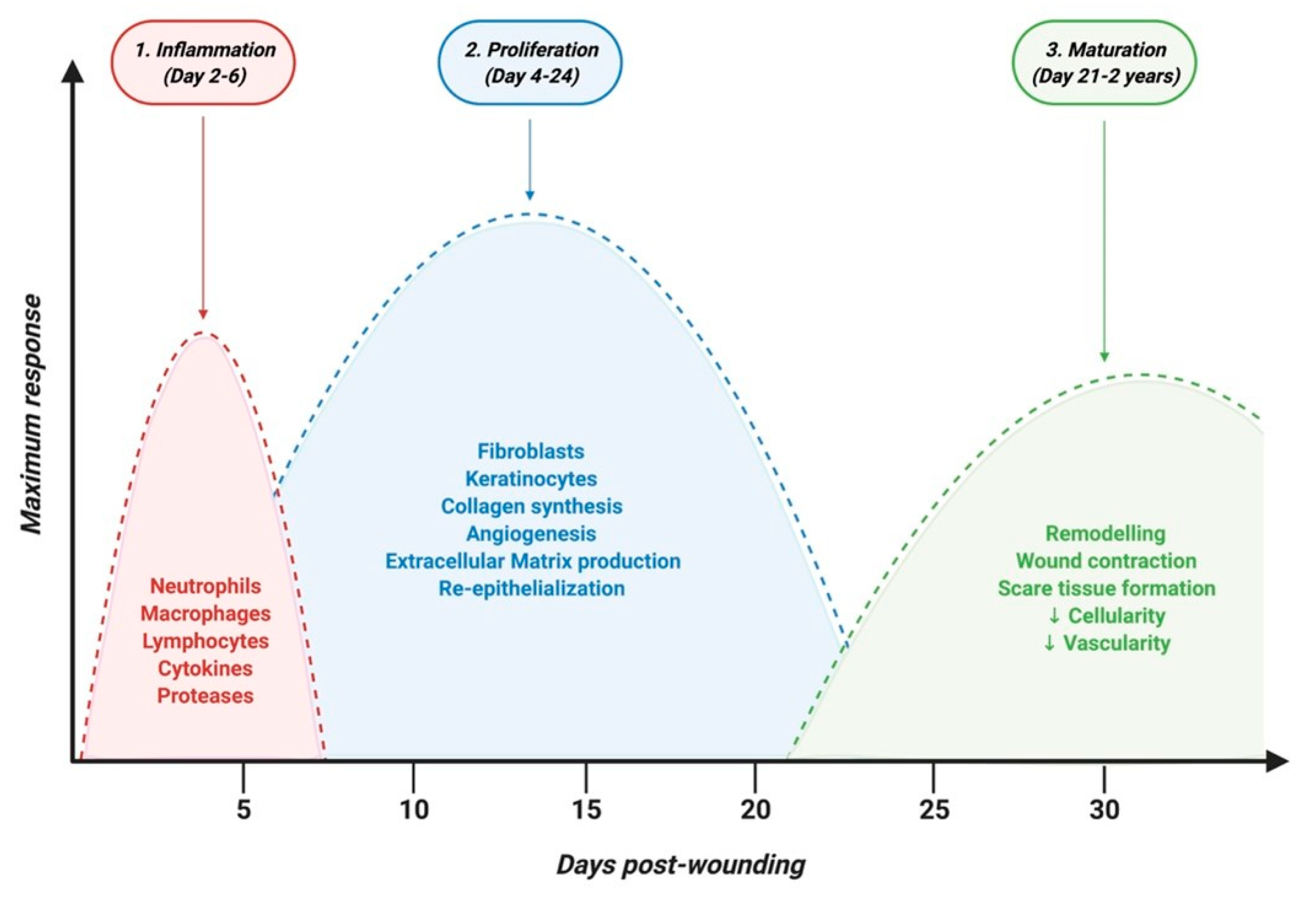
Figure 2. Timeline of physiological skin wound healing phases.
