The rapid separation and efficient recycling of catalysts after a catalytic reaction are considered important requirements along with the high catalytic performances. In this view, although heterogeneous catalysis is generally less efficient if compared to the homogeneous type, it is generally preferred since it benefits from the easy recovery of the catalyst. Recycling of heterogeneous catalysts using traditional methods of separation such as extraction, filtration, vacuum distillation, or centrifugation is tedious and time-consuming. They are uneconomic processes and, hence, they cannot be carried out in the industrial scale.
- catalysis
- heterogeneous catalysts
- separation methods
- recycling
- recovery
1. Traditional Methods of Recovery: Filtration and Centrifugation
In accordance with the need of green and sustainable development, a long lifetime and an easily recycling are important requirements for practical application of a catalyst in industry [1]. In gas/solid systems, the catalyst is easily recovered from the reaction medium mechanically. Instead, in liquid media reaction, a rapid and efficient method of separation is required to remove the catalyst from products in order to recycle it as many times as possible. Therefore, the appropriate separation process should be chosen based on the nature of the catalytic material, the time required, and the energy consumed.
and
are two of the methods traditionally employed in laboratory scale to allow handling, separation, recovery, and recycling of heterogeneous catalysts. Like all the other techniques of separation, these techniques are based on the different chemical or physical properties between the components of the reaction mixture.
exploits the different size of the components of the mixture to separate an undissolved solid from a liquid or a gas. The technique involves the use of a porous structure (filter/membrane) called septum that allows the fluid phase of mixture to pass through but keeps larger solid particles back. As the liquid/solid material passes through the filter, the solid material is collected and removed. In this process, the collected solid material is called “residue” while the fluid material more or less thoroughly separated from the solids is called “filtrate”, “effluent”, or “permeate”.
The permeation of the fluid phase through the filter medium is influenced by the gradient of pressure across the septum [2]. Filtration processes can be classified in accordance with driving forces that produce such difference of pressure. In most cases, the liquid is allowed to flow through the filter medium only by gravity, and the process is known as “gravity filtration”. Generally, gravity filtration is used to remove solid impurities such as drying agent, an undesired side product, or leftover reactant from a liquid media. Since this driving force is relatively weak, the liquid phase must be of low viscosity and the mixture sufficient dilute.
The difference of pressure for the “centrifugal filtration” is due to the centrifugal force acting on the fluid that, at high rotation speeds, can be many orders of magnitude higher than the gravitational force. The process requires more technical equipment, but, as a general rule, it is much faster and more efficient than the gravitational one. “Vacuum filtration” and “pressured filtration” usually are used in the laboratory in preference to gravity filtration.
A vacuum filter operates by creating a partial vacuum on the opposite side of the filter. Therefore, the vacuum filtration technique requires a vacuum pump able to aspire the liquid through the septum. In pressure filtration, the liquid phase is driven through the filter by mechanical pressure on the feed side of the filter. Since the driving force (pressurized fluid or vacuum) is much greater than gravity, the pressure difference generated is sufficient to filter a filtrate too viscus, thus permitting higher filtration rates and a lower consumption of solvents. Processes of filtration are schematized in
1.
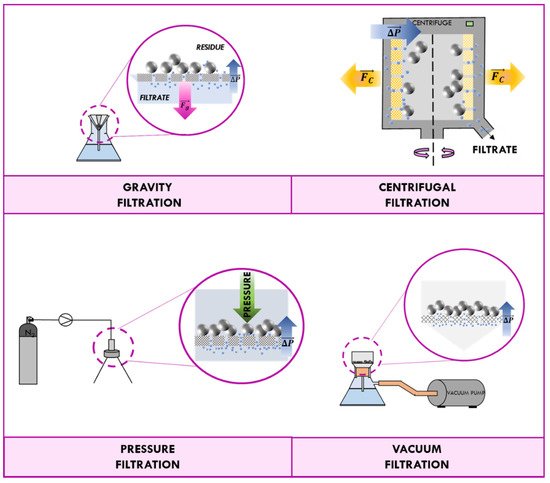
Schematization of filtration processes: gravity, centrifugal, pressure, and vacuum.
As in other separation processes, the separation of phases by filtration is never complete: liquid adheres to the separated solids, and the filtrate often contains some solids. Therefore, the recovery of solid catalysts after separation from reaction mixture by filtration methods very often requires further treatments such as washing, deliquoring (elimination of liquid from the filter by mechanical forces), and thermal drying [2].
Recently, Mina Amirsoleimani and her collaborators synthetized a highly effective heterogeneous and environmentally benign catalyst for the conversion of a variety of amines to their corresponding formamides and acetamides, which was recovered by filtering the reaction mixture and recycled seven times without any loss in activity [3].
is a technique frequently used to separate mixtures of two or more phases on the basis of their different density. Separation of heterogeneous mixtures through sedimentation happens spontaneously due to earth gravity, but it would take a long time. If separation is carried out by the application of a centrifugal force, this natural process is accelerated (
2).
