Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Guido Viel.
Novel psychoactive substances (NPS) represent a severe health risk for drug users. The molecular mechanisms underlying the action of NPS are more complex than expected, with a wide range of overlap among activated receptors and neurotransmitter systems.
- new psychoactive substances (NPS)
- mass spectrometry
1. Introduction
Novel Psychoactive Substances (NPS) are an inhomogeneous group of substances which are typically sold as “legal” alternatives to the classical scheduled drugs of abuse, such as heroin, cocaine, amphetamines, benzodiazepines etc. [1]. The term “novel” derives from the fact that, contrarily to classical drugs of abuse, NPS were not covered by the International Drug Control Conventions of 1961–1971 [1,2][1][2]. Nowadays, the term could be considered somehow misleading, since many of the compounds have been later included in the list of scheduled substances at a national or international level [2]. Nonetheless, the “legality” of these compounds still represents one of the main attractions for consumers [2]. One of the characteristics of the NPS phenomenon resides in the ease of producing novel compounds by minimal twisting or modifications of the chemical structures, producing a nonscheduled molecule and circumventing existing legislations. Some authors have underlined that the huge efforts of national/international organizations, striving to include a molecule within the list of prohibited substances, are the main trigger for the innovation and production of novel compounds (the so-called “cat and mouse model”) [2[2][3],3], which have rated more than 50 novel compounds per year since early 2000. Thus, even if many of these substances are now controlled, several are still nonscheduled, undetected, and unidentified. These substances are not even consumed or produced, but certainly will be in the next future. To date, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) has monitored 790 new psychoactive substances [1,4,5][1][4][5]. The main drives for consuming NPS are also the reported “safety” and “natural origin” by the supplier, both concepts that have led to an extraordinary growth in popularity of NPS since 2007, especially among younger users browsing the Internet [5,6,7][5][6][7]. Although they are claimed as “safe” or sold “not for human consumption,” these substances pose severe health risks, the prevention of which cannot disregard from an in-depth understanding of their pharmacokinetic and pharmacodynamic properties.
2. Synthetic Opioids
Although they are still of limited diffusion across the European market, synthetic opioids represent a massive health risk due to their high potency and severe adverse effects. Indeed, they have been reported as one of the main causes of the waves of opioid deaths in the USA [9,10,11,12,13][8][9][10][11][12]. The term “synthetic opioids” includes a wide range of antinociceptive and analgesic compounds (fentanyl derivatives, benzamide, acetamide and piperazine families) [14][13] that act as partial or full agonists at G-protein-coupled receptors (μ, κ, and δ) [15,16,17][14][15][16]. μ-opioid receptors, as shown in knock-out mice, are mainly located in brain and gastrointestinal tract and lead to anxiolysis, relaxation, sedation, antinociception, euphoria, and respiratory depression [7,17,18,19,20,21][7][16][17][18][19][20]. Other effects include hypothermia, miosis, nausea, and the inhibition of gastrointestinal propulsion. The activation of κ and δ-receptors also leads to hallucination, dissociate feelings, and dysphoria, as shown for U-50488H, and immunomodulation [14,22,23][13][21][22]. The peculiar profile of opioid receptor agonism might explain also unusual toxicity, e.g., a deep level of unconsciousness for MT-45 [21][20]. G-proteins (Gαi), determining the inhibition of cyclic adenosine monophosphate (cAMP) production, inhibition of Ca2+ channels of the L-type, and activation of the inward-rectifying K+ channels, leading to hyperpolarization and reduced neuronal excitability, are mainly responsible for analgesia, while β-arrestins are additional transducers, which could be involved in the unwanted effects of synthetic opioids [24][23].
Generally, synthetic opioids present stronger analgesic activity compared to morphine and classical opioid. Fentanyl and carfentanyl are approximately 50–100- and 10000-times respectively more potent than classical opioids [25,26,27,28][24][25][26][27]. Affinity to opioid receptors significantly differs between stereoisomers, e.g., only the trans form has opioid activity for U-47700 and U-50488 [27][26], and R-enantiomers are thought to be more potent than the S ones [29][28]. Even though the in vitro efficacy and potency of several new compounds, such as AP-237, bromadol, brorphine, tianeptine, isotonitazene, and piperidylthiambuetene, has been characterized [9[8][29],30], their exact psychopharmacological and neurotoxicological profiles remain scarcely known [25][24].