5. SC-Derived EVs/Exs as Nanomedicine Therapeutics for Chronic Skin Inflammation
Evs/Exs from various immune and non-immune cells contribute to the pathogenesis of various inflammatory skin diseases including psoriasis, atopic dermatitis (AD), as well as autoimmune disorders [48]. For instance, EVs/Exs from both keratinocytes and mast cells contribute to psoriasis inflammation through the activation of neutrophils and CD1a-reactive T cells, respectively [49][50]. In autoimmune inflammatory bullous pemphigoid, blister fluid-derived EVs/Exs containing a variety of inflammatory proteins contribute to the pathogenesis of this severe disorder [51]. Within these findings, EVs/Exs have been considered as potential biomarkers of inflammatory skin disorders but also regarded as ultimate therapeutic agents that can be even further engineered to deliver various drugs. Thus, the current state of knowledge on EVs/Exs accentuated the therapeutic potential of these SC-derived nanoparticles in the context of chronic skin inflammation and its associated chronic wounding, and encouraged the development of nanomedicine strategies to manage such disorders.
The ability of EVs/Exs to impact cells depends on their protein markers and cargo, which mimic the properties of their origin. Therefore, among the first considerations when developing an EVs/Exs-based therapeutic strategy is the cellular source and whether it is autologous or allogeneic, in order to avoid unwanted biological activity inherent to parent cells. For instance, MSC from healthy individuals or cancer patients may deliver a bioactive EVs/Exs cargo that inhibits or promotes tumor growth, respectively. Exosomal PD-L1 expression also changes during treatment with anti-PD-1 antibodies in melanoma as well as in head and neck cancers, and certain anti-cancer drugs can induce the release of exosomes that can effectively induce natural killer cell cytotoxicity. Whether anti-inflammatory drugs and monoclonal antibodies used as inflammatory skin diseases treatment regimen alter the bioactive cargo of EVs/Exs derived from patient cells remains an open question. Yet, within the above findings one may speculate similar scenarios. Thus, it would be preferable to use SC cells-derived EVs/Exs obtained from healthy allogeneic individuals, rather than patients’ autologous cells, to develop therapeutic strategies. In addition, besides being more pragmatic, the therapeutic potential of EVs/Exs from allogeneic sources has been proven higher or at least equal to that of EVs/Exs from autologous source in various in vitro and in vivo experimental model systems, including skin injuries [14][41][52][53]. The concept of “allogeneic-driven-benefit” is today fairly admitted, but, the optimal allogeneic cells, if any, are still under active research.
The human placenta as a unique temporary organ that ensures the mutual coexistence of the allogeneic organism of mother and fetus [54][55][56], is considered a natural model of human transplantation. Accordingly, it was suggested as a potential “universal source” for the development of allogeneic biotherapeutics. This notion is further facilitated by the non-invasive and fairly ethical accessibility of the organ and by the fact that placental EVs/Exs perform a myriad of functions from regulation of maternal immune reaction to the physiological development of the fetus [57][58][59]. Thus, placenta-derived EVs/Exs are likely endowed with intrinsic immunomodulatory regenerative/reparative capacity [60], and might be considered as “universal EVs/Exs” for the development of efficient nanomedicine strategies to manage chronic inflammatory disorders.
SC-derived EVs/Exs as biotherapeutics can be delivered through intravenous, subcutaneous, intraperitoneal, oral or even nasal administration. Regardless of the delivery route, EVs/Exs primarily accumulate in organs such as the liver, spleen, kidney, and lung [61][62]. Currently, information on SC-derived EVs/Exs biodistribution and retention time in skin wounds is still scarce. Nonetheless, because the retention time of EVs/Exs in different organs is in general short and depends on EVs/Exs origins [62][63], increasing EVs/Exs’ half-life as well as resistance to biodegradability through numerous engineering techniques is under active development. Among the recent advances, some in vivo studies showing that encapsulation of EVs/Exs in hydrogel extends their retention at injured sites and endows them with higher wound healing capacity [64][65].
Development of wound dressings containing SC-derived EVs/Exs is another innovative, non-invasive and simple way of delivering EVs/Exs to patients suffering from chronic skin inflammation. The composition of wound dressing can be also modulated to provide a proper microenvironment that would increase their benefits. In this regard, it has been shown in a rat model of chronic diabetic wounds that an EVs/Exs-based wound dressing composed of antioxidant polyurethane (PUAO) cryogel and supplemented with ADSCs-derived EVs/Exs, efficiently promotes angiogenesis, collagen remodeling, granulation tissue formation, and re-epithelialization, thus wound healing [66].
