Viral respiratory infections are recognized risk factors for the loss of control of allergic asthma and the induction of exacerbations. Severe asthma is more susceptible to virus-induced asthma exacerbations, especially in the presence of high IgE levels.
Viral respiratory infections are recognized risk factors for the loss of control of allergic asthma and the induction of exacerbations. Severe asthma is more susceptible to virus-induced asthma exacerbations, especially in the presence of high IgE levels.
- viral respiratory infections
- severe asthma
- immune response
- biologicals
- omalizumab
1. Introduction
Viral respiratory infections are recognized risk factors for the loss of control of allergic asthma and the induction of exacerbations, both in adults and children [1,2,3]. Almost 80% of asthma exacerbations were associated with viral infections [1,2,3]. Severe asthma is more susceptible to virus-induced asthma exacerbations, especially in the presence of high IgE levels [4,5]. Respiratory viruses mainly involved in asthma exacerbations are human rhinoviruses (HRV), respiratory syncytial viruses (RSV), influenza viruses, parainfluenza virus, metapneumovirus, bocavirus, adenovirus, coronavirus [1,3,6]. HRV are the most common causative agents found in the 5 days preceding the development of exacerbations [3].
Viral respiratory infections are recognized risk factors for the loss of control of allergic asthma and the induction of exacerbations, both in adults and children [1][2][3]. Almost 80% of asthma exacerbations were associated with viral infections [1][2][3]. Severe asthma is more susceptible to virus-induced asthma exacerbations, especially in the presence of high IgE levels [4][5]. Respiratory viruses mainly involved in asthma exacerbations are human rhinoviruses (HRV), respiratory syncytial viruses (RSV), influenza viruses, parainfluenza virus, metapneumovirus, bocavirus, adenovirus, coronavirus [1][3][6]. HRV are the most common causative agents found in the 5 days preceding the development of exacerbations [3].
Asthmatic exacerbations consist of episodes characterized by an increase in respiratory symptoms (dyspnoea, cough, wheezing, chest tightness) and worsening of lung function requiring changes of therapy, the intake of oral corticosteroids (OCS), emergency treatment, and hospitalization in the most severe cases [2,3]. In terms of pulmonary function tests, asthma exacerbation is defined as a >20% reduction in FEV
Asthmatic exacerbations consist of episodes characterized by an increase in respiratory symptoms (dyspnoea, cough, wheezing, chest tightness) and worsening of lung function requiring changes of therapy, the intake of oral corticosteroids (OCS), emergency treatment, and hospitalization in the most severe cases [2][3]. In terms of pulmonary function tests, asthma exacerbation is defined as a >20% reduction in FEV
1 (Forced Expiratory Volume in 1 s) from baseline or a >30% reduction in peak expiratory flow (PEF) for 2 consecutive days. Asthmatic exacerbations can accelerate the decline in lung function and increase the risk of subsequent exacerbations [2,3,8].
(Forced Expiratory Volume in 1 s) from baseline or a >30% reduction in peak expiratory flow (PEF) for 2 consecutive days. Asthmatic exacerbations can accelerate the decline in lung function and increase the risk of subsequent exacerbations [2][3][7].
Viral infections can cause asthma exacerbations through multiple mechanisms [3,5,6]: increased serum IgE levels, promotion of respiratory reactivity (including through epithelial damage), increased eosinophilic inflammation of the respiratory tract, increased neutrophilic inflammation of the lower airways, direct infection of the lower respiratory tract.
Viral infections can cause asthma exacerbations through multiple mechanisms [3][5][6]: increased serum IgE levels, promotion of respiratory reactivity (including through epithelial damage), increased eosinophilic inflammation of the respiratory tract, increased neutrophilic inflammation of the lower airways, direct infection of the lower respiratory tract.