Synthetic opioids might interact also with other receptors, especially with the serotoninergic ones or with monoamine transporters such as norepinephrine transporter (NET) and serotonin transporter (SERT) [7], as seen for AH-7921, the effects of which were prolonged by the co-injection of serotonin (5HT) and attenuated by norepinephrine [31][30]. Contrarily to morphine, which has antagonistic interactions with 5HT3A receptors [32][31], interaction of fentanyl with 5HT1A and 2A receptors might lead to additional toxicity due to serotonin syndrome, especially in combination with other drugs active on the serotonin system [33][32]. This might explain why rescue therapy with naloxone (receptor antagonist) are noneffective, or less effective than what expected [34,35,36][33][34][35].
Fentanyl and carfentanil also showed relevant affinity for α1 adrenoceptors, possibly explaining severe muscle rigidity at the laryngeal, tracheal, and chest musculature and the closure of vocal cords, as well as for dopamine receptors (D4.4 and D1). Moreover, they blocked the uptake by monoamine transporter 2 and this might further explain the relevant respiratory and cardiothoracic effects [37][36].
3. Synthetic Cannabinoids
Synthetic cannabinoids, also called “Spice,” are synthetic cannabinoid receptor agonists (SCRAs) which have been originally developed for their potential therapeutic role by exploiting the endocannabinoid system [38,39,40][37][38][39]. Since then, “Spice” products have been sold as legal marijuana surrogate, becoming very popular among younger people and now representing the widest class of NPS. Synthetic cannabinoids are full agonists at CB1 and CB2, G-coupled human cannabinoid receptors [41[40][41][42][43][44][45][46][47][48],42,43,44,45,46,47,48,49], which are weakly bound by delta-9-tetrahydrocannabinol (THC) and which inhibit adenylyl cyclase and activate mitogen-activated protein kinases [50,51][49][50]. CB receptors can also activate inwardly, rectifying potassium channels and mediating an inhibition of N- and P/Q-type calcium currents (more details are given in Figure 2 [50][49].
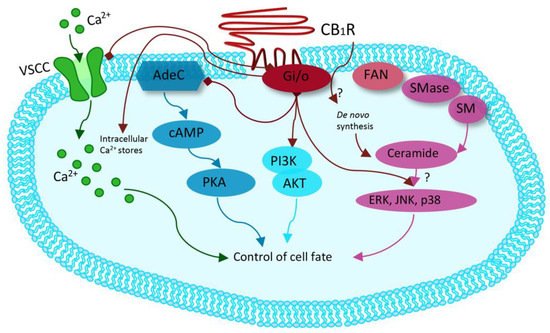

Figure 2. Modified from Guzman et al. Cannabinoids: Potential anticancer agents. Nat Rev Cancer. Mechanism activated by the receptor of human cannabinoids 1 (CB1R), ranging from binding to G-protein-coupled receptors (Gi/o) with inhibition of the adenylyl cyclase (AdeC), and therefore of the cyclicAMP (cAMP) and of the protein kinase A (PKA). Inhibition of voltage-sensitive Ca2+ channels (VSCC); release of Ca2+ from intracellular stores; activation of the phosphatidylinositol 3-kinase (PI3K)–AKT pathway; activation of mitogen-activated protein kinase cascades as extracellular-signal-regulated kinase (ERK), JUN amino-terminal kinase (JNK), and p38 and ceramide generation through FAN–sphingomyelinase (factor associated with neutral sphingomyelinase activation–SMase).
