Rainwater harvesting (RWH) can be used to mitigate global water crises; however, they have been poorly received by communities because of the unsuitable quality of stored rainwater. Heterotrophic bacteria present in the water can degrade the water’s microbiological quality and create health issues. Moreover, exposure to visible light can affect both suspended and surface-attached heterotrophic bacteria, a phenomenon that is poorly investigated.
- visible light
- rainwater harvesting
- heterotrophs
- biofilm
1. Introduction
Water is critical for the survival of human beings. In developing nations, a constant population growth corresponds to increased pressure on water resources and water consumption [1][2]. Thus, millions of people live in areas with extreme water vulnerability and do not have access to clean water [2]. Furthermore, it has been predicted that by 2050, more than half of the global population will live in areas that suffer from water scarcity at least one month each year [3]. Serious actions to tackle these underlying threats should then be implemented through the improvement of water management, increased efforts of water conservation, and adoption of nature-based solutions [3], one of which is via RWH [4].
RWH technology consists of collecting rainwater from a roof or other catchment surface and directing it into a storage area [5]. This storage area ranges from a simple rainwater barrel to a more complex multiple tank system [6]. Despite the inclusion of simple methods, RWH is not widely used in developing countries due to the excess cost of the storage tanks, the uncertainty of water quality, and poor installation or maintenance [5][7]. In households that use RWH, cheap recycled or scavenged items like plastic barrels, jerrycans, or plastic drums, that allow for the penetration of visible light, are often used in lieu of more ideal opaque tanks [4][8] to help address water shortage for both potable and non-potable purposes [5].
A common issue with RWH is the quality of rainwater harvested. Because of the presence of dry and wet particles that settle on the roof catchment, such as dust and bird droppings, many researchers have observed poor quality of harvested water [9][10] which may contain pathogens that are harmful to humans health [11]. Few studies, which utilized opaque and large storage tanks, have investigated the factors affecting microbial growth in RWH [12][13][14]. However, to the knowledge of the authors, no research has been done on the quality of rainwater using storage items that households in developing countries commonly use, which are cheaper, more accessible, and allow for the penetration of visible light [8]. Thus, the purpose of this paper is to fill the current knowledge gap on the microbial water quality of rainwater kept in storage of small capacity allowing light penetration. The quality of harvested rainwater is influenced by several physicochemical, spatial, temporal, and microbiological factors [15][16]. Baseline low concentration of total dissolved solids and organic carbon in rainwater has the potential to inhibit the growth of SB [17]. On the other hand, high water temperature inside storage tanks can stimulate bacterial proliferation [16]. The number of bacteria in rainwater also differs depending on the storage water depth and over time for both suspended as well as SAB [18][19][20]. In addition, while SAB in tap water has been found to carry waterborne pathogens in drinking water distribution systems [21], several studies have observed a positive effect of SAB particularly on harvested rainwater quality [15][17]. The advantages, however, were found only in investigations that involved stored rainwater not exposed to visible light.
A study by Kim and Han (2011) observed that after inoculating Pseudomonas aeruginosa in rainwater storage with SAB, 99% of the Pseudomonas was removed after five days in full-scale tanks [17]. In addition, Coombes et al. (2006) stated that in underground rainwater tanks, SAB removed toxic metals and compounds from the water column by playing the role of a bioreactor [12]. Suspended heterotrophic bacteria can also digest dissolved organic compounds, attach to the SAB, and die naturally due to starvation [12][22] Thus, in an RWH tank that is not exposed to visible light, SAB improves microbiological water quality [19][22]. Although these studies highlight the potential benefits of SAB, studies on their growth and their effect on heterotrophic bacteria in stored rainwater exposed to visible light are lacking. Nevertheless, the influence of visible light on water quality has been investigated in other sources of water.
