The critical role of the immune system in host defense against foreign bodies and pathogens has been long recognized. With the introduction of a new field of research called osteoimmunology, the crosstalk between the immune and bone-forming cells has been studied more thoroughly, leading to the conclusion that the two systems are intimately connected through various cytokines, signaling molecules, transcription factors and receptors.
- biomaterials
- bone regeneration
- osteoimmunomodulation
- immune response
1. Introduction
Annually, millions of people suffer from common bone defects caused by trauma, infection, tumor resection and pathological processes [1][2][1,2]. Even though healthy bone tissue has an extraordinary capacity for self-repair, around 10% of patients develop severe complications, such as delayed healing and non-union, leading to more expensive and often invasive treatment strategies [3]. A common method of treatment involves the use of bone grafts for the rapid restoration of larger defects/injuries, but a series of disadvantages, such as an insubstantial amount of satisfactory graft material available for use and morbidity at the donor site (e.g., nerve injury, pain, hemorrhage, infection), limits its use as a therapeutic tool for bone regeneration [4][5][6][7][4,5,6,7]. Because of the limitations associated with the conventional treatment, in the last few decades, various implantable biomaterials have been developed and tested as promising bone substitute alternatives. Their wide usability relies on their ability to act as biocompatible supports and delivery platforms for biologically active molecules, which can be easily tailored for a specific purpose (e.g., modification of the chemical and physical properties) [8][9][10][11][8,9,10,11]. However, regardless of their inert and non-toxic character, adverse immune reactions such as excessive inflammation, impairment of healing, fibrotic encapsulation and implant rejection can occur due to their exogenous nature [11][12][11,12]. The traditional method of designing implantable biomaterials focuses on developing bone substitutes that can elicit a favorable osteogenic and osseointegration process, by tailoring their mechanical and physicochemical characteristics [2][13][14][2,13,14]. Nevertheless, this concept does not always lead to satisfying results, with studies reporting certain inconsistencies between the in vitro and in vivo results, thus leading to the hypothesis according to which the mechanism underlying the capacity of the materials to mediate bone regeneration is known only partially and it is far more complex than it was thought [13]. For a long time, it was believed that the bone dynamics involve only cells from the skeletal system, such as osteoblasts and osteoclasts, but a more sophisticated understanding of the bone biology suggests that the osteogenic process is a result of the interplay between the skeletal and immune systems. Essential events in the bone remodeling process, such as hematopoiesis, structural support and mineralization, require intimate cooperation between the skeletal and immune systems, through numerous common regulatory molecules, such as cytokines, transcription factors, receptors and other signaling molecules [15][16][17][18][15,16,17,18]. For example, cytokines such as the transforming growth factor (TGF-β) and interleukin (IL)-4 have been reported to induce osteoblast migration, proliferation and secretion of the extracellular matrix (ECM) in the early stage of cell differentiation [19][20][19,20], while tumor necrosis factor-alpha (TNF-α) and IL-1β have the opposite effect, being involved in the inhibition of the differentiation process [21]. Moreover, the immune system has been recognized as being involved in the first stage of the natural healing process and studies have shown that treatment with anti-inflammatory drugs leads to impaired fracture healing [22][23][24][25][22,23,24,25]. This crosstalk between the immune and skeletal systems was first identified in a series of pioneering studies on osteoclast-activated factors derived from immune cells in the 1970s [26]. However, almost 30 years later, the term “osteoimmunology” was first coined and used to describe the introduction of a new direction in research and of a new interdisciplinary field [26][27][26,27]. With this advancement in bone biology, it was detrimental that a shift in the design paradigm should take place, from biomaterials capable of direct activation of cells responsible for the osteogenic process, towards biomaterials capable of modulating the local immune environment in favor of bone healing and regeneration [13][28][29][13,28,29]. Biomaterials endowed with immunomodulatory properties are classified as osteoimmunomodulatory biomaterials. Materials presenting favorable osteoimmunomodulatory properties are capable of inducing a suitable inflammatory response that results in the formation of new bone tissue by issuing factors from inflammatory cells capable of increasing osteogenic cell recruitment and differentiation. On the other hand, biomaterials with poor osteoimmunomodulatory properties will lead to an excessive chronic inflammatory process coupled with increased osteoclast formation, resulting in bone destruction, fibrous capsule formation and, in the end, implant failure [13][29][13,29]. Therefore, osteoimmunomodulation brings forth a promising strategy for designing bone biomaterials with multifactorial effects, such as tuning the immune system, promoting osteogenesis and regulating osteoclastogenesis [30][31][32][30,31,32].
