In rheumatoid arthritis (RA), extracellular vesicles (EVs) are associated with both the propagation and attenuation of joint inflammation and destruction. However, the specific EV content responsible for these processes is largely unknown. Investigations into identifying EV content are confounded by the challenges in obtaining high-quality EV preparations from synovial fluid. Implementing a size exclusion chromatography-based method of EV isolation, coupled with small RNA sequencing, we accurately characterised EV miRNAs in synovial fluid obtained from RA patients and investigated the differences between joints with high- and low-grade inflammation.
- rheumatoid arthritis
- extracellular vesicles
- miRNA
- synovial fluid
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
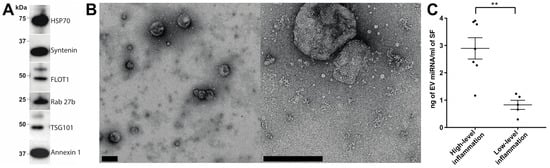
2. Characterisation of EV Isolation
| RA (High-Grade Inflammation) | RA (Low-Grade Inflammation) | p-Value | |
|---|---|---|---|
| Average Expression (CPM) | |||
| n | 7 | 5 | |
| hsa-miR-99a-5p | |||
| 45,879 | |||
| arget Gene ID | Target Gene Name | miRNA Regulators |
|---|---|---|
| Target Gene Name | miRNA Regulators | |
| - | ||
| Age—mean (s.d.) | 64.4 (11.8) | |
| 1 | hsa-miR-100-5p | 132,441 |
| IGF1R | Insulin like growth factor 1 receptor | hsa-let-7b-5p hsa-miR-100-5p hsa-miR-21-5p hsa-miR-99a-5p |
| 67.6 (10.0) | ||
| IGF1R | Insulin like growth factor 1 receptor | hsa-miR-143-3p hsa-miR-150-3p |
| 2 | 0.64 | |
| hsa-miR-99b-5p | hsa-miR-185-5p | |
| ZEB1 | ||
| Target Gene ID | Target Gene Name | miRNA Regulators | |||||||
|---|---|---|---|---|---|---|---|---|---|
| hsa-miR-21-5p | hsa-miR-223-3p hsa-miR-223-5p hsa-miR-378a-3p hsa-miR-503-5p hsa-miR-7-5p |
||||||||
| PTEN | Phosphatase and tensin homolog | hsa-miR-214-3p | hsa-miR-21-5p | 179,929 | PTEN | Phosphatase and tensin homolog | hsa-miR-103a-3p hsa-miR-106b-3p hsa-miR-142-5p hsa-miR-155-5p hsa-miR-21-3p hsa-miR-21-5p hsa-miR-25-3p |
||
| Sex—number of females/males | 3/4 | 2/3 | >0.99 | ||||||
| 3 | |||||||||
| hsa-miR-23b-3p | hsa-miR-486-5p |
hsa-miR-148a-3p | 86,059 | White cell count—mean (s.d.) cells µL−1 | 8940 * | 171.8 | <0.001 | ||
| 4 | |||||||||
| hsa-miR-92b-3p | CCND2 | Cyclin D2 | hsa-let-7a-3p hsa-let-7a-5p hsa-let-7b-5p hsa-miR-26a-5p |
||||||
| E2F2 | E2F transcription factor 2 | hsa-let-7a-3p hsa-let-7a-5p |
VEGFA | hsa-let-7b-5p | Vascular endothelial growth factor A | hsa-miR-26a-5p |
hsa-miR-101-3p hsa-miR-150-5p hsa-miR-185-5p hsa-miR-21-5p hsa-miR-378a-3p |
hsa-let-7a-5p | 43,627 |
| hsa-miR-503-5p | PTEN | Phosphatase and tensin homolog | hsa-miR-10b-5p hsa-miR-21-5p hsa-miR-26a-5p hsa-miR-92a-3p |
hsa-miR-503-5p hsa-miR-7-5p |