Research on the effects of visible light on SB present in the aquatic ecosystem yielded conflicting results. Visible light was found to hinder microbial growth by producing reactive oxygen species, which cause oxidative damage in bacterial cells [23][24]. However, a literature review done by Ruiz-Gonzales et al. (2013) discussed the effects of sunlight on heterotrophic bacterial activity, suggesting that visible light induces photosynthesis, which affects the number of organic compounds passing through the microbial food chains. The photochemical transformation of dissolved compounds in the water in turn increases the amount of food that is available for the existing heterotrophic bacteria, leading to its multiplication [25]. Furthermore, research done by Schmidt et al. (2018) showed that SAB in water exposed to visible light, despite its stable counts over time, were significantly higher in number compared to the non-exposed condition at the end of the study period [26]. These findings, however, are yet to be seen in studies on harvested rainwater.
2. Effect of Visible Light on Surface-Attached and Suspended Heterotrophic Bacteria in a Typical Household Rainwater Harvesting Tank
2.1. Effect of Visible Light on SAB
2.1.1. Effect of Visible Light on SAB at Different Water Depths
All the coupons that were analyzed in this study contained heterotrophic bacteria on their surfaces. The surface-attached heterotrophic bacterial counts are shown in Figure 2Figure 1.
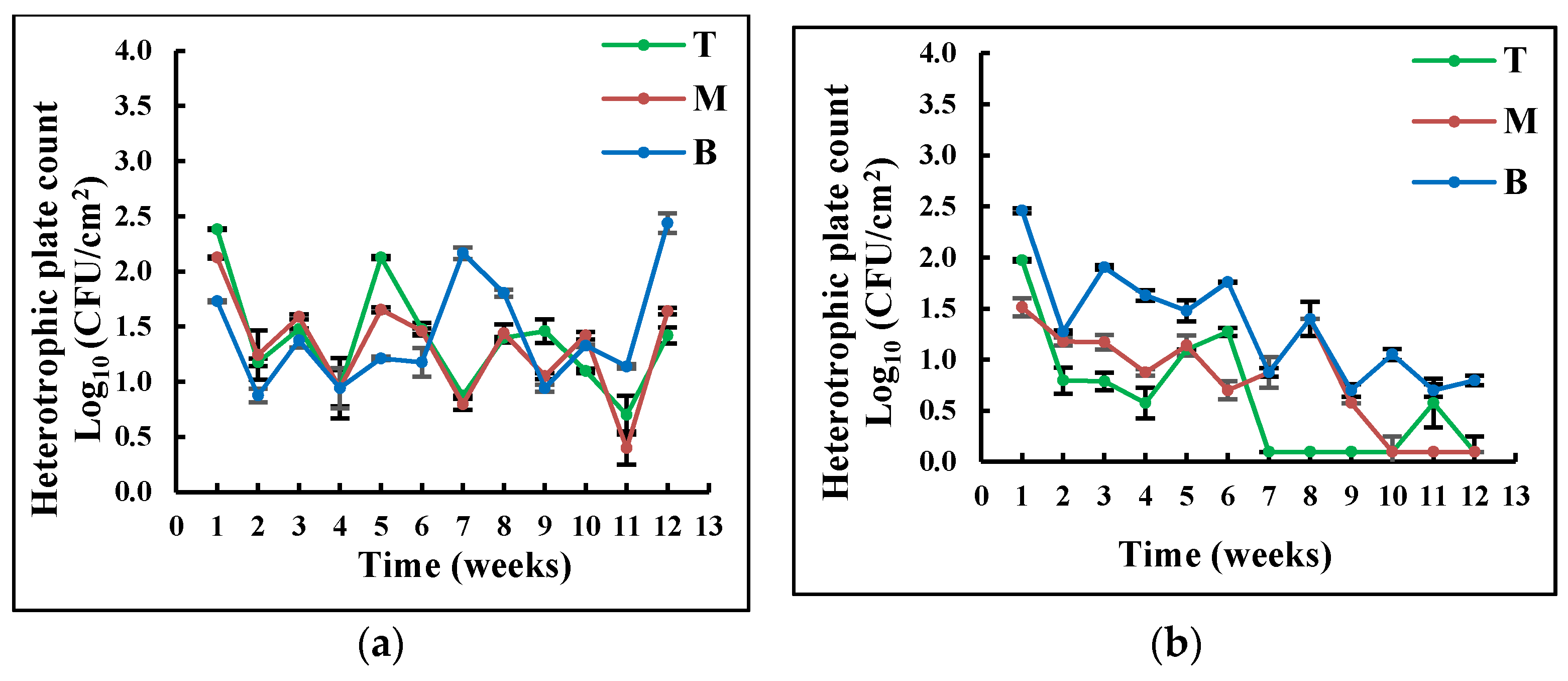
Exposure to visible light had a significant effect on the amount of surface-attached bacteria in the tank (p ≤ 0.02). While the number of heterotrophic bacteria was relatively stable in the TES, it decreased in the TNES. The stability of heterotrophic bacteria is probably caused by the higher availability of food produced by phototrophs during photosynthesis [26]. This hypothesis is corroborated by a significant difference in the concentration of total organic carbon in the two tanks, illustrated in Figure 3Figure 2, (Student t-test p < 0.05). According to Schmidt et al. (2018), phototrophic bacteria in biofilms contribute to their stabilization and cultivation; additionally, low light intensity results in a significant reduction in biofilm development [26].
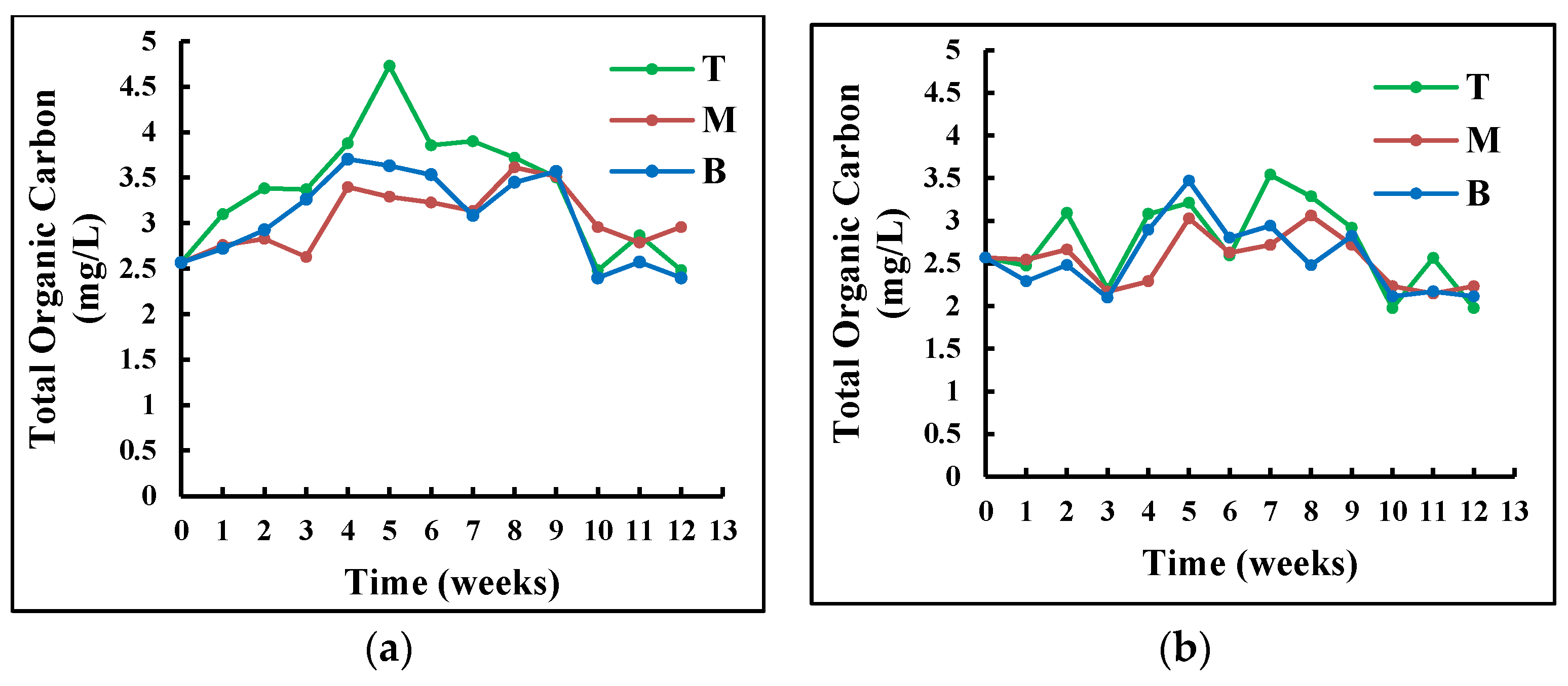
Figure 32. Variation of concentration of total organic carbon (a) in the tank exposed to sun and (b) in the tank not exposed to sun at different water depths (T: top, M: middle, and B bottom).