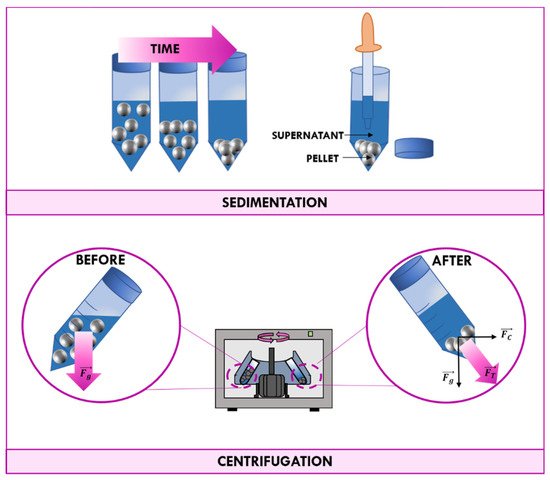
Schematic representation of the sedimentation and the centrifugation processes.
According to Newton’s first law, a body tends to remain in a state on rest or linear uniform motion in the absence of forces acting upon it. When the body moves with uniform speed along a circular, a force directed towards the center of the circle acts upon it. This force is called centripetal force. Changing the frame of reference, the body moving in the curve path feels an apparent force called
that has the same magnitude and dimensions as the centripetal force but points in the opposite direction. Such force is dependent on the speed of rotation in RPM and the distance of the body from the center of rotation.
The centrifugal force provided by a centrifuge in combination with gravitational force cause particles to move radially away from the axis of rotation. More-dense particles of the mixture tend to move at the bottom of the tube, while less-dense particles of the mixture migrate towards the axis of the centrifuge. The solid deposited on the bottom of the tube is called “pellet”, while the remaining solution is called the “supernate” or “supernatant liquid”. The supernatant liquid can be quickly decanted from the tube without disturbing the precipitate, or aspirated with a Pasteur pipette [4].
Nasresfahani et al., in their recent study, prepared an efficient heterogeneous Nickel−Copper bimetallic catalyst for the synthesis of substituted amines that was successfully recovered through centrifugation and reused four times without notable decrease in its activity [5].
However, recycling of heterogeneous catalysts using traditional methods of separation such as filtration and centrifugation is tedious and time-consuming. In addition, these separation techniques are not usually able to collect the nano-sized catalysts without weight loss. Recovery by filtration is not advisable because smaller sized nanoparticles could pass through the filter paper. On the other hand, centrifugation is uneconomic processes and, consequently, it cannot be carried out on an industrial scale. Recently, magnetic separation for catalyst recycling provided an interesting alternative to the separation methods mentioned above in terms of time, energy, and efficiency of catalysis [6][7][8].
2. Magnetic Separation
By using an external magnet, the separation of magnetic solid catalysts has been demonstrated to be fast, simple, convenient, and efficient; this new type of approach makes the work-up procedure economically and technically feasible with a minimal loss of solid catalyst (
3) [9]. In accordance with the principles of green chemistry, magnetic separation reduces secondary waste generation and energy consumption, and also offers the opportunity to expand the use of expensive catalysts based on noble metals and rare-earth elements [10].
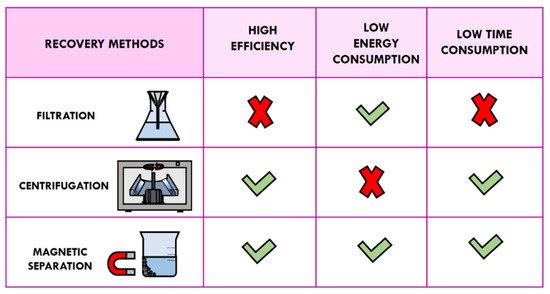
Comparison of recovery methods: filtration, centrifugation, and magnetic separation.
2.1. Magnetic Nanoparticles (MNPs)
In recent years, magnetic nanoparticles (MNPs) have received considerable attention among researchers due to their wide use in the preparation of recyclable catalysts. These nanoparticles can be directly used as catalysts or as magnetic carriers for the catalytic species immobilized on their surface. Utilizing magnetic nanoparticles as catalysts or supports helps to combine the advantages of both nano-sized particles and magnetic materials. The large surface-to-volume ratio of these types of materials allows excellent loading of the homogeneous catalyst and then obtaining a magnetic catalyst with high catalytic activity.