Anti-citrullinated protein antibody (% positive) | 71% | 20% | 0.24 | |
| 5 | hsa-miR-92a-3p | 45,881 | |||||||
| STAT3 | Signal transducer and activator of transcription 3 | hsa-let-7a-5p hsa-miR-148a-3p hsa-miR-21-5p hsa-miR-92a-3p |
Rheumatoid factor (% positive) | 86% | 60% | 0.52 | |||
| 6 | hsa-let-7b-5p | 31,280 | Disease Activity Score 28—median (range) | 4.7 (3.31–5.41) | 3.5 (2.74–5.0) | 0.15 | |||
| C-reactive protein—median (range) mg L−1 | 20 (6–164) | 2 (1.4–2) | 0.16 |
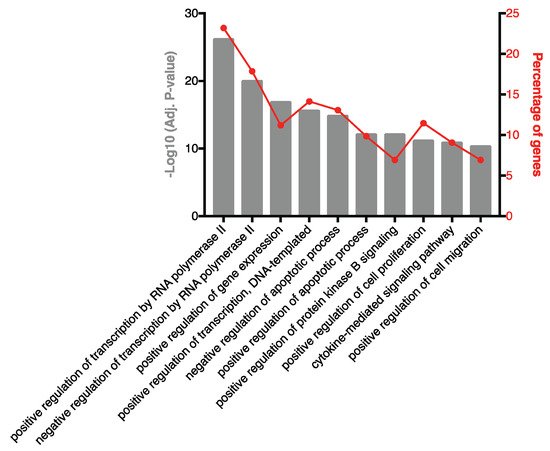
3. Highly Ranked SF EV miRNAs Target Immunomodulatory SF EV Proteins
| Rank | miRNA |
|---|
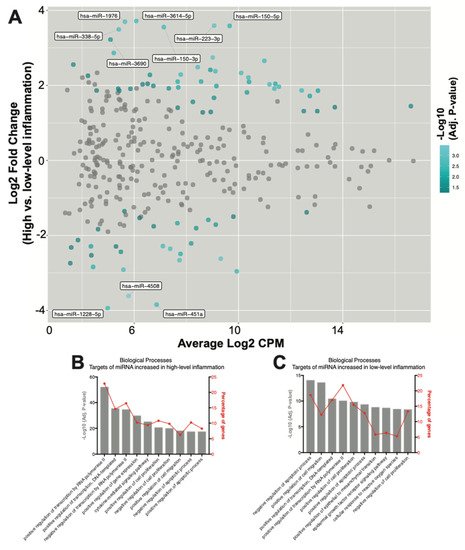
4. Seventy-Eight SF EV miRNAs Are Differentially Expressed between RA Patients with High- and Low-Grade Inflammation
| miRNA | Average Read Count (Log2 CPM) | Log2 Fold Change(High- vs. Low−Grade Inflammation) | Adjusted p-Value | ||||
|---|---|---|---|---|---|---|---|
| hsa-miR-4508 | 5.78 | −3.61 | 3.94 × 10−4 | ||||
| hsa-miR-223-3p | 9.07 | 3.59 | 6.42 × 10−4 | ||||
| hsa-miR-3529-3p | 9.01 | 2.74 | 6.42 × 10−4 | ||||
| hsa-miR-615-3p | 9.32 | ||||||
| −2.62 | 6.42 × 10 | −4 | |||||
| FBXW7 | F-box and WD repeat domain containing 7 | hsa-miR-155-5p hsa-miR-223-3p hsa-miR-223-5p | |||||
| −2 | |||||||
| BCL2 | BCL2, apoptosis regulator | hsa-miR-143-3p hsa-miR-192-5p hsa-miR-21-5p |
hsa-miR-1976 | 5.65 | 3.70 | hsa-miR-25-3p | 1.15 × 10−3 |
| hsa-miR-503-5p | hsa-miR-543 | 7.74 | −2.66 | ||||
| MYB | MYB proto-oncogene, transcription factor | 1.15 × 10 | −3 | hsa-miR-103a-3p hsa-miR-150-3p hsa-miR-150-5p hsa-miR-155-5p hsa-miR-503-5p |
7 | hsa-miR-10b-5p | 35,499 |
| hsa-miR-338-5p | 5.41 | 3.49 | 1.36 × 10−3 | ||||
| EGFR | 8 | hsa-miR-99b-5p | |||||
| hsa-miR-146b-3p | 31,569 | ||||||
| 8.43 | 2.49 | ||||||
| hsa-miR-185-5p | |||||||
| 8.63 | |||||||
| 1.28 | |||||||
| 4.48 × 10 | |||||||
| −2 | |||||||
| hsa-miR-23b-5p | |||||||
| 6.21 | |||||||
| −1.53 | |||||||
| 4.58 × 10 | |||||||
| −2 | |||||||
| hsa-miR-378f | |||||||
| 5.39 | |||||||
| 1.91 | |||||||
| 4.73 × 10 | |||||||
| −2 | |||||||
| hsa-miR-27b-5p | |||||||
| 5.63 | |||||||
| −1.36 | |||||||
| 4.76 × 10 | |||||||
| −2 | |||||||
| hsa-miR-1306-5p | |||||||
| 3.58 | |||||||
| −2.32 | |||||||
| 4.98 × 10 | |||||||
| −2 | |||||||
| Target Gene ID | ||||||
|---|---|---|---|---|---|---|
| Epidermal growth factor receptor | ||||||
| hsa-miR-146b-5p | ||||||
| hsa-miR-21-5p | ||||||
| hsa-miR-27a-5p | hsa-miR-7-5p | |||||
| 1.36 × 10 | −3 | |||||
| RAC1 | Rac family small GTPase 1 | hsa-miR-101-3p hsa-miR-142-3p hsa-miR-142-5p |
9 | hsa-miR-26a-5p | 34,975 | |
| hsa-miR-155-5p | hsa-miR-433-3p | 7.38 | −2.36 | 1.36 × 10−3 | ||