2.1.2. Effect of Visible Light on Total Biomass of SAB at Different Water Depths
Figure 4Figure 3 shows that the amount of total biomass that was exposed to visible light was significantly larger than that of the non-exposed tank. Visible light induces the production of organic compounds necessary for the metabolism of heterotrophic bacteria as seen in Figure 3Figure 2. Augusti et al. (2020) and Music et al. (2019) have emphasized that light is a primary energy source for autotrophic organisms, linking photosynthesis directly to the growth of biomass and uptake of nutrients [27][28]. In addition, the secretion of the extracellular substance bu SAB might also contribute to the disparity between the amount of total biomass in TES and TNES. Schmidt et al. (2018), found a higher amount of extracellular polymeric substances with light exposure [26].
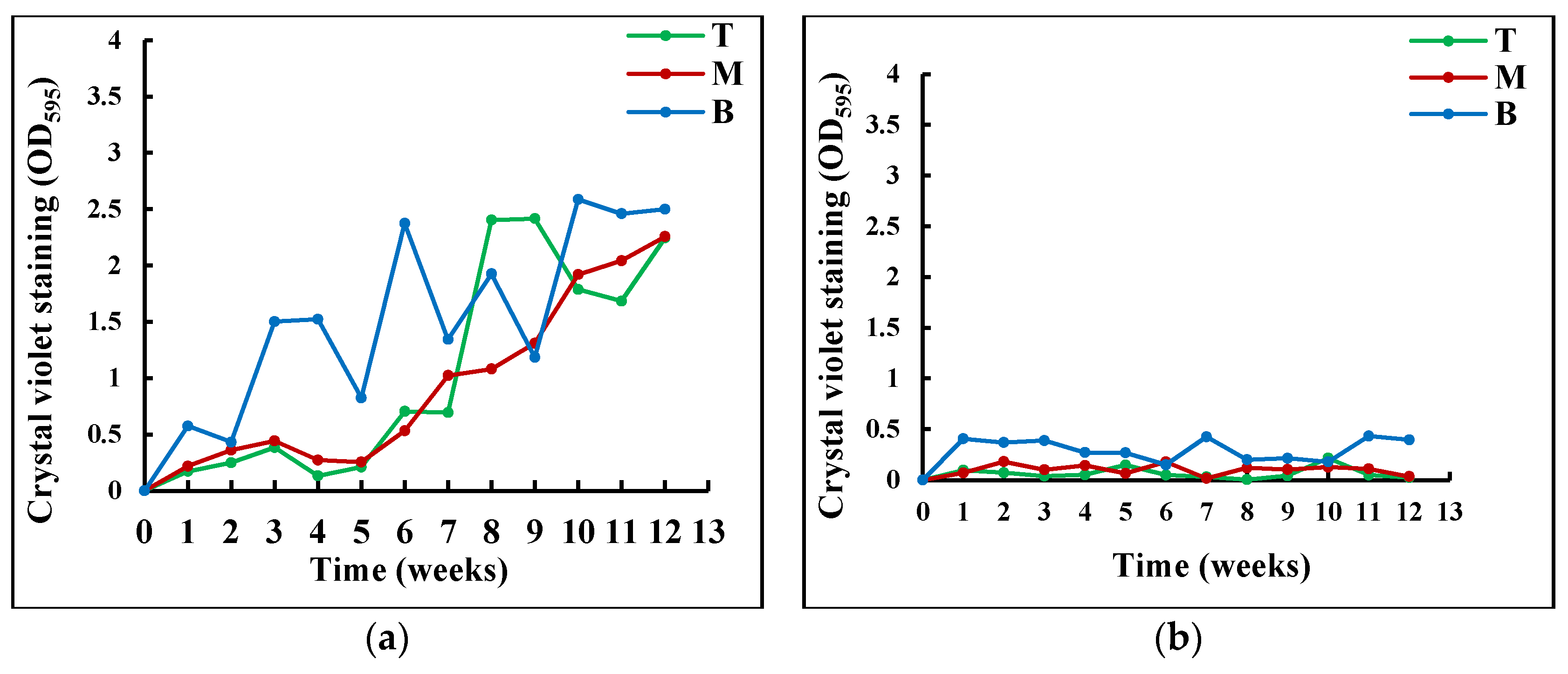
Figure 43. Variation of the total SAB biomass (a) in the TES and (b) in the TNES at different water depths over time (T: top, M: middle, and B bottom).
2.2. Effect of Visible Light on SB
Figure 5Figure 4 shows the comparison of SB between TES and TNES over time and different water depths. The decay rate of heterotrophic bacteria summarized in Table 3 (0.024 CFU day−1) in the TNES was significantly higher than that in the TES (0.004 CFU day−1; p < 0.05). According to Ruiz-Gonzalez et al. (2013) and Hameed et al. (2020), visible light triggers the uptake of DOM and cell division of heterotrophic bacteria [25][29]. Therefore, a decrease in the bacterial count was alleviated by