Magnetic nanoparticles (MNPs) usually exhibit a phenomenon known as superparamagnetism. Superparamagnetism is a form of magnetism that takes place when the size of ferromagnetic or ferrimagnetic nanoparticles is as low as 10–20 nm [11]. As a consequence of an externally applied magnetic field, superparamagnetic and paramagnetic materials become magnetized up to their saturation magnetization. As soon as the field goes to zero, any residual magnetic interaction disappears. In comparison with paramagnetic materials, superparamagnetic nanoparticles have a higher magnetic susceptibility, rendering them highly sensitive to externally applied magnetic fields [12].
If nanoparticles are sufficiently small, each of them can be considered as characterized by a single magnetic domain able to act as a “single super spin”. Unlike ferromagnetic materials, in the absence of the magnetic field, these nanoparticles randomly change the orientation of their spins even at room temperature. Instead, the ordered magnetic moments of ferromagnetic materials become disordered in response to thermal fluctuations only above the Curie Temperature (CT), or Curie point, losing their permanent magnetism.
The magnetic component of MNPs provides a fast separation from the liquid phase by application of an external magnetic field and an easy redispersion in the absence of an applied magnetic field, avoiding in this way the conventional filtration, centrifugation, or other tedious workup processes. Most magnetic-supported catalysts can be reused to several runs almost keeping their initial activity [13].
When a permanent magnet is placed in contact with a beaker containing a suspension of magnetic nanomaterial, magnetic dipoles of nanoparticles point in the same direction of the field lines. As shown in
4, the applied magnetic field induces nanoparticles to migrate towards the magnet. After a few minutes, all particles end up concentrated at the wall in correspondence with the magnet; in this way, the supernatant can be easily aspirated with a syringe. When the magnet is removed, the random orientation of nanoparticle is so fast that the measured magnetic moment gets down to zero quickly and nanoparticles behave as no-magnetic materials.
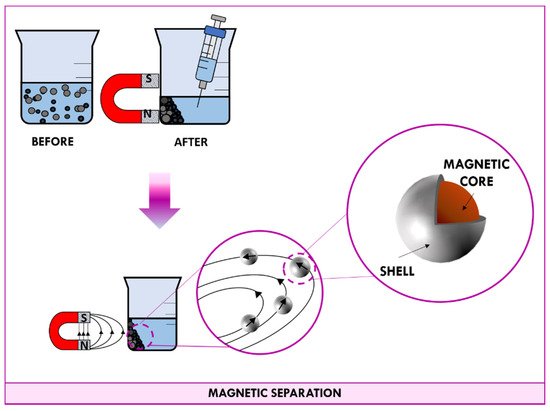
Schematic representation of the magnetic separation.
Properties such as large surface area, relatively low preparation cost and toxicity, good stability, coercivity (the field required to bring the magnetism to zero), low Curie temperature [14], and rapid recovery make them ideal supports in the field of catalysis. Thanks to these particular properties, these materials are also applied in a variety of fields such as biotechnology/biomedicine [15], magnetic resonance imaging (MRI) [16], controlled drug delivery [16], biosensors [14], hyperthermia [17], gene therapy [18], gas sensor [19], data storage [20], adsorbent materials [21], batteries [22], pigments [23], water purification [24], tissue repair [25], detoxification of biological fluids [26], and labeling and separation of cells [27].
The most common MNPs are pure metal nanoparticles (Fe, Co, Ni), metal alloys (FePt, CoPt), iron oxides (FeO, Fe
O
, Fe
O
), or ferrites MFe
O
(M = Co, Mn, Cu, Zn) [28]. These materials can be easily modified and functionalized without altering their magnetic properties. Appropriate surface changes are required to improve the dispersion, the catalytic activity, and the physicochemical and mechanical properties of MNPs, and make them biocompatible [29].
For example, cobalt (0) nanoparticles supported on ceria were used as catalyst in the hydrolysis of ammonia borane for hydrogen production. The presence of cerium(III) defects improved the catalytic activity of metal nanoparticles through a more favorable substrate–metal interaction [30]. After completion of reaction, the catalyst was isolated by a permanent magnet and then redispersed for a subsequent run of hydrolysis retaining its initial catalytic activity even after the fifth use [31].
Among the magnetic nanoparticles, iron oxides are widespread used in the field of MNPs catalysis owing to their optical, electrical, magnetic, and catalytic properties [32]. Up to now, 16 pure phases of iron oxides, in the form of oxides, hydroxides, and oxyhydroxides have been found [33]. Particularly, magnetite (Fe
O
), an important type of ferrite, is highly regarded and applied due to their low cost, availability, nontoxicity, and easy functionalization with other metallic species or organocatalysts [8]. Fe
O
is characterized by strong superparamagnetic and electron properties due to the presence of iron cations in both capacities (Fe
, Fe
) [33]. Their superparamagnetic behavior makes magnetite NPs possible to use such as the magnetic core to an eventual core–shell structure. Adding Fe
O
into catalysts endows the catalysts with excellent magnetic properties and improves even further the catalytic performances [8].