| TP53 | Tumor protein p53 | hsa-miR-150-3p hsa-miR-150-5p hsa-miR-25-3p hsa-miR-28-3p |
10 | |||
| hsa-miR-485-3p | 5.59 | −2.91 | 1.36 × 10−3 | |||
| hsa-miR-101-3p | 10.10 | 2.33 | 1.52 × 10−3 | |||
| hsa-miR-27a-5p | 11.19 | 2.03 | 1.53 × 10−3 | |||
| hsa-miR-361-3p | 11.38 | 1.91 | 1.76 × 10−3 | |||
| hsa-miR-3614-5p | 6.08 | 3.72 | 1.86 × 10−3 | |||
| hsa-miR-150-3p | 7.14 | 3.55 | 1.89 × 10−3 | |||
| hsa-miR-223-5p | 9.11 | 2.38 | 1.89 × 10−3 | |||
| hsa-miR-142-5p | 7.78 | 2.29 | 1.89 × 10−3 | |||
| hsa-miR-106b-3p | 10.38 | 2.21 | 1.89 × 10−3 | |||
| hsa-miR-28-3p | 12.42 | 1.87 | 1.89 × 10−3 | |||
| hsa-miR-455-5p | 7.10 | −2.28 | 1.89 × 10−3 | |||
| hsa-miR-451a | 6.86 | −3.84 | 1.89 × 10−3 | |||
| hsa-miR-143-3p | 10.99 | 2.66 | 2.04 × 10−3 | |||
| Zinc finger E-box binding homeobox 1 | hsa-miR-101-3p | hsa-miR-142-5p hsa-miR-150-5p hsa-miR-223-3p |
hsa-miR-1228-5p | 4.99 | −3.94 | 2.11 × 10−3 |
| hsa-miR-30e-3p | 11.55 | 1.96 | 2.25 × 10−3 | |||
| hsa-miR-486-5p | 9.93 | −2.96 | 2.30 × 10−3 | |||
| hsa-miR-1273h-3p | 5.22 | 2.87 | 2.56 × 10−3 | |||
| hsa-miR-150-5p | 9.66 | 3.59 | 3.01 × 10−3 | |||
| hsa-miR-378c | 7.67 | 2.10 | 3.61 × 10−3 | |||
| hsa-miR-92b-5p | 7.78 | −2.49 | 3.61 × 10−3 | |||
| hsa-miR-4448 | 4.42 | −2.84 | 3.61 × 10−3 | |||
| hsa-miR-103b | 8.57 | 2.10 | 4.76 × 10−3 | |||
| hsa-miR-941 | 11.41 | 2.04 | 4.76 × 10−3 | |||
| hsa-miR-103a-3p | 7.69 | 1.95 | 5.24 × 10−3 | |||
| hsa-miR-1246 | 10.40 | 2.09 | 5.64 × 10−3 | |||
| hsa-miR-125b-1-3p | 7.66 | −1.67 | 5.64 × 10−3 | |||
| hsa-miR-769-5p | 9.15 | 1.98 | 6.68 × 10−3 | |||
| hsa-miR-378a-3p | 13.05 | 1.86 | 6.68 × 10−3 | |||
| hsa-miR-140-3p | 12.73 | 1.72 | 6.68 × 10−3 | |||
| hsa-miR-214-5p | 4.34 | −2.29 | 6.68 × 10−3 | |||
| hsa-miR-574-3p | 8.84 | −1.59 | 7.83 × 10−3 | |||
| hsa-miR-1180-3p | 8.18 | −2.21 | 8.45 × 10−3 | |||
| hsa-miR-155-5p | 10.33 | 1.81 | 8.69 × 10−3 | |||
| hsa-miR-629-5p | 8.92 | 2.41 | 9.41 × 10−3 | |||
| hsa-miR-328-3p | 9.13 | −1.71 | 1.05 × 10−2 | |||
| hsa-miR-3690 | 5.09 | 3.22 | 1.18 × 10−2 | |||
| hsa-miR-7704 | 8.38 | −1.81 | 1.48 × 10−2 | |||
| hsa-miR-221-5p | 9.06 | 1.67 | 1.51 × 10−2 | |||
| hsa-miR-486-3p | 5.49 | −2.44 | 1.51 × 10−2 | |||
| hsa-miR-589-5p | 6.60 | 2.28 | 1.56 × 10−2 | |||
| hsa-miR-142-3p | 7.33 | 1.92 | 1.56 × 10−2 | |||
| hsa-miR-192-5p | 8.25 | 1.56 | 1.98 × 10−2 | |||
| hsa-miR-618 | 3.67 | 2.55 | 2.18 × 10−2 | |||
| hsa-miR-21-5p | 16.61 | 1.45 | 2.22 × 10−2 | |||
| hsa-miR-21-3p | 6.73 | 1.99 | 2.57 × 10−2 | |||
| hsa-miR-345-5p | 5.86 | 1.91 | 2.59 × 10−2 | |||
| hsa-miR-214-3p | 6.75 | −1.76 | 2.59 × 10−2 | |||
| hsa-miR-532-5p | 12.50 | 1.31 | 2.70 × 10−2 | |||