the production of new cells. In addition, with visible light exposure, phototrophs produce carbohydrates that sustain heterotrophic bacteria [30][31]. Furthermore, the oligotrophic condition of the rainwater tank not exposed to sunlight, therefore with fewer bacteria due to lack of food, might have accentuated the difference in the plate counts of the two tanks [22].
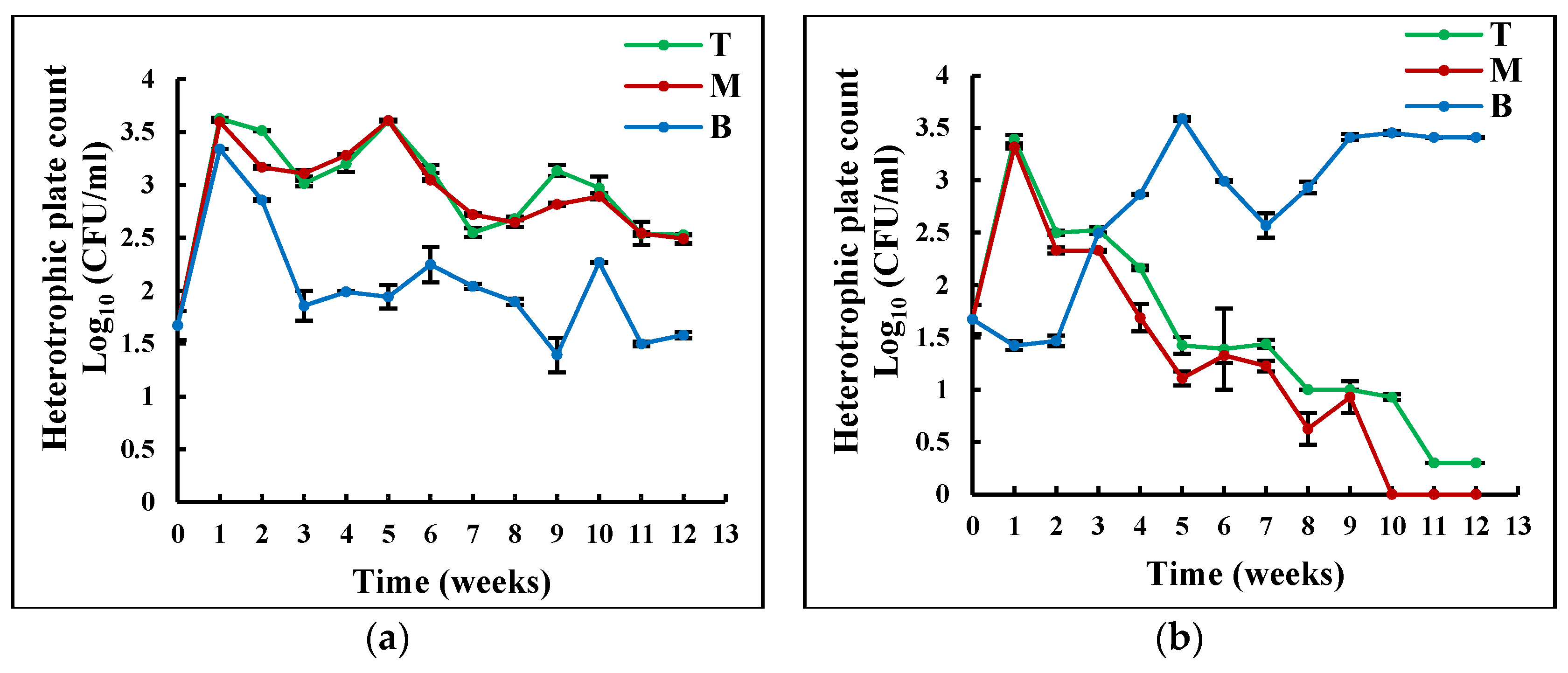
Figure 54. Variation in suspended heterotrophic bacteria (a) in TES and (b) in TNES at different water depths over time (T: top, M: middle, and B bottom).
2.3. Effect of Visible Light on Physiochemical Parameters
The pH, TDS, and DO concentration were not affected by different water depths, as shown in Table 4Table 1. The difference in temperature between the top and bottom levels was around 1 °C for more than half of the experimental period and the temperature ranged from 20.7 to 28.9 °C. Heterotrophic bacteria grow rapidly when the temperature ranges between 20 and 30 °C; that is higher the temperature, the faster the replication [30][31]. The difference in temperature between the TES and TNES was approximately 1.5 °C, as depicted in Table 4Table 1, and was not statically significant. A noticeable difference in the physicochemical characteristics of water between the tanks was the slightly higher concentration of dissolved oxygen in the TES. This difference was possibly due to photosynthesis by phototrophs in the presence of visible light [32].
Table 41. Variation in the physicochemical parameters of rainwater: pH, temperature, total dissolved solids (TDS), and dissolved oxygen concentration.