2.2. Magnetically Recoverable Transition Metal Ferrite Nanoparticles
In the last few years, magnetic spinel ferrite nanoparticles with a general molecular formula MFe
O
(M is transition metal, such as Fe, Cu, Zn, and Mn) have attracted significant interest on the field of heterogeneous catalysis. They offer several advantages such as chemical and thermal stability [34], and high surface area and mechanical hardness. Ferrite nanocatalysts not only are environmentally benign and compatible with green chemistry aspects but also can be simply recovered from reaction mixture and recycled up to several times almost without significant loss of their catalytic activity [35].
Ali et al. studied catalytic performance of cuprospinel CuFe
O
nanoparticles in the biodiesel production via the waste frying oil (WFO) transesterification process. This catalyst, synthesized by a combination of co-precipitation and hydrothermal methods, showed high activity and reactivity, simplicity, easy reusability, and excellent stability under different reaction conditions. After the reaction time, the catalyst was recovered by an external magnetic field, washed with acetone to remove the adhered oil and glycerol, and dried and reused again without any leaching or any catalytic efficiency reduction even for five reaction cycles [36].
2.3. Magnetically Recoverable Orthoferrites of Rare-Earth Nanoparticles
The rare earths (REEs) are a class of seventeen transition metal elements, including 15 lanthanides, scandium (Sc), and yttrium (Y), widely applied in various fields such as metallurgy, electronic devices, high-performance alloys, glass, ceramics, and the phosphorous industries. They are also used to make permanent magnets (PMs) [37][38]. The variable valence states and electronic structures of rare earth ions allow their use for several catalytic applications. For example, same rare earth elements are applied in catalysis as dopants or components to modulate the specific physical and chemical properties of different materials [39], cerium-based materials act as catalysts in various reactions such as CO
hydrogenation [40], Fischer–Tropsch synthesis [41], and CO
methanation [42][43][44][45] due to redox properties and excellent oxygen mobility.
Orthoferrites of rare-earth elements (REE) with the formula RFeO
(R = Sc, Y, La-Lu) are a novel class of acid-base catalysts of great interest for researchers due to their high activity and the possibility of magnetic recovery. They are applied mainly in redox and photoinduced processes [10]. For example, K.D Martinson and his research group successfully synthetized a phase-pure porous magnetic nanopowder based on holmium orthoferrites by glycine-nitrate combustion approach for the first time. The porous holmium orthoferrite has been tested for n-hexane conversion at 500 °C and 1 atm. The observed catalytic activity can be attributed to high concentration of acid-base sites on its surface. After reaction, the spent sample was separated by an external magnetic field of a permanent magnet from the reaction followed by heat treatment at 600 °C for 1 h, with an efficiency of 97% [10].
2.4. Surface Modification of Magnetic Nanoparticles
Unfortunately, it has been found that MNPs commonly tend to agglomerate into a large cluster due to their small interparticle distances, high surface energy, and the existence of van der Waals forces. As a result, all of this involves a non-uniform dispersion of the magnetic catalyst in the reaction mixture and then a rapid loss of catalytic activity and magnetism [46]. This problem is solved by coating their surface with suitable protecting agents to prevent direct contact between the nanoparticles and accordingly improve their chemical stability. Generally, magnetic nanoparticles (NPs) are coated with carbon (graphene) or silica so that they form magnetic core–shell structures. Carbon or silica coating provides the possibility to prevent metal oxidation and/or to facilitate the introduction of functional groups via sp
carbon of graphene or silanol groups of silica [47], which allow, in turn, the attachment of various catalytic species on the magnetic NPs surface including ionic liquids through covalent bonds or electrostatic interactions. Recently, much attention has been paid to other metal oxides, such as ceria and titania, for coating of the magnetic core in order to obtain magnetically recoverable catalyst supports. Vono et al. have found that the pre-coating of magnetic supports containing ceria or titania with a uniform layer of silica provides thermal resistant magnetic materials without modifying the magnetic properties of the iron oxide core [48].
The process of synthesis of functionalized magnetic nanoparticles usually consists of three steps: the formation of the magnetic core, the coating of the nanoparticles with an outer shell and the modification of the resultant core–shell structure.