| hsa-miR-378e | 5.43 | 1.86 | 2.86 × 10−2 | |||
| hsa-miR-185-3p | 6.32 | 2.03 | 3.05 × 10−2 | |||
| hsa-miR-6787-3p | 3.55 | −2.74 | 3.07 × 10−2 | |||
| hsa-miR-503-5p | 4.23 | 2.26 | 3.11 × 10−2 | |||
| hsa-miR-25-3p | 13.02 | 1.31 | 3.11 × 10−2 | |||
| hsa-miR-3120-5p | 4.61 | −1.83 | 3.24 × 10−2 | |||
| hsa-miR-203b-5p | 3.84 | −2.12 | 3.35 × 10−2 | |||
| hsa-miR-500a-3p | 7.67 | 1.40 | 3.42 × 10−2 | |||
| hsa-miR-7-5p | 4.56 | 1.86 | 3.56 × 10−2 | |||
| hsa-miR-3622a-5p | 4.36 | −2.53 | 3.56 × 10−2 | |||
| hsa-miR-365a-5p | 5.30 | −2.29 | 3.76 × 10−2 | |||
| hsa-miR-23b-3p | 9.80 | −1.50 | 3.85 × 10 |
| TP53 | Tumor protein p53 | hsa-miR-125b-1-3p hsa-miR-214-3p hsa-miR-214-5p |
| −2 | ||
| hsa-miR-424-3p | ||
| 6.52 | ||
| 2.04 | ||
| 4.29 × 10 | ||
| −2 | ||
| hsa-miR-501-3p | ||
| 8.94 | ||
| 1.29 | ||
| 4.30 × 10 | ||
| −2 | ||
| hsa-miR-92b-3p | ||
| 12.61 | ||
| −1.38 | ||
| 4.37 × 10 | ||
| −2 | ||
| hsa-miR-146b-5p | ||
| 13.69 | ||
| 1.42 | ||
| 4.42 × 10 |
References
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Foers, A.D.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Review: Extracellular Vesicles in Joint Inflammation. Arthritis Rheumatol. 2017, 69, 1350–1362.
- Malda, J.; Boere, J.; Van De Lest, C.H.A.; Van Weeren, P.R.; Wauben, M.H.M. Extracellular vesicles—New tool for joint repair and regeneration. Nat. Rev. Rheumatol. 2016, 12, 243–249.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Murata, K.; Yoshitomi, H.; Tanida, S.; Ishikawa, M.; Nishitani, K.; Ito, H.; Nakamura, T. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2010, 12, 1–14.
- Li, J.; Wan, Y.; Guo, Q.; Zou, L.; Zhang, J.; Fang, Y.; Zhang, J.; Zhang, J.; Fu, X.; Liu, H.; et al. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R81.
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014, 41, 89–103.
- Bosch, S.; Young, N.A.; Mignot, G.; Bach, J.-M. Epigenetic Mechanisms in Immune Disease: The Significance of Toll-Like Receptor-Binding Extracellular Vesicle-Encapsulated microRNA. Front. Genet. 2020, 11, 578335.
- Bernard, M.A.; Zhao, H.; Yue, S.C.; Anandaiah, A.; Koziel, H.; Tachado, S.D. Novel HIV-1 MiRNAs Stimulate TNFα Release in Human Macrophages via TLR8 Signaling Pathway. PLoS ONE 2014, 9, e106006.
- Foers, A.D.; Chatfield, S.; Dagley, L.F.; Scicluna, B.J.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J. Extracell. Vesicles 2018, 7, 1490145.
- Foers, A.D.; Dagley, L.F.; Chatfield, S.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Proteomic analysis of extracellular vesicles reveals an immunogenic cargo in rheumatoid arthritis synovial fluid. Clin. Transl. Immunol. 2020, 9, e1185.
 Encyclopedia
Encyclopedia