| Physiochemical Parameters | Tank Exposed to Sun | Tank Not Exposed to Sun | Average Difference between TES and TNES | |||
|---|---|---|---|---|---|---|
| Min | Max | Min | Max | |||
| pH | Top | 5.42 | 8.29 | 5.97 | 8.4 | 0.48 ± 0.4 |
| Middle | 4.9 | 8.29 | 5.72 | 7.95 | 0.46 ± 0.4 | |
| Bottom | 4.09 | 7.83 | 6.07 | 7.83 | 0.49 ± 0.4 | |
| Temperature (°C) | Top | 21.9 | 28.9 | 20.3 | 27 | 1.51 ± 1.0 |
| Middle | 20.2 | 28.9 | 20.6 | 27 | 1.34 ± 0.9 | |
| Bottom | 20.7 | 28.6 | 20 | 26.9 | 1.39 ± 1.0 | |
| Dissolved oxygen (mg/L) | Top | 5.28 | 8.94 | 5.28 | 7.54 | 1.26 ± 0.4 |
| Middle | 5.28 | 8.94 | 5.28 | 7.56 | 1.24 ± 0.4 | |
| Bottom | 5.28 | 8.7 | 5.28 | 7.44 | 1.31 ± 0.5 | |
| Total dissolved solid (mg/L) | Top | 41 | 57 | 37 | 57 | 4.33 ± 3.8 |
| Middle | 38 | 57 | 35 | 60 | 4.58 ± 4.4 | |
| Bottom | 31 | 57 | 39 | 57 | 3.08 ± 2.7 | |
3. Conclusions
We experimentally investigated the effect of visible light on the microbiological and physicochemical quality of harvested rainwater. The bacterial count of SB decreased faster at TNES than TES case, resulting in better microbial quality at TNES. The number of SAB decreased over time when it is not exposed to visible light, while it remained stable when exposed to visible light. There was no big difference between TES and TNES in other physicochemical parameters except that the total organic carbon and concentration of dissolved oxygen were higher in TES than in TNES. Overall, the water quality can be maintained well without the effect of visible light.