CB1 receptors are mainly located in the central nervous system, thus covering most of the psychoactive effects of SCRAs. Due to the distribution of CB1 and CB2 receptors on the terminals of neuron, which mediate a modulation and inhibition of synaptic transmission, cannabinoids have effects on neuronal development, motor function, cognition, and memory, appetite, sleep, thermoregulation, analgesia, reward processes, cardiovascular, respiratory, immune, and reproductive functions [7,52,53,54,55][7][51][52][53][54]. Reward, euphoria, memory loss, altered vigilance, anxiety and cognitive deficit, proconvulsant, antinociceptive, cataleptic, hypolocomotion, and hypothermic effects of SCRAs, such as JWH-018, JWH-073, 5F-AMB, 5F-AB-PINACA, and Cumyl-4CN-BINACA, are mediated by CB1 receptor activation, as demonstrated in CB1 knock-out mice or by CB1-blocking agents [56,57,58,59,60,61,62,63,64,65][55][56][57][58][59][60][61][62][63][64]. These neurological effects differ from that of classical cannabinoids, e.g., cannabidiol (CBD), one of the main non-psychotropic cannabinoids, which has been shown to interact with peroxisome proliferator-activated receptors and acetylcholinesterase and to modulate beta-amyloid deposition and tau protein phosphorylation, with several promising therapeutic uses [66][65].
In adolescent and adult mice, in vivo brain administration of 5-MDMB-PICA produced anxiety-like and compulsive states [67][66]. The effects on neuronal development have been also studied. Brain malformations have also been shown due to inhibition of Pax-6, which is necessary for the closure of the nascent neural tube, as well as CB1-mediated ocular malformation, lack of memory retention and hyperactivity, and inhibition of new synapses formation in hippocampal neurons [68][67]. Moreover, SCRAs induced hyperreflexia and myoclonias, not induced by THC, with effects prevented by the administration of CB1 receptor antagonist/reverse agonist AM 251, while this is not the case for sensory-motor impairments [69,70][68][69]. CB receptor antagonists also prevent SCRAs from producing cytotoxic effects on cytotrophoblasts cells, forebrain cultures, and skeletal muscle cells by CP-55.940 and CP 47.497-C8 [71,72,73,74,75][70][71][72][73][74].
CB1 receptor have been shown to have a role in the interaction between ethanol and SCRAs, with an increase in ethanol-induced motor impairments after JWH-018 administration [76][75], and in analgesia, with a synergistic effect between SCRAs and opioids [77][76].
The selectivity, affinity, and activity of SCRAs appear to be related to their chemical structure [60,78,79[59][77][78][79],80], e.g., the fluorination of the alkyl side chain of Cumyl-PEGACLONE led to a more affine and active compound, 5F-Cumyl-PEGACLONE [81][80]. The pharmacological profile (affinity and activity) of 5F-Cumyl-PICA 5F-Cumyl-PINACA and 5F-Cumyl-P7AICA has been also recently determined [82][81]. Halogenated JWH-018 was less effective in causing seizures, myoclonia, and hyperreflexia than JWH-018 [83][82]. Moreover, the enantiomeric configuration might have a role in the affinity to receptors [84,85][83][84].
One of the main issues of SCRAs, which might also lead to death, is represented by cardiotoxicity and cannabinoid-receptor associated arrhythmias [86][85], which might be a CB2-mediated effect, resulting in prolonged QT interval [87][86]. CB2 might also mediate a vasodilator effect, additionally triggered by independent (nitric-oxide-related) mechanisms [88][87]. However, no chronotropic effect by CB2 was shown on isolated rat atria treated with SCRAs, and the exact mechanism of SCRAs-related arrhythmias remains unknown [89,90][88][89].
Metabolites have been shown to retain activity at CB1 and/or CB2 receptors [43][42] as shown for JWH-018, JWH-073, 5F-AKB48, and AB-PINACA, with implications for toxicity [91,92,93,94][90][91][92][93]. However, a non-receptor-mediated mechanism has been proposed for the toxicity of the JWH-018 main metabolite when compared to the parent drug, and for WIN55,212-2 in spatial memory tasks, which causes a CB-receptor-independent decrease of cholinergic activation [95,96][94][95].