32. Immune Response to Respiratory Viruses and SARS-CoV-2
During immune responses to viruses, an initial activation of innate immunity typically occurs and the production of type I and III interferons (IFN-α/β and -λ, respectively) is essential in the control of viral spread. Plasmacytoid dendritic cells (pDCs) are the primary blood source of IFN-α [11]. It is known that a reduction in the production of IFN by pDCs and epithelial cells is frequent in atopic patients, with a consequent delayed and inefficient antiviral defence [12]. Another important aspect is that the intensity of the response to IFN-α after the ex vivo viral challenge of pDCs is inversely related to serum IgE levels. This evidence suggests that pDCs antiviral responses may be suppressed in atopic patients. In fact, asthmatics show great susceptibility to respiratory viral infections, which are often a trigger for exacerbations [13].
During immune responses to viruses, an initial activation of innate immunity typically occurs and the production of type I and III interferons (IFN-α/β and -λ, respectively) is essential in the control of viral spread. Plasmacytoid dendritic cells (pDCs) are the primary blood source of IFN-α [8]. It is known that a reduction in the production of IFN by pDCs and epithelial cells is frequent in atopic patients, with a consequent delayed and inefficient antiviral defence [9]. Another important aspect is that the intensity of the response to IFN-α after the ex vivo viral challenge of pDCs is inversely related to serum IgE levels. This evidence suggests that pDCs antiviral responses may be suppressed in atopic patients. In fact, asthmatics show great susceptibility to respiratory viral infections, which are often a trigger for exacerbations [10].
However, the inflammatory Th2 environment appears to be able to favor viral infections, through the altered release of IFN-α in the peripheral blood mononuclear cells (PBMCs) of asthmatic patients, the reduction of NF-kB and the lack of activation of Toll-Like Receptor (TLR)7 and TLR8. In contrast, Th2 inflammation appears protective against SARS-CoV-2 infection [15].
However, the inflammatory Th2 environment appears to be able to favor viral infections, through the altered release of IFN-α in the peripheral blood mononuclear cells (PBMCs) of asthmatic patients, the reduction of NF-kB and the lack of activation of Toll-Like Receptor (TLR)7 and TLR8. In contrast, Th2 inflammation appears protective against SARS-CoV-2 infection [11].
Furthermore, the real role of eosinophils in the context of infections has not been precisely established yet. Eosinophils are reduced in peripheral blood from SARS-CoV-2 infected patients [19]. Eosinophilic cells blood count seems to have a major role in COVID-19 diagnosis and prognosis. Eosinopenia has been associated in up to 81% of cases and was proposed as a possible diagnostic marker for the disease. Persistent eosinopenia was associated with higher mortality [20]. Numerous and recent studies showed that eosinopenia was present in patients with severe COVID-19 phenotypes and in many cases with fatal evolution [21,22,23,24].
Furthermore, the real role of eosinophils in the context of infections has not been precisely established yet. Eosinophils are reduced in peripheral blood from SARS-CoV-2 infected patients [12]. Eosinophilic cells blood count seems to have a major role in COVID-19 diagnosis and prognosis. Eosinopenia has been associated in up to 81% of cases and was proposed as a possible diagnostic marker for the disease. Persistent eosinopenia was associated with higher mortality [13]. Numerous and recent studies showed that eosinopenia was present in patients with severe COVID-19 phenotypes and in many cases with fatal evolution [14][15][16][17].
The pathophysiology of eosinopenia in COVID-19 has not been fully clarified yet, but it is probably multifactorial. Possible explanations concern the inhibition of eosinophils egress from the bone marrow, the blockage of eosinophilopoiesis, the reduced expression of chemokine receptors and adhesion factors and finally the direct eosinophilic apoptosis induced by type 1 IFN released during acute infection [25,26].
The pathophysiology of eosinopenia in COVID-19 has not been fully clarified yet, but it is probably multifactorial. Possible explanations concern the inhibition of eosinophils egress from the bone marrow, the blockage of eosinophilopoiesis, the reduced expression of chemokine receptors and adhesion factors and finally the direct eosinophilic apoptosis induced by type 1 IFN released during acute infection [18][19].
43. Severe Asthma and COVID-19: Epidemiological Data
As for now, literature data, although limited and preliminary, show that severe asthma patients treated with biologics don’t have an increased risk of SARS-CoV-2 infection or progression to severe forms compared to the non-asthmatic population [27]. These data, in agreement with other international assessments, indicate that treatments with biologics during the COVID-19 pandemic appear to be safe and can be continued in patients with severe allergic asthma, interrupting it only in case of confirmed SARS-CoV-2 positivity [28] and up to negativity of the nasopharyngeal swab [27,28,29].