2. Overview of the Immune System
The immune system represents a powerful and diverse defensive tool, involved in host protection against foreign threats and body homeostasis control. The main function of the immune system is to resolve infections, repair the injured tissue and return the organism to a state of homeostasis. Its efficacy relies on the ability to exhibit a specific yet limiting, rapid and destructive response, appropriate for the inflammatory trigger [33]. At the most basic level, the human immune system can be classified into two interconnected branches, the innate immune system and the adaptive immune system, both involved in defending the organism against numerous threats, such as injuries, microbes, bacteria and toxins or any other causes [34][35][34,35]. The innate immune system is intimately connected to the inflammatory pathways and wound healing system, being capable of eliciting a non-specific immune response on contact with a foreign material or injured tissue, without previous programming [11][36][11,36]. Due to its non-specific nature, the innate immune system represents the first line of defense, being activated when certain molecules known as non-infectious damage-associated molecular patterns (DAMPs) and infectious pathogen-associated molecular patterns (PAMPs) [37] bind to specific molecular structures called surface-expressed pattern recognition receptors (PRRs) presented by the immune cells (e.g., macrophages, dendritic cells) resident in the normal healthy tissue [33][38][33,38]. The PRRs can function as soluble proteins involved in the opsonization process, as phagocytic transmembrane receptors (e.g., the mannose receptor and dectin-1) and can be involved in complement activation [33]. To date, many classes of PRRs, including toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLR) and retinoic acid inducible gene-I (RIG-I)-like receptors (RLR), have been studied and characterized [38]. These receptors elicit an immune response through the activation of the transcription nuclear factor kappa B (NF-kB), which prompts the upregulation of proinflammatory cytokine genes such as IL-1 and TNF-α, responsible for the recruitment and activation of different subsets of leukocytes (e.g., neutrophils, macrophages) at the site of injury or infection [38][39][38,39]. This represents the starting point of a cascade of events responsible for changing the local environment of the surrounding tissue and vasculature [40]. The first cells to act are neutrophils, which engulf pathogens and attract other immune cells such as macrophages and other neutrophils, through the secretion of various inflammatory molecules and growth factors [41][42][43][41,42,43]. The infiltrating macrophages, together with the tissue-resident macrophages, adopt a proinflammatory phenotype, clearing the foreign particles/debris through phagocytosis and secreting pro-inflammatory cytokines such as IL-6, TNF-α and interferon-γ (IFN-γ), vital for the early phase of normal tissue healing and the activation of the adaptive immune system [44]. In contrast to the innate immunity, the adaptive immune system contains cells (B and T cells) capable of recognizing specific antigens, after multiple contacts, developing the so-called “immunological memory” [45]. A special subset of immune cells, which act as messengers between the innate and the adaptive immune systems, is represented by the dendritic cells. This cell population is capable of processing antigens into short peptides and presenting them on their cell surface (antigen presentation), thus exposing the antigens to the T cells for phagocytosis [46]. Another important subpopulation of cells is represented by the mastocytes, tissue-resident sentinels, capable of releasing various mediators (e.g., cytokines, chemokines) involved in leukocyte recruitment and venular permeability enhancement. Moreover, data found in the literature showed that by suppressing the activation and function of the mastocytes, an indirect interference in the mast-cell-induced recruitment of other immune cells could be observed [47].
Figure 1 offers a basic overview of the innate and adaptive immune system components that contribute to the immune reaction towards a pathogen agent/foreign body.
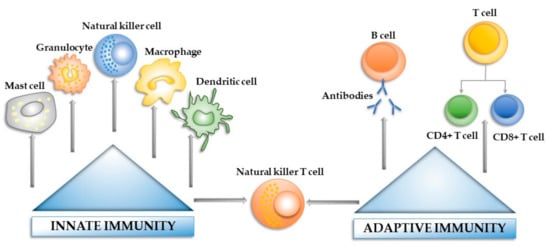
Figure 1. The main components of the innate and adaptive immune systems.