It is possible to suggest a practical implication in the design and operation of typical household rainwater tanks to maintain good water quality. The storage should be installed under a shade or putting cover at the opening to avoid penetration of visible light. Disinfection in the tank should be avoided to maintain the microbial balance and self-purification in the rainwater tank.
References
- UN. World Fertility and Family Planning 2020: Highlights; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2020; Available online: (accessed on 17 April 2021).
- Water UN. Coping with Water Scarcity a Strategic Issue and Priority for System-Wide Action; New York, NY, USA, 2006; Available online: (accessed on 17 April 2021).
- World Water Assessment Programme. The United Nations World Water Development Report. New York, NY, USA, 2018. Available online: (accessed on 17 April 2021).
- Bernard, B.; Joyfred, A. Contribution of Rainfall on Rooftop Rainwater Harvesting and Saving on the Slopes of Mt. Elgon, East Africa. Sci. World J. 2020, 2020, 7196342.
- Yannopoulos, S.; Giannopoulou, I.; Kaiafa-Saropoulou, M. Investigation of the current situation and prospects for the development of rainwater harvesting as a tool to confront water scarcity worldwide. Water 2019, 11, 2168.
- Rainwater Harvesting 101. Available online: (accessed on 24 April 2021).
- Thomas, T.H. The limitations of roofwater harvesting in developing countries. Waterlines 2014, 33, 139–145.
- UN Habitat for a Better Future: Blue Drop Series on Rainwater Harvesting and Utilisation—Book 3 Project Managers and Implemetation Agency. Available online: (accessed on 23 April 2021).
- Lee, J.Y.; Bak, G.; Han, M. Quality of roof-harvested rainwater-Comparison of different roofing materials. Environ. Pollut. 2012, 162, 422–429.
- Alim, M.A.; Rahman, A.; Tao, Z.; Samali, B.; Khan, M.M.; Shirin, S. Suitability of roof harvested rainwater for potential potable water production: A scoping review. J. Clean. Prod. 2020, 248, 119226.
- Al-Batsh, N.; Al-Khatib, I.A.; Ghannam, S.; Anayah, F.; Jodeh, S.; Hanbali, G.; Khalaf, B.; van der Valk, M. Assessment of rainwater harvesting systems in poor rural communities: A case study from Yatta Area, Palestine. Water 2019, 11, 585.
- Coombes, P. Key Messages from a Decade of Water Quality Research into Roof Collected Rainwater Supplies; Burswood Convention Centre Perth: Burswood, WA, Australia, 2006; pp. 1–9. Available online: (accessed on 20 February 2021).
- Kim, M.; Han, M. Role of Biofilm in Rainwater Tank. Microb. Biofilms. Importance Appl. 2016.
- Van Der Merwe, V.; Duvenage, S.; Korsten, L. Comparison of biofilm formation and water quality when water from different sources was stored in large commercial water storage tanks. J. Water Health 2013, 11, 30–40.
- Evans, C.A.; Coombes, P.J.; Dunstan, R.H.; Harrison, T. Extensive bacterial diversity indicates the potential operation of a dynamic micro-ecology within domestic rainwater storage systems. Sci. Total Environ. 2009, 407, 5206–5215.