As for now, literature data, although limited and preliminary, show that severe asthma patients treated with biologics don’t have an increased risk of SARS-CoV-2 infection or progression to severe forms compared to the non-asthmatic population [20]. These data, in agreement with other international assessments, indicate that treatments with biologics during the COVID-19 pandemic appear to be safe and can be continued in patients with severe allergic asthma, interrupting it only in case of confirmed SARS-CoV-2 positivity [21] and up to negativity of the nasopharyngeal swab [20][21][22].
4.1. Impact of Monoclonal Antibodies for Severe Asthma on Viral Infections
3.1. Impact of Monoclonal Antibodies for Severe Asthma on Viral Infections
A study showed that mepolizumab had no clinical effects in terms of immune response to this infection [34]. A patient with severe eosinophilic asthma, who had been receiving benralizumab for two years, had a confirmed SARS-CoV-2 infection. The course of COVID-19 was very mild, with a rapid and complete resolution of the clinical picture [35].
A study showed that mepolizumab had no clinical effects in terms of immune response to this infection [23]. A patient with severe eosinophilic asthma, who had been receiving benralizumab for two years, had a confirmed SARS-CoV-2 infection. The course of COVID-19 was very mild, with a rapid and complete resolution of the clinical picture [24].
4.2. Effect of Omalizumab in Patients with Severe Allergic Asthma and Respiratory Viral Infections
3.2. Effect of Omalizumab in Patients with Severe Allergic Asthma and Respiratory Viral Infections
Omalizumab was the first biologic drug authorized for clinical use for the treatment of severe allergic asthma refractory to maximally dosed standard therapies. In addition to the effects described above, recent studies showed that omalizumab was able to improve the antiviral response in subjects with allergic asthma, otherwise inadequate; these patients are more susceptible to virus-induced asthma exacerbations, especially those with elevated IgE levels [28]. By sequestering IgE, omalizumab also positively influences aspects of the innate immune response to viral respiratory infections, which is deficient in patients with severe allergic asthma [49,50,51]. People with severe allergic asthma are more susceptible to respiratory viruses (85% of children and 50% of adults) and more likely to experience virus-induced asthma exacerbations, which are more severe in the presence of elevated IgE levels [48,49,50,51,52,53] (
Omalizumab was the first biologic drug authorized for clinical use for the treatment of severe allergic asthma refractory to maximally dosed standard therapies. In addition to the effects described above, recent studies showed that omalizumab was able to improve the antiviral response in subjects with allergic asthma, otherwise inadequate; these patients are more susceptible to virus-induced asthma exacerbations, especially those with elevated IgE levels [21]. By sequestering IgE, omalizumab also positively influences aspects of the innate immune response to viral respiratory infections, which is deficient in patients with severe allergic asthma [25][26][27]. People with severe allergic asthma are more susceptible to respiratory viruses (85% of children and 50% of adults) and more likely to experience virus-induced asthma exacerbations, which are more severe in the presence of elevated IgE levels [28][25][26][27][29][30] (
).
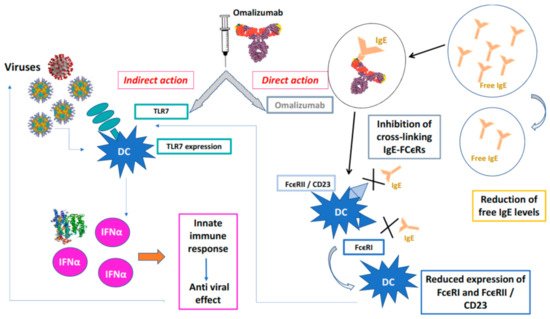
Figure 1.
Protective action of omalizumab towards viral infections.Omalizumab acts directly forming IgE / anti-IgE immune complexes that prevent the interaction of IgE with high affinity (FcϵRI) and low affinity (FcϵRII / CD23) membrane receptors of PD cells, indirectly, PD cells binds viruses by TLCR7 that release INFα and activate innate immune response. X is the blockade of binding on the receptor.