2.5. Ionic Liquid Coated Magnetic Nanoparticles
Recently, Ionic liquids (ILs)-coated MNPs have attracted growing interest as catalysts for a wide range of new industrial applications. Ionic liquids (ILs) are commonly defined as salts composed of organic cations and organic or inorganic anions with melting point below the boiling point of water. According to the nature of the cation and/or the counter anion moiety, the ionic liquid may behave as an acidic, basic, or organ catalyst [49]. Many of these ILs have been investigated in a variety of organic transformations in which ILs can act as solvents and/or catalysts [50]. They have a number of advantages such as wide liquid range and potential window, extremely low vapor pressure, high ionic conductivity, and extraordinary ability to functionalization [51]. However, their industrial applications are hampered by their high viscosity [46], high cost, large consumption, and homogeneousness (difficulty to separate) [52]. To overcome these drawbacks, supported ionic liquid catalysts (SIL) could be a valid alternative to homogeneous analogues since they maintain the excellent catalytic properties of ionic liquids, but also manifest the advantages of easy separation and recovery typical of the heterogeneous catalyst. On the basis of this awareness, different support materials, such as amorphous or ordered mesoporous silica, zeolites, polystyrene, magnetic nanoparticles, and many others have been used as carrier for loading ionic liquids [53]. Particularly, ionic liquids immobilized on the magnetic nanoparticles surface, have emerged as good catalysts because of their facile separation by an external magnet.
Cano et al. synthesised a new, highly active, and efficiently recoverable catalytic system modifying nano-magnetite materials with a paramagnetic halometallate-based ionic liquid (MILs), Fe
O
@SiO
@(mim)[FeCl
] (mim: methylimidazolium). This system was applied in the glycolysis of PET into BHET under conventional heating. The catalyst exhibited an excellent recycling behaviour, achieving nearly 100% yield and selectivity over twelve consecutive reaction cycles at 180 °C and was easily recovered with an external magnetic field avoiding tedious work-up or purification processes. Further analyses by ICP revealed that the amount of catalyst lost after each cycle was minimal and the amount of Fe in the purified product was negligible [52].
Ren et al. synthesized an alkaline ionic liquid immobilized on protective copolymers coated magnetic nanoparticles ([Im][OH]/P(VBC-DVB) @MNPs). The catalyst consisted of three layers: a magnetic core, an intermediate protective shell, and an outer catalytic layer. The magnetic core, mainly composed of CoFe
O
magnetic nanoparticles (CFNPs), makes easier separation of the catalyst from reaction medium. The protective layer, formed by the copolymer of divinylbenzene (DVB) and vinyl benzyl chloride (VBC), incorporated into the catalyst, on the other hand, prevents the corrosion of magnetic nanoparticle when the catalyst is used in polar solvents, thus preserving recyclability and improving its stability. The catalytic layer, which consisted of 1-propyl-3-alkylimidazole hydroxide ionic liquid, is where the reaction takes part. This catalyst showed an excellent catalytic activity for Knoevenagel condensation between benzaldehyde and ethyl cyanoacetate due to the large amount of OH
ions loading on the surface and a lower activation energy than of homogeneous catalyst NaOH. The catalyst was conveniently recovered upon applying an external magnetic field, and it was recycled at least six times without significant changes [54]. However, since the loading of ILs on the surface cannot be too high, catalytic activity turns out to be limited owing to the relatively low number of catalytic units compared to the large surface area of magnetic nanoparticles [46]. To improve the catalytic activity, it is necessary to increase the amount of catalyst used for the catalytic process, and this could require a greater use of solvents as well as expensive recovery cost and possible toxicological concern [54]. This problem could be solved by the surface modification of the magnetic nanoparticles with different polymer grafting methods [55]. The introduction of polymer onto the MNPs increases the loading content of grafted homogenous material since more catalytic units can be attached onto magnetic support [55]. On the other hand, the polymer layer could prevent the formation of clusters in high temperature processes [56]. Arghan et al. developed a novel poly (ionic liquid) (PIL) coated magnetic nanoparticles catalyst, noted as n-Fe
O
/PVAm-SO
H, and used it as a magnetic heterogeneous acid catalyst for the one-pot synthesis of tri and tetrasubstituted imidazole derivatives under optimized condition in excellent yields. The catalyst was synthesized by directly grafting polyvinyl amine layer onto surface of Fe
O
without using any bridged organosilane compounds. In the following, the sulfonation treatment was carried out by covalent grafting of chlorosulfonic acid on amine groups to convert n-Fe
O
/PVAm into an efficient acid catalyst. In addition, the resulted catalyst was readily separated in a green way by using a magnetic force and reused eight times without any significant deterioration in catalytic activity. The study results show that this new acid magnetic catalyst exhibits more advantages than soluble acids [55].
Nowadays, dicationic ionic liquids (DILs), a new subclass of ILs, have attracted significant attention. Typically, these molecules contain two cationic head groups connected together with a rigid or flexible spacer, associated with two counter anions, respectively [51]. Modifying the length of the chain and the type of spacer or the cation, their physicochemical properties can be tuned [46]. In comparison to various reported monocationic ionic liquid in the literature, dicationic ionic liquids exhibit several interesting advantages such as higher thermal stability, surface activity, more extensive liquid range, and higher selectivity [51]. These ILs can be used in many applications from the “classical” use as solvents, catalysts, or catalytic supports in organic reactions, to more specific uses as high temperature lubricants/heat transfer fluids, including a variety of roles in analytical sciences [46].