With the fast-track advances of gene editing techniques, such as CRISPR-Cas9, generating EVs/Exs tailored to enhance their immunomodulatory/anti-inflammatory regenerative/reparative capacities is today highly doable. Tailoring could be achieved either by engineering parent cells to overexpress molecule(s) or miRNA, which would result in production of EVs/Exs expressing these molecules, or directly engineering EVs/Exs with miRNA or molecules that enhance their benefits [61][67][68][69] (Figure 3).
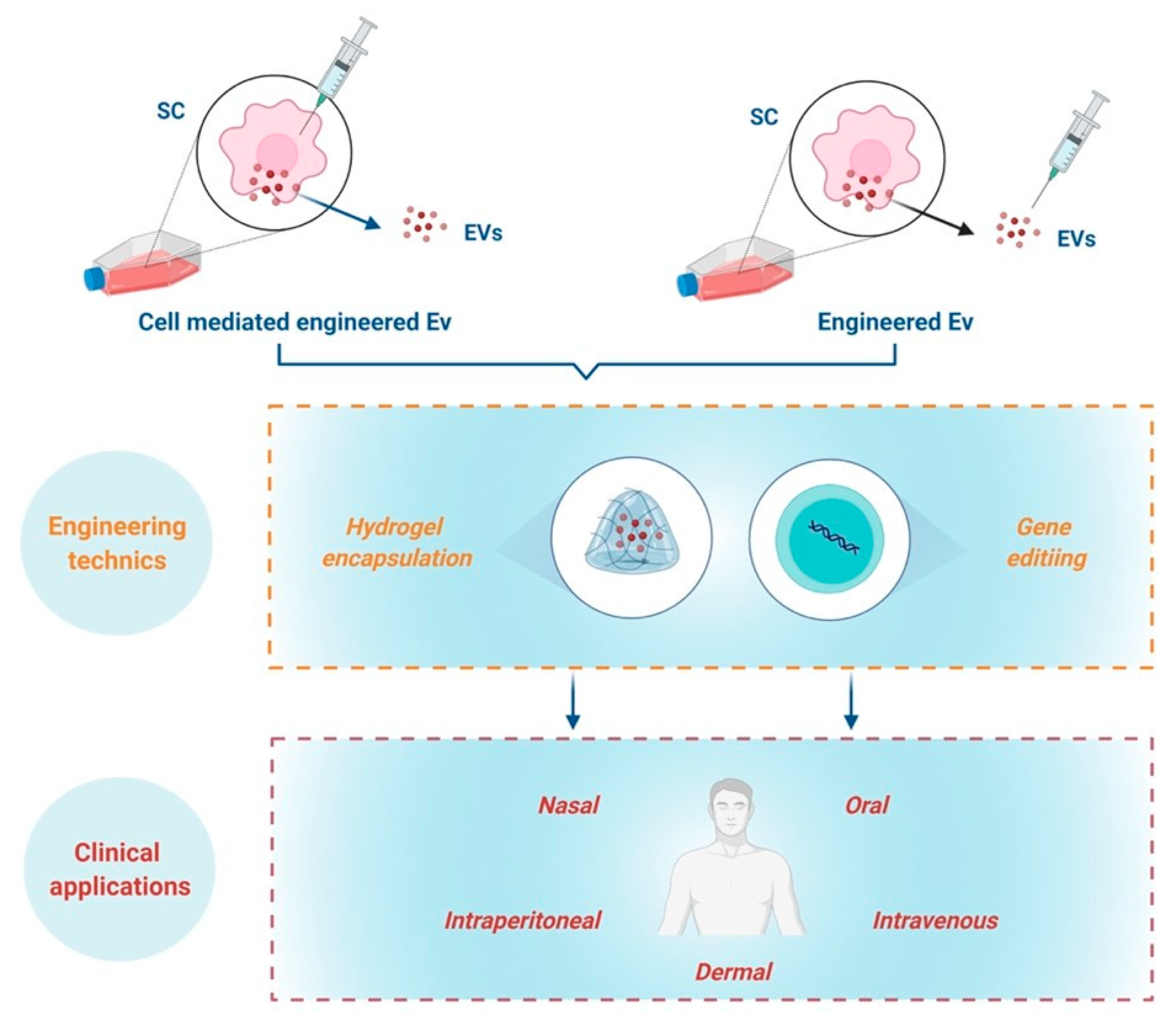
Figure 3. Schematic representation of engineering approaches towards clinical applications.