4.3. Omalizumab and Reduction of Virus-Induced Seasonal Exacerbations: Trial Data
3.3. Omalizumab and Reduction of Virus-Induced Seasonal Exacerbations: Trial Data
Patients with severe asthma and viral infections (not only in paediatric age) appear more susceptible to exacerbations, especially during fall, leading to significant consequences: greater morbidity, higher healthcare costs and disease progression [14]. The efficacy of omalizumab in improving antiviral response and consequently reducing potentially virus-induced seasonal exacerbations has been demonstrated in several clinical trials conducted in both adult/adolescent patients and paediatric populations with moderate-to-severe allergic asthma.
Patients with severe asthma and viral infections (not only in paediatric age) appear more susceptible to exacerbations, especially during fall, leading to significant consequences: greater morbidity, higher healthcare costs and disease progression [31]. The efficacy of omalizumab in improving antiviral response and consequently reducing potentially virus-induced seasonal exacerbations has been demonstrated in several clinical trials conducted in both adult/adolescent patients and paediatric populations with moderate-to-severe allergic asthma.
54. Summary
All mAbs currently used for the treatment of severe asthma are considered to be safe even in this historical moment with an important risk of viral infections, in particular from the SARS-CoV-2. Despite the fears that emerged during the initial stages of the pandemic, asthma is not a disease that carries a risk of developing severe forms of COVID-19. Biologics for severe asthma are all effective, they are carefully chosen and are safe for viral infections. An important and still not fully understood problem is the management of patients with severe asthma during the COVID-19 pandemic.
All mAbs currently used for the treatment of severe asthma are considered to be safe even in this historical moment with an important risk of viral infections, in particular from the SARS-CoV-2. Despite the fears that emerged during the initial stages of the pandemic, asthma is not a disease that carries a risk of developing severe forms of COVID-19. Biologics for severe asthma are all effective, they are carefully chosen and are safe for viral infections. An important and still not fully understood problem is the management of patients with severe asthma during the COVID-19 pandemic.
References
- Busse, W.W.; Lemanske, R.F., Jr.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. Available online: (accessed on 1 March 2021).
- Adeli, M.; El-Shareif, T.; Hendaus, M.A. Asthma exacerbation related to viral infections: An up to date summary. J. Fam. Med. Prim. Care 2019, 8, 2753–2759.
- Cardet, J.C.; Casale, T.B. New insights into the utility of omalizumab. J. Allergy Clin. Immunol. 2019, 143, 923–926.
- Gill, M.A.; Liu, A.H.; Calatroni, A.; Krouse, R.Z.; Shao, B.; Schiltz, A.; Gern, J.E.; Togias, A.; Busse, W.W. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J. Allergy Clin. Immunol. 2018, 14, 1735–1743.
- Novak, N.; Cabanillas, B. Viruses and asthma: The role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology 2020, 161, 83–93.
- Bai, T.R.; Vonk, J.M.; Postma, D.S.; Boezen, H.M. Severe exacerbations predict excess lung function decline in asthma. Eur. Respir. J. 2007, 30, 452–456.
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I interferon and inflammatory monocyte-macrophage responses cause lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193.
- Gonzales-van Horn, S.R.; Farrar, J.D. Interferon at the crossroads of allergy and viral infections. J. Leukoc. Biol. 2015, 98, 185–194.
- Gern, J.E. How rhinovirus infections cause exacerbations of asthma. Clin. Exp. Allergy 2015, 45, 32–42.
- Cameron, M.J.; Ran, L.; Xu, L.; Danesh, A.; Bermejo-Martin, J.F.; Cameron, C.M.; Muller, M.P.; Gold, W.L.; Richardson, S.E.; Poutanen, S.M.; et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007, 81, 8692–8706.
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741.
- Dastoli, S.; Bennardo, L.; Patruno, C.; Nisticò, S.P. Are erythema multiforme and urticaria related to a better outcome of COVID-19? Dermatol. Ther. 2020, 33, e13681.
- Flores-Torres, A.S.; Salinas-Carmona, M.C.; Salinas, E.; Rosas-Taraco, A.G. Eosinophils and respiratory viruses. Viral. Immunol. 2019, 32, 198–207.