Fahimeh Rezaei and co-workers designed Brønsted acidic dicationic ionic liquid immobilized on silica-coated iron oxide support with core–shell structure and applied it as a recyclable nanocatalyst under solvent-free conditions in the synthesis of pyrazole derivatives [57]. The catalyst has maintained his catalytic activity at least five times. In addition, Reyhaneh Karimi-Chayjani demonstrated that magnetic γFe
O
@SiO
NPs-supported Bis[(3-aminopropyl) triethoxysilane] dichloride bis-dicationic ionic liquid (DIL) catalyst can catalyse the Knoevenagel condensation achieving high reaction rates and yields. The synthesized nanomagnetic catalyst possesses a high superparamagnetic characteristic and can be simply recovered and reused applying an external magnetic field at least six times without loss of catalytic activity [14].
Metal-organic frameworks (MOFs), also known as porous coordination polymers (PCPs), are a class of porous crystalline materials, highly investigated in the last twenty years. Several features of MOFs such as controllable composition, large surface areas, regular and accessible pores, and crystalline open structures allows them to encapsulate within their frameworks magnetic nanoparticles, making them promising heterogeneous catalysts or catalytic carriers of various species including ionic liquids [58].
For example, very recently, Xie et al. developed an efficient and magnetically recyclable acidic catalyst based on the amino-functionalized metal-organic framework structures, for the biodisel production via simultaneous transesterification-esterifications of low-quality oil feedstock. To prepare the acidic catalyst with Brönsted–Lewis acid sites, the POM-ILs with sulfonated organic cations and Keggin POM counter anions were immobilized on the CoFe
O
/MIL-88B(Fe)-NH
support combining in this way the advantages of ILs and heterogeneous character of MOFs. The catalyst showed a good stability, probably, due to the charge interaction of acidic groups of POM-based sulfonated ILs with amino groups of the magnetic support. On the other hand, the encapsulation of magnetic nanoparticles (CoFe
O
) into cavities of the metal organic framework nanocomposites gives magnetic properties to catalysts, improving its separation performances. In order to investigate the reusability of the solid catalyst, its recovery was conveniently carried out by using a permanent magnet. The catalyst was reused for five transesterification runs under the optimized reaction conditions without significant decay of the catalytic activity [59].
References
- Molnár, Á.; Papp, A. Catalyst recycling—A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65.
- Ripperger, S.; Gösele, W.; Alt, C.; Loewe, T. Filtration, 1. Fundamentals. Ullmann’s Encycl. Ind. Chem. 2013, 1–38.
- Amirsoleimani, M.; Khalilzadeh, M.A.; Zareyee, D. Preparation and catalytic evaluation of a palladium catalyst deposited over modified clinoptilolite () for chemoselective N-formylation and N-acylation of amines. J. Mol. Struct. 2021, 1225, 129076.
- Majekodunmi, S.O. A review on centrifugation in the pharmaceutical industry. Am. J. Biomed. Eng. 2015, 5, 67–78.
- Nasresfahani, Z.; Kassaee, M.Z. Nickel−Copper bimetallic mesoporous nanoparticles: As an efficient heterogeneous catalyst for N -alkylation of amines with alcohols. Appl. Organomet. Chem. 2021, 35, e6032.
- Li, H.; Ma, Y. Magnetically separable Fe3O4-supported Ru–Ni bimetallic catalysts for diformyltricyclodecanes hydrogenation to value-added fine chemicals. Prog. React. Kinet. Mech. 2020, 45.
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nanosized materials for efficient biodiesel synthesis via acid–base/enzyme catalysis. Green Chem. 2020, 22, 2977–3012.
- Li, F.; Zhu, W.; Jiang, S.; Wang, Y.; Song, H.; Li, C. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over Fe3O4 modified Ru/Carbon nanotubes catalysts. Int. J. Hydrog. Energy 2020, 45, 1981–1990.
- Alaei, S.; Haghighi, M.; Toghiani, J.; Vahid, B.R. Magnetic and reusable MgO/MgFe2O4 nanocatalyst for biodiesel production from sunflower oil: Influence of fuel ratio in combustion synthesis on catalytic properties and performance. Ind. Crop. Prod. 2018, 117, 322–332.
- Martinson, K.; Kondrashkova, I.; Omarov, S.; Sladkovskiy, D.; Kiselev, A.; Kiseleva, T.; Popkov, V. Magnetically recoverable catalyst based on porous nanocrystalline HoFeO3 for processes of n-hexane conversion. Adv. Powder Technol. 2020, 31, 402–408.
- Wahajuddin, A.S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471.