Interactions with other neuroceptors, leading to inhibition of cholinergic contraction in the respiratory system, inhibition of glutamate release, and release of dopamine in the nucleus accumbens, leading in vivo to abuse potential and psychomotor agitation, might be partly explained by a presynaptic CB1 mediated effect [97,98,99][96][97][98]. Interactions of SCRAs has been described with dopamine, serotonin, and glutamate systems, with possible effects on schizophrenia and psychosis after SCRAs intake [100][99]. Other non-cannabinoid-mediated interactions include those with other G-coupled protein receptors, capsaicin receptor, and the vanilloid receptor 1 [52,101,102][51][100][101]. It should be mentioned that transient receptor potential (TRP) channels might also mediate significant effects of SCRAs, since endogenous endocannabinoids such as anandamide are TRP agonists [103][102]. Moreover, as shown for AM2201 and JWH-018, SCRAs might act as allosteric modulators of other receptors, e.g., 5-HT1A receptors, determining a hypothermic response in mice lacking CB receptors [104][103] or producing behavioral responses [105][104]. SCRAs such as WIN55,212-2 can also inhibit a 5-HT mediated current in a non-CB-receptor-dependent manner [106][105].
4. Stimulants, Psychedelics, and Hallucinogens
Stimulants such as cocaine, amphetamine, MDMA, and cathinones typically determine a sympathomimetic action, with tachycardia and hypertension, hallucinogenic (including psychosis and delirium), and psychoactive stimulants effects, e.g., agitation, euphoria, and increased emotional empathy [7,107,108,109,110,111,112][7][106][107][108][109][110][111]. Novel stimulants are considered to lead to the same effects, though with higher potency [113[112][113],114], by interacting with monoamine transporters, particularly with dopamine transporter (DAT), NET, and SERT. This interaction might be of the “blocking type,” i.e., by inhibition of the uptake of neurotransmitter from the extracellular space, thus leading to an increase of the respective monoamines [115][114]. In addition or alternatively to the blocking of monoamine transporters, some drugs might act as “substrates,” entering the intracellular space, releasing monoamine, and mediating a so-called non-exocytotic monoamine efflux, as occurs for MDMA and methamphetamine [7,115,116,117,118][7][114][115][116][117].
Novel psychostimulant drugs are mostly classified on the basis of the greater noradrenergic vs. dopaminergic vs. serotoninergic activity [119,120,121,122,123][118][119][120][121][122]. Indeed, a high DAT/SERT ratio and a substrate-type monoamine releasers action is typical of amphetamine-type stimulant-like properties, with high potential of abuse [124][123], whereas a lower ratio (0.01–0.1) leads mainly to empathogenic effects, similarly to MDMA, with low intracranial self-stimulation [7,116,125][7][115][124]. The DAT/SERT ratios of the main stimulants are shown in Figure 3.
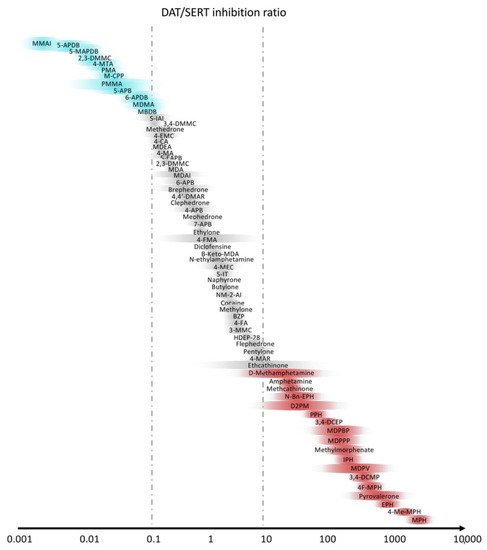

Figure 3. Selectivity of stimulants with the ratio between dopamine (DAT) and serotonin (SERT) transporters. Slightly modified from Luethi et al. [7].
Serotonergic compounds usually lead to a subjective sense of well-being and increased sociability in humans. These compounds have been associated with 5-HT syndrome, hyperthermia, and resulting organ failure [116][115]. Hyperthermia might be reduced using adrenergic antagonists, highlighting the importance of adrenergic receptors in the determination of this adverse effect [126][125].
The effects of psychostimulants seem to be also influenced by the chiral configuration, e.g., S-enantiomer may have greater serotoninergic features, and R-enantiomers may have higher dopaminergic features [127][126].