The combination of gene editing technics to create modified EVs/Exs with addition of biomaterials such as hydrogel encapsulation and composite-loaded wound dressing are highly promising for management of chronic skin inflammation. However, these concepts are still at their infancy and call upon furthering investigations to reach the clinical translation of EVs/Exs-based nanomedicine to help patients suffering from chronic skin inflammation.
6. Current Limitations of EVs/Exs-Based Therapeutic Strategies
Cell-free EVs/Exs-based therapeutics permit to avoid some limitations of SC-based strategies including eventual rejection and short persistence while almost acting in a similar manner as whole cells. Despite considerable advances in the field, successful clinical translation of these promising therapeutic strategies is still facing various challenges.
The low productivity of pure EVs/EVs is among the first concerns because therapy requires large amounts. The poor yield after isolation, could indeed limit sufficient production for large-scale development for therapy. Some conditions like hypoxia or pH modifications can increase EVs/Exs yield, but such cells conditioning prior to EVs/Exs isolation could modify their “cargo” and decrease their purity. To what extent EVs/Exs should be pure depends on the experimental question and end usage. High purity is a must to attribute a function, but less purity and higher quantity are in general required for most therapeutic strategies where function is paramount not the definitive association of function with EVs/Exs. Several studies also indicated that the composition of the EVs/Exs “cargo” be it proteins or RNAs can be different and depends on different purification methods [70]. miRNA and potentially other molecules from the EVs/Exs “cargo” could cause undesirable effects, such as tumorigenesis, which may be caused by differences contaminating products. Thus, EVs/Exs collected with different methods, could differ in many aspects and properties, and there is no one-size-fits all procedure; mainly a balance between purity and quantity in function of EVs/Exs end-use to preserve particles properties and reduce the risk of adverse effects.
The eventual misallocation of EVs/Exs at sites other than the intended target upon administration is another current concern calling upon furthering the understanding of EVs/Exs bio-distribution to increase benefit and avoid undesired effects. The biology of miRNA is complex, and a given molecule could induce distinct and even opposite effects in different tissues. It means that administration of EVs/Exs therapy needs improvement to permit specific organs targeting.
References
- Wood, K.J.; Issa, F.; Hester, J. Understanding Stem Cell Immunogenicity in Therapeutic Applications. Trends Immunol. 2016, 37, 5–16.
- Charron, D.; Suberbielle-Boissel, C.; Tamouza, R.; Al-Daccak, R. Anti-HLA antibodies in regenerative medicine stem cell therapy. Hum. Immunol. 2012, 73, 1287–1294.
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531.
- Lauden, L.; Boukouaci, W.; Borlado, L.R.; Lopez, I.P.; Sepulveda, P.; Tamouza, R.; Charron, D.; Al-Daccak, R. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ. Res. 2013, 112, 451–464.
- Boukouaci, W.; Lauden, L.; Siewiera, J.; Dam, N.; Hocine, H.R.; Khaznadar, Z.; Tamouza, R.; Borlado, L.R.; Charron, D.; Jabrane-Ferrat, N.; et al. Natural killer cell crosstalk with allogeneic human cardiac-derived stem/progenitor cells controls persistence. Cardiovasc Res. 2014, 104, 290–302.
- Dam, N.; Hocine, H.R.; Palacios, I.; DelaRosa, O.; Menta, R.; Charron, D.; Bensussan, A.; El Costa, H.; Jabrane-Ferrat, N.; Dalemans, W.; et al. Human Cardiac-Derived Stem/Progenitor Cells Fine-Tune Monocyte-Derived Descendants Activities toward Cardiac Repair. Front. Immunol. 2017, 8, 1413.
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624.