- Kneller, E.F.; Luborsky, F.E. Particle Size Dependence of Coercivity and Remanence of Single-Domain Particles. J. Appl. Phys. 1963, 34, 656–658.
- Kooti, M.; Nasiri, E. Synthesis of a novel magnetic nanocatalyst based on rhodium complex for transfer hydrogenation of ketone. Appl. Organomet. Chem. 2019, 33, e4886.
- Karimi-Chayjani, R.; Daneshvar, N.; Shirini, F.; Tajik, H. New magnetic nanocatalyst containing a bis-dicationic ionic liquid framework for Knoevenagel condensation reaction. Res. Chem. Intermed. 2019, 45, 2471–2488.
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021.
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334.
- Sasikala, A.R.K.; GhavamiNejad, A.; Unnithan, A.R.; Thomas, R.G.; Moon, M.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. A smart magnetic nanoplatform for synergistic anticancer therapy: Manoeuvring mussel-inspired functional magnetic nanoparticles for pH responsive anticancer drug delivery and hyperthermia. Nanoscale 2015, 7, 18119–18128.
- SJiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064.
- Zhai, Q.; Du, B.; Feng, R.; Xu, W.; Wei, Q. A highly sensitive gas sensor based on Pd-doped Fe3O4nanoparticles for volatile organic compounds detection. Anal. Methods 2014, 6, 886–892.
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632.
- Chen, S.; Bai, B.; He, Y.; Hu, N.; Wang, H.; Suo, Y. Controllable conversion of Prussian bio-template into 3D cage-like magnetic Fe3O4@N-doped carbon absorbent and its cohesive regeneration by persulfate activation. RSC Adv. 2019, 9, 1151–1164.
- Malara, A.; Pantò, F.; Santangelo, S.; Antonucci, P.L.; Fiore, M.; Longoni, G.; Ruffo, R.; Frontera, P. Comparative life cycle assessment of Fe2O3-based fibers as anode materials for sodium-ion batteries. Environ. Dev. Sustain. 2020, 23, 6786–6799.
- Wang, Z.; Zhai, S.; Lv, J.; Qi, H.; Zheng, W.; Zhai, B.; An, Q. Versatile hierarchical Cu/Fe3O4 nanocatalysts for efficient degradation of organic dyes prepared by a facile, controllable hydrothermal method. RSC Adv. 2015, 5, 74575–74584.
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 2019, 162, 538–568.
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Valladares, L.D.L.S.; Aguiar, J.A. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143.
- Lian, S.; Kang, Z.; Wang, E.; Jiang, M.; Hu, C.; Xu, L. Convenient synthesis of single crystalline magnetic Fe3O4 nanorods. Solid State Commun. 2003, 127, 605–608.
- Neoh, K.G.; Kang, E.T. Surface modification of magnetic nanoparticles for stem celllabeling. Soft Matter 2011, 8, 2057–2069.
- Wang, D.; Astruc, D. Fast-Growing Field of Magnetically Recyclable Nanocatalysts. Chem. Rev. 2014, 114, 6949–6985.
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810.
- He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Cerium-Oxide-Modified Nickel as a Non-Noble Metal Catalyst for Selective Decomposition of Hydrous Hydrazine to Hydrogen. ACS Catal. 2015, 5, 1623–1628.
- Akbayrak, S.; Taneroğlu, O.; Özkar, S. Nanoceria supported cobalt(0) nanoparticles: A magnetically separable and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. New J. Chem. 2017, 41, 6546–6552.
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67.
- Vahidian, M.; Elhamifar, D.; Shaker, M. Protected, Email Core–shell structured magnetic mesoporous silica-titania: A novel, powerful and recoverable nanocatalyst. Polyhedron 2020, 178, 114326.
- Hamad, H.A.; El-Latif, M.M.A.; Kashyout, A.B.; Sadik, W.A.; Feteha, M.Y. Study on synthesis of superparamagnetic spinel cobalt ferrite nanoparticles as layered double hydroxides by co-precipitation method. Russ. J. Gen. Chem. 2014, 84, 2205–2210.
- Amiri, M.; Eskandari, K.; Salavati-Niasari, M. Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv. Colloid Interface Sci. 2019, 271, 101982.
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297.
- Sun, X.; Yuan, K.; Zhang, Y. Advances and prospects of rare earth metal-organic frameworks in catalytic applications. J. Rare Earths 2020, 38, 801–818.
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.-Y. Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048.
- Zeng, Z.; Xu, Y.; Zhang, Z.; Gao, Z.; Luo, M.; Yin, Z.; Zhang, C.; Xu, J.; Huang, B.; Luo, F.; et al. Rare-earth-containing perovskite nanomaterials: Design, synthesis, properties and applications. Chem. Soc. Rev. 2020, 49, 1109–1143.