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146.
- Renner, A.; Marth, K.; Patocka, K.; Idzko, M.; Pohl, W. COVID-19 in two severe asthmatics receiving benralizumab: Busting the eosinophilia myth. ERJ Open Res. 2020, 6, 00457–2020.
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379.
- Hassani, M.; Leijte, G.; Bruse, N.; Kox, M.; Pickkers, P.; Vrisekoop, N.; Koenderman, L. Differentiation and activation of eosinophils in the human bone marrow during experimental human endotoxemia. J. Leukoc. Biol. 2020, 108, 1665–1671.
- Butterfield, J.H. Treatment of hypereosinophilic syndromes with prednisone, hydroxyurea, and interferon. Immunol. Allergy Clin. N. Am. 2007, 27, 493–518.
- Matucci, A.; Caminati, M.; Vivarelli, E.; Vianello, A.; Micheletto, C.; Menzella, F.; Crisafulli, E.; Passalacqua, G.; Bagnasco, D.; Lombardi, C.; et al. COVID-19 in severe asthmatic patients during ongoing treatment with biologicals targeting type 2 inflammation: Results from a multicenter Italian survey. Allergy 2020.
- Licari, A.; Castagnoli, R.; Votto, M.; Brambilla, I.; Ciprandi, G.; Marseglia, G.L. Biologic Use in Allergic and Asthmatic Children and Adolescents During the COVID-19 Pandemic. Pediatric Allergy Immunol. Pulmonol. 2020, 33, 155–158.
- Criado, P.R.; Pagliari, C.; Criado, R.F.J.; Marques, G.F.; Belda, W., Jr. What the physicians should know about mast cells, dendritic cells, urticaria, and omalizumab during COVID-19 or asymptomatic infections due to SARS-CoV-2? Dermatol. Ther. 2020, 33, e14068.
- Vultaggio, A.; Agache, I.; Akdis, C.A.; Akdis, M.; Bavbek, S.; Bossios, A.; Bousquet, J.; Boyman, O.; Chaker, A.M.; Chan, S.; et al. Considerations on biologicals for patients with allergic disease in times of the COVID-19 pandemic: An EAACI statement. Allergy 2020, 75, 2764–2774.
- Piñeros, Y.S.S.; Bal, S.M.; van de Pol, M.A.; Dierdorp, B.S.; Dekker, T.; Dijkhuis, A.; Brinkman, P.; van der Sluijs, K.F.; Zwinderman, A.H.; Majoor, C.J.; et al. Anti-IL-5 in Mild Asthma Alters Rhinovirus-induced Macrophage, B-Cell, and Neutrophil Responses (MATERIAL). A Placebo-controlled, Double-Blind Study. Am. J. Respir. Crit. Care Med. 2019, 199, 508–517.
- Menzella, F.; Galeone, C.; Bertolini, F.; Castagnetti, C.; Facciolongo, N. Innovative treatments for severe refractory asthma: How to choose the right option for the right patient? J. Asthma Allergy 2017, 10, 237–247.
- Casale, T.B.; Stokes, J. Anti-IgE therapy: Clinical utility beyond asthma. J. Allergy Clin. Immunol. 2009.
- Kantor, D.B.; Stenquist, N.; McDonald, M.C.; Schultz, B.J.; Hauptman, M.; Smallwood, C.D.; Nelson, K.A.; Perzanowski, M.S.; Matsui, E.C.; Phipatanakul, W.; et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J. Allergy Clin. Immunol. 2016, 138, 1467–1471.
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2020, 55, 1900588.37.
- López-Abente, J.; Benito-Villalvilla, C.; Jaumont, X.; Pfister, P.; Tassinari, P.; Palomares, O. Omalizumab restores the ability of human plasmacytoid dendritic cells to induce Foxp3+Tregs. Eur. Respir. J. 2021, 57, 2000751.
- Yamaya, M. Virus infection-induced bronchial asthma exacerbation. Pulm. Med. 2012, 2012, 834826.
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J., Jr.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485.
 Encyclopedia
Encyclopedia
