Selenium (Se) is an essential micronutrient for mammals, and its deficiency seriously threatens human health. A series of biofortification strategies have been developed to produce Se-enriched foods for combating Se deficiency. Although there have been some inconsistent results, extensive evidence has suggested that Se supplementation is beneficial for preventing and treating several chronic diseases. Understanding the association between Se and chronic diseases is essential for guiding clinical practice, developing effective public health policies, and ultimately counteracting health issues associated with Se deficiency. The current review will discuss the food sources of Se, biofortification strategies, metabolism and biological activities, clinical disorders and dietary reference intakes, as well as the relationship between Se and health outcomes, especially cardiovascular disease, diabetes, chronic inflammation, cancer, and fertility.
- selenium biofortification
- chronic diseases
- baseline selenium status
- methylated selenium compounds
1. Introduction
2. Food Sources of Se
2.1. The Overview of Se Contents and Forms in Different Foods
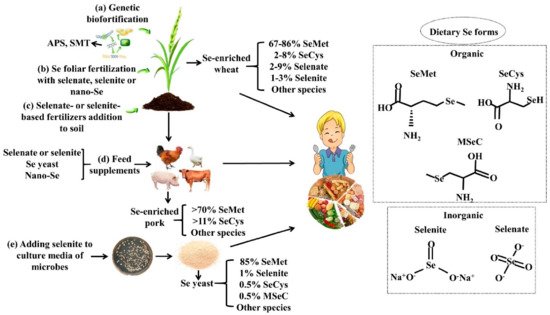
2.2. Se Biofortification
2.2.1. Agronomic Biofortification
2.2.2. Genetic Biofortification
2.2.3. Se-Biofortified Agricultural Products
2.3. Se Nutritional Fortifiers and Se Fortified Foods
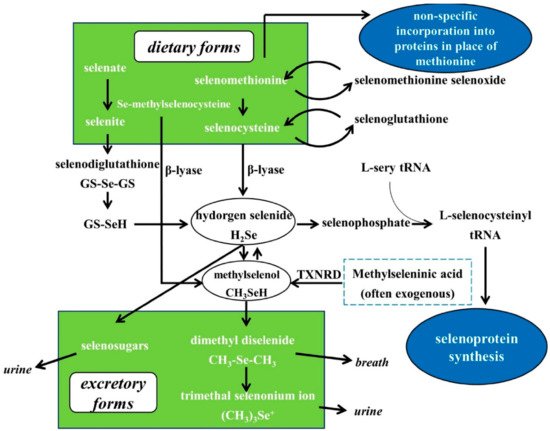
3. Se Nutritional Status Assessment, Metabolism, Bioavailability and Biological Functions
| Selenoprotein (Abbreviation) | Tissue Distribution a | Localization | Functions |
|---|---|---|---|
| Glutathione peroxidase 1 (GPX1) | Blood, kidney, liver, placenta | Cytosol | Reduces cellular H2O2 and lipid peroxides |
| Glutathione peroxidase 2 (GPX2) | Gastrointestinal tract, liver, mammary |
Cytosol | Reduces peroxide in the gut |
| Glutathione peroxidase 3 (GPX3) | Epididymis, kidney, plasma | Plasma | Reduces peroxide in blood |
| Glutathione peroxidase 4 (GPX4) | Liver, testis | Cytosol; mitochondria; nucleus (testis-specific) |
Reduces phospholipid peroxide |
| Glutathione peroxidase 6 (GPX6) | Embryos, olfactory epithelium | Cytosol | Reduces cellular H2O2 in the olfactory epithelium |
| Thioredoxin reductase 1 (TXNRD1) | Heart, kidney, liver | Cytosol | Regenerates reduced thioredoxin |
| Thioredoxin reductase 2 (TXNRD2) | Adrenal gland, heart, kidney, liver | Cytosol | Catalyzes a variety of reactions, specific for thioredoxin and glutaredoxin systems |
| Thioredoxin reductase 3 (TXNRD3) | Testis, heart, kidney, liver | Mitochondria | Reduces the oxidized form of thioredoxin and glutaredoxin 2 |
| Iodothyronine deiodinase 1 (DIO1) | Kidney, liver, thyroid | Plasma membrane | Important for systemic active thyroid hormone levels |
| Iodothyronine deiodinase 2 (DIO2) | Brain, brown adipose tissue, pituitary |
Endothelial reticulum | Important for local active thyroid hormone levels |
| Iodothyronine deiodinase 3 (DIO3) | Brain, placenta, skin | Plasma membrane | Inactivates thyroid hormone |
| Methionine sulfoxide reductase B1 (MSRB1) | Liver, kidney | Cytosol | Reduces methionine- R-sulfoxide residues in proteins to methionine |
| Selenophosphate synthetase 2 (SEPHS2) | Kidney, liver, testis | Cytosol | Synthesis of selenophosphate |
| Selenoprotein F (SELENOF) | Liver, prostate | Endoplasmic reticulum (ER) | Involved in protein folding |
| Selenoprotein H (SELENOH) | Unknown b | Nucleus | Involved in redox sensing and transcription |
| Selenoprotein I (SELENOI) |
Unknown b | Membrane | Involved in phospholipid biosynthesis |
| Selenoprotein K (SELENOK) | Unknown b | ER membrane | Modulates Ca2+ influx that affects immune cell function; component of ER-associated degradation |
| Selenoprotein M (SELENOM) | Brain | ER | Protein folding in ER |
| Selenoprotein N (SELENON) | Brain, heart, liver, muscle | ER membrane | Proper muscle development |
| Selenoprotein O (SELENOO) | Unknown b | Mitochondria | Unknown c |
| Selenoprotein P (SELENOP) | Liver, plasma | Plasma | Se transport and antioxidant function |
| Selenoprotein S (SELENOS) |
Unknown b | ER membrane | Involved in ER-associated degradation |
| Selenoprotein T (SELENOT) |
Unknown b | ER and Golgi | Involved in redox regulation and cell anchorage |
| Selenoprotein V (SELENOV) | Testes | Cytosol | Unknown c |
| Selenoprotein W (SELENOW) | Brain, muscle, testes | Cytosol | Necessary for muscle function |
4. Chronic Diseases
4.1. Cardiovascular Disease
4.2. Metabolic Diseases
4.2.1. Diabetes Mellitus
4.2.2. Thyroid Diseases
4.3. Chronic/Acute Inflammations
4.4. Cancer
4.4.1. Human Studies on Se and Cancer
-
Epidemiological studies on Se exposure and cancer risk
- Human intervention studies with Se
4.4.2. Preclinical Studies on the Anticarcinogenic Effects of Different Forms of Se
4.4.3. Possible Mechanisms for Anticarcinogenic Actions of Se
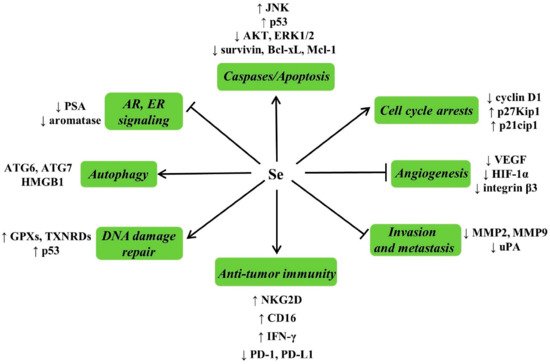
4.4.4. Se and Cancer Adjuvant Therapy
- Enhancing antitumor efficacy
- Reduction in toxicity