- Frontera, P.; Macario, A.; Malara, A.; Modafferi, V.; Mascolo, M.C.; Candamano, S.; Crea, F.; Antonucci, P. CO2 and CO hydrogenation over Ni-supported materials. Funct. Mater. Lett. 2018, 11, 1850061.
- Spadaro, L.; Arena, F.; Granados, M.L.; Ojeda, M.; Fierro, J.; Frusteri, F. Metal–support interactions and reactivity of Co/CeO2 catalysts in the Fischer–Tropsch synthesis reaction. J. Catal. 2005, 234, 451–462.
- Frontera, P.; Macario, A.; Malara, A.; Santangelo, S.; Triolo, C.; Crea, F.; Antonucci, P. Trimetallic Ni-Based Catalysts over Gadolinia-Doped Ceria for Green Fuel Production. Catalysts 2018, 8, 435.
- Frontera, P.; Malara, A.; Modafferi, V.; Antonucci, V.; Antonucci, P.; Macario, A. Catalytic activity of Ni-Co supported metals in carbon dioxides methanation. Can. J. Chem. Eng. 2020, 98, 1924–1934.
- Frontera, P.; Macario, A.; Malara, A.; Antonucci, V.; Modafferi, V.; Antonucci, P.L. Simultaneous methanation of carbon oxides on nickel-iron catalysts supported on ceria-doped gadolinia. Catal. Today 2020, 357, 565–572.
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.; Antonucci, P. The role of Gadolinia Doped Ceria support on the promotion of CO2 methanation over Ni and Ni Fe catalysts. Int. J. Hydrog. Energy 2017, 42, 26828–26842.
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719.
- Shifrina, Z.B.; Bronstein, L.M. Magnetically Recoverable Catalysts: Beyond Magnetic Separation. Front. Chem. 2018, 6, 298.
- Vono, L.L.R.; Damasceno, C.C.; Matos, J.R.; Jardim, R.F.; Landers, R.; Masunaga, S.H.; Rossi, L.M. Separation technology meets green chemistry: Development of magnetically recoverable catalyst supports containing silica, ceria, and titania. Pure Appl. Chem. 2018, 90, 133–141.
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842.
- Zhang, S.-Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.-K.; Yuan, J. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755.
- Sorkhi, S.E.S.; Hashemi, M.M.; Ezabadi, A. Introduction of a novel dicationic Brönsted acidic ionic liquid based on pyrazine and its application in the synthesis of xanthenediones and 3, 4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Res. Chem. Intermed. 2020, 46, 2229–2246.
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic ionic liquid-coated 3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110.
- Campisciano, V.; Giacalone, F.; Gruttadauria, M. Supported Ionic Liquids: A Versatile and Useful Class of Materials. Chem. Rec. 2017, 17, 918–938.
- Ren, Y.; Li, H.; Yang, W.; Shi, D.; Wu, Q.; Zhao, Y.; Feng, C.; Liu, H.; Jiao, Q. Alkaline Ionic Liquids Immobilized on Protective Copolymers Coated Magnetic Nanoparticles: An Efficient and Magnetically Recyclable Catalyst for Knoevenagel Condensation. Ind. Eng. Chem. Res. 2019, 58, 2824–2834.
- Arghan, M.; Koukabi, N.; Kolvari, E. Sulfonated-polyvinyl amine coated on Fe3O4 nanoparticles: A high-loaded and magnetically separable acid catalyst for multicomponent reactions. J. Iran. Chem. Soc. 2019, 16, 2333–2350.
- Pourjavadi, A.; Hosseini, S.H.; Aghayeemeibody, S.A.; Hosseini, S.T. Poly(basic ionic liquid) coated magnetic nanoparticles: High-loaded supported basic ionic liquid catalyst. Comptes Rendus Chim. 2013, 16, 906–911.
- Rezaei, F.; Amrollahi, M.A.; Khalifeh, R. Brønsted Acidic Dicationic Ionic Liquid Immobilized on Fe3O4/SiO2 Nanoparticles as an Efficient and Magnetically Separable Catalyst for the Synthesis of Bispyrazoles. ChemistrySelect 2020, 5, 1760–1766.
- Wu, Z.; Chen, C.; Wan, H.; Wang, L.; Li, Z.; Li, B.; Guo, Q.; Guan, G. Fabrication of Magnetic NH2-MIL-88B (Fe) Confined Brønsted Ionic Liquid as an Efficient Catalyst in Biodiesel Synthesis. Energy Fuels 2016, 30, 10739–10746.
- Xie, W.; Wang, H. Synthesis of heterogenized polyoxometalate-based ionic liquids with Brönsted-Lewis acid sites: A magnetically recyclable catalyst for biodiesel production from low-quality oils. J. Ind. Eng. Chem. 2020, 87, 162–172.