- Hocine, H.R.; Costa, H.E.; Dam, N.; Giustiniani, J.; Palacios, I.; Loiseau, P.; Bensussan, A.; Borlado, L.R.; Charron, D.; Suberbielle, C.; et al. Minimizing the risk of allo-sensitization to optimize the benefit of allogeneic cardiac-derived stem/progenitor cells. Sci. Rep. 2017, 7, 41125.
- Al-Daccak, R.; Charron, D. Editorial: Alloimmune Response From Regenerative Medicine. Front. Immunol. 2018, 9, 3121.
- Munir, H.; Ward, L.S.C.; McGettrick, H.M. Mesenchymal Stem Cells as Endogenous Regulators of Inflammation. Adv. Exp. Med. Biol. 2018, 1060, 73–98.
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363.
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728.
- Barile, L.; Gherghiceanu, M.; Popescu, L.M.; Moccetti, T.; Vassalli, G. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J. Biomed. Biotechnol. 2012, 2012, 354605.
- Hocine, H.R.; Brunel, S.; Chen, Q.; Giustiniani, J.; San Roman, M.J.; Ferrat, Y.J.; Palacios, I.; de la Rosa, O.; Lombardo, E.; Bensussan, A.; et al. Extracellular Vesicles Released by Allogeneic Human Cardiac Stem/Progenitor Cells as Part of Their Therapeutic Benefit. Stem Cells Transl. Med. 2019, 8, 911–924.
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11.
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418.
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol. Cancer Res. 2019, 17, 337–347.
- Fu, Q.; Zhang, Q.; Lou, Y.; Yang, J.; Nie, G.; Chen, Q.; Chen, Y.; Zhang, J.; Wang, J.; Wei, T.; et al. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene 2018, 37, 6105–6118.
- Haque, N.; Widera, D.; Govindasamy, V.; Soesilawati, P.; Abu Kasim, N.H. Extracellular Vesicles from Stem and Progenitor Cells for Cell-Free Regenerative Therapy. Curr. Mol. Med. 2021.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750.
- Liangsupree, T.; Multia, E.; Riekkola, M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773.
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153.
- Nolan, J.P.; Duggan, E. Analysis of Individual Extracellular Vesicles by Flow Cytometry. Methods Mol. Biol. 2018, 1678, 79–92.
- Montecalvo, A.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.G.; Wang, Z.; Divito, S.J.; Papworth, G.D.; Watkins, S.C.; Robbins, P.D.; Larregina, A.T.; et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J. Immunol. 2008, 180, 3081–3090.
- Segura, E.; Nicco, C.; Lombard, B.; Veron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Thery, C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005, 106, 216–223.
- Lindenbergh, M.F.S.; Wubbolts, R.; Borg, E.G.F.; van’T Veld, E.M.; Boes, M.; Stoorvogel, W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J. Extracell Vesicles 2020, 9, 1798606.
- Admyre, C.; Johansson, S.M.; Paulie, S.; Gabrielsson, S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur. J. Immunol. 2006, 36, 1772–1781.
- Qazi, K.R.; Gehrmann, U.; Domange Jordo, E.; Karlsson, M.C.; Gabrielsson, S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood 2009, 113, 2673–2683.
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Thery, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303.
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244.
- Guay, C.; Kruit, J.K.; Rome, S.; Menoud, V.; Mulder, N.L.; Jurdzinski, A.; Mancarella, F.; Sebastiani, G.; Donda, A.; Gonzalez, B.J.; et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic beta Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019, 29, 348–361.e6.
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116.
- Tung, S.L.; Fanelli, G.; Matthews, R.I.; Bazoer, J.; Letizia, M.; Vizcay-Barrena, G.; Faruqu, F.N.; Philippeos, C.; Hannen, R.; Al-Jamal, K.T.; et al. Regulatory T Cell Extracellular Vesicles Modify T-Effector Cell Cytokine Production and Protect Against Human Skin Allograft Damage. Front. Cell Dev. Biol. 2020, 8, 317.
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146.
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS ONE 2010, 5, e11469.
- Hood, J.L.; Pan, H.; Lanza, G.M.; Wickline, S.A.; Consortium for Translational Research in Advanced Imaging and Nanomedicine. Paracrine induction of endothelium by tumor exosomes. Lab Investig. 2009, 89, 1317–1328.