- Evison, L.; Sunna, N. Microbial regrowth in household water storage tanks. J. Am. Water Work Assoc. 2001, 93, 85–94.
- Kim, M.; Han, M. Composition and distribution of bacteria in an operating rainwater harvesting tank. Water Sci. Technol. 2011, 63, 1524–1530.
- Amin, M.T.; Kim, T.; Amin, M.N.; Han, M.Y. Effects of Catchment, First-Flush, Storage Conditions, and Time on Microbial Quality in Rainwater Harvesting Systems. Water Environ. Res. 2013, 85, 2317–2329.
- Spinks, A.T. Water Quality, Incident Treatment Train Mechanism and Health Risks Associated with Rainwater Harvesting System in Australia; University Newcastle: Newcastle, Australia, 2007.
- Peter, J.; Spinks, A.; Evans, C.; Dunstan, H. Performance of Rainwater Tanks at an Inner City House in Carrington NSW during a Drought; Callaghan, Newcastle, Australia, 2004; Available online: (accessed on 20 February 2021).
- Fu, Y.; Peng, H.; Liu, J.; Nguyen, T.H.; Hashmi, M.Z.; Shen, C. Occurrence and quantification of culturable and viable but non-culturable (VBNC) pathogens in biofilm on different pipes from a metropolitan drinking water distribution system. Sci. Total Environ. 2021, 764, 142851.
- Kim, M.; Han, M. Role of biofilms in improving microbial quality in rainwater tanks. Desalin. Water Treat. 2015, 53, 2579–2584.
- Santos, A.L.; Henriques, I.; Gomes, N.C.M.; Almeida, A.; Correia, A.; Cunha, A. Effects of ultraviolet radiation on the abundance, diversity and activity of bacterioneuston and bacterioplankton: Insights from microcosm studies. Aquat. Sci. 2011, 73, 63–77.
- Hockberger, P.E. A History of Ultraviolet Photobiology for Humans, Animals and Microorganisms. Photochem. Photobiol. 2002, 76, 561.
- Ruiz-González, C.; Simó, R.; Sommaruga, R.; Gasol, J.M. Away from darkness: A review on the effects of solar radiation on heterotrophic bacterioplankton activity. Front. Microbiol. 2013, 4, 131.
- Schmidt, H.; Thom, M.; Wieprecht, S.; Manz, W.; Gerbersdorf, S.U. The effect of light intensity and shear stress on microbial biostabilization and the community composition of natural biofilms. Res. Rep. Biol. 2018, 9, 1–16.
- Agustí, S.; WKrause, J.A.; Marquez, I.; Wassmann, P.; Kristiansen, S.; Duarte, C.M. Arctic (Svalbard islands) active and exported diatom stocks and cell health status. Biogeosciences 2020, 17, 35–45.
- Misic, C.; Covazzi Harriague, A. Development of marine biofilm on plastic: Ecological features in different seasons, temperatures, and light regimes. Hydrobiologia 2019, 835, 129–145.
- Hameed, A.; Lai, W.A.; Shahina, M.; Stothard, P.; Young, L.S.; Lin, S.Y.; Sridhar, K.R.; Young, C.C. Differential visible spectral influence on carbon metabolism in heterotrophic marine flavobacteria. FEMS Microbiol. Ecol. 2020, 96, fiaa011.
- Villa, F.; Pitts, B.; Lauchnor, E.; Cappitelli, F.; Stewart, P.S. Development of a laboratory model of a phototroph-heterotroph mixed-species biofilm at the stone/air interface. Front. Microbiol. 2015, 6, 1251.
- Valverde, A.; Makhalanyane, T.P.; Seely, M.; Cowan, D.A. Cyanobacteria drive community composition and functionality in rock-soil interface communities. Mol. Ecol. 2015, 24, 812–821.
- Roeselers, G.; Loosdrecht, M.C.M.V.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235.