- Zhang, H.G.; Grizzle, W.E. Exosomes and cancer: A newly described pathway of immune suppression. Clin. Cancer Res. 2011, 17, 959–964.
- Su, D.; Tsai, H.I.; Xu, Z.; Yan, F.; Wu, Y.; Xiao, Y.; Liu, X.; Wu, Y.; Parvanian, S.; Zhu, W.; et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J. Extracell Vesicles 2019, 9, 1709262.
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noel, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410.
- Rogers, R.G.; Fournier, M.; Sanchez, L.; Ibrahim, A.G.; Aminzadeh, M.A.; Lewis, M.I.; Marban, E. Disease-modifying bioactivity of intravenous cardiosphere-derived cells and exosomes in mdx mice. JCI Insight 2019, 4.
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc Res. 2021, 117, 292–307.
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355.
- Domenis, R.; Cifu, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325.
- Fan, G.C. Hypoxic exosomes promote angiogenesis. Blood 2014, 124, 3669–3670.
- Showalter, M.R.; Wancewicz, B.; Fiehn, O.; Archard, J.A.; Clayton, S.; Wagner, J.; Deng, P.; Halmai, J.; Fink, K.D.; Bauer, G.; et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem. Biophys. Res. Commun. 2019, 512, 729–735.
- Zhao, T.; Sun, F.; Liu, J.; Ding, T.; She, J.; Mao, F.; Xu, W.; Qian, H.; Yan, Y. Emerging Role of Mesenchymal Stem Cell-derived Exosomes in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2019, 14, 482–494.
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94.
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955.
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412.
- Jiang, M.; Fang, H.; Shao, S.; Dang, E.; Zhang, J.; Qiao, P.; Yang, A.; Wang, G. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019, 33, 13241–13253.
- Fang, H.; Shao, S.; Jiang, M.; Dang, E.; Shen, S.; Zhang, J.; Qiao, P.; Li, C.; Wang, G. Proinflammatory role of blister fluid-derived exosomes in bullous pemphigoid. J. Pathol. 2018, 245, 114–125.
- Samuel, M.; Gabrielsson, S. Personalized medicine and back-allogeneic exosomes for cancer immunotherapy. J. Int. Med. 2021, 289, 138–146.
- Lu, M.; Peng, L.; Ming, X.; Wang, X.; Cui, A.; Li, Y.; Wang, X.; Meng, D.; Sun, N.; Xiang, M.; et al. Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine 2019, 42, 443–457.
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 10, 1397.
- Le Bouteiller, P.; Bensussan, A. Up-and-down immunity of pregnancy in humans. F1000Res 2017, 6, 1216.
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235.
- Tolosa, J.M.; Schjenken, J.E.; Clifton, V.L.; Vargas, A.; Barbeau, B.; Lowry, P.; Maiti, K.; Smith, R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012, 33, 933–941.
- Stenqvist, A.C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013, 191, 5515–5523.
- Hedlund, M.; Stenqvist, A.C.; Nagaeva, O.; Kjellberg, L.; Wulff, M.; Baranov, V.; Mincheva-Nilsson, L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: Evidence for immunosuppressive function. J. Immunol. 2009, 183, 340–351.
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78.
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug. Deliv. Rev. 2016, 106, 148–156.
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494.
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell Vesicles 2015, 4, 26316.
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. A 2020, 108, 545–556.
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2019, 8, 313–324.
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020.
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles As Nanomedicine: Hopes And Hurdles In Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859.
- Rayyan, M.; Zheutlin, A.; Byrd, J.B. Clinical research using extracellular vesicles: Insights from the International Society for Extracellular Vesicles 2018 Annual Meeting. J. Extracell Vesicles 2018, 7, 1535744.
- Ibrahim, A.G.E.; Li, C.; Rogers, R.; Fournier, M.; Li, L.; Vaturi, S.D.; Antes, T.; Sanchez, L.; Akhmerov, A.; Moseley, J.J.; et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat. Biomed. Eng. 2019, 3, 695–705.
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell Vesicles 2014, 3.