Amphetamines are substrates of vesicular monoamine transporters and inhibitors of monoamine oxidases and interact with trace amine-associated receptor 1 (TAAR1) [7,112,128,129,130,131][7][111][127][128][129][130]. Stimulants also present complex interactions with neuroendocrine molecules, e.g., they increase oxytocin levels, although the latter, as demonstrated for 4-Fluoroamphetamine, might be unrelated to cognitive and emotional behavior and empathy [132][131]. Amphetamine-type psychostimulant include derivatives of aminorex, such as 4-methylaminorex (4-MAR) and 4,4′-dimethylaminorex (4,4′-DMAR) [133,134][132][133]. Although both are derivatives of aminorex, the former appears as a more typical stimulant, with a high DAT/SERT ratio, while the latter is thought to lead mainly to empathogenic effects [125][124].
Both 3,4-dichloromethylphenidate (3,4-CTMP) and ethylphenidate are analogs of methylphenidate, a prescription drug used in the treatment of the attention-deficit hyperactivity disorder (ADHD), and are commonly consumed to produce euphoria or as cognitive enhancers [7,135][7][134]. Even though 3,4-CTMP was originally studied as a treatment for cocaine abuse [136][135], methylphenidate derivatives determine a dopamine and a noradrenaline efflux in the nucleus accumbens and stria terminalis, which are involved in the hedonic processing system and which explain the abuse potential of the drugs, with NET and DAT inhibitor activity [7]. 3,4-CTMP is mainly considered as a a “cocaine-like” instead of “amphetamine-like” drug, since it increases the release of dopamine when stimulated, but not in baseline conditions [135][134]. As a transporter inhibitor, diclofensine has also a similar pharmacological profile to cocaine. However, it also has high affinity for D2 and for adrenergic α1A and α2A receptors [137][136].
Phenmetrazines derivatives, e.g., 3-fluorophenmetrazine (3-FPM), diphenylprolinol (D2PM), and desoxypipradrol (2-DPMP), similarly to methylphenidate, are DAT and NET inhibitors, with prolonged psychostimulants effects and low serotoninergic effects [7,116][7][115].
Synthetic cathinones, typically called “bath salts,” are both indirect releasers by transporter blocking action, e.g., pyrovalerone derivatives, and direct substrate effects, e.g., 4-methylmethcathinone (mephedrone) and methylone [7,118,138,139,140][7][117][137][138][139]. Pyrrolidine-containing cathinones, such as methylenedioxypyrovalerone (MDPV,) are blockers at DAT and NET with lower potency at SERT and do not show a substrate activity [118][117]. MDPV, one of the most popular bath salts, has been shown to induce an EEG synchronization associated with delirium syndrome in rats treated by microdialysis, blocked by D1 and D2 receptor antagonists [141][140]. Moreover, it led to the reduction of social play behavior in young adult male rats, while effects were blocked by RX821002 and flupenthixol, respectively, α2 and dopamine receptor antagonists [142][141]. Drug-induced dopaminergic activity parallels the locomotor stimulation and rewarding effect [118,143,144][117][142][143]. Methylone is a nonspecific substrate [118][117], producing an inward current at SERT but not at DAT, similarly to MDMA [145][144], and oxidative stress, which is responsible for the neurotoxicity of methylone and, to a greater extent, MDPV [146][145]. 4-MEC, 4-MePP, and α-PVP also mainly block DAT, with greater abuse potential compared to other stimulants [147,148][146][147]. Unusual neuropsychiatric symptoms have been attributed to some synthetic cathinones, suggesting additional pharmacological features. Among synthetic cathinones, α-pyrrolidinohexiophenone (α-PHP) also exhibit anticholinergic activity (at M1 and M2 receptors), which might have a role in clinical features such as delusions, cognitive impairment, and cardiovascular effect such as tachycardia and hypertension [149][148]. α-PPP has an antagonistic interaction with 5-HT2A-receptors, which could be responsible for its limited abuse potential compared to other compounds of the same class [150][149].
Among benzofurans (e.g., 5-APB) indole derivatives and amino-indane, 5-iodoaminoindane (5-IAI), and 5,6-methylenedioxy-2-aminoindane (MDAI) preferentially inhibit SERT and NET, and the latter also has shown NE-releasing properties [116,151,152,153,154,155][115][150][151][152][153][154]. Among piperazines, 1-benzylpiperazine (BZP) has a more selective action on NET, with no or low serotoninergic effects, leading to cardiostimulant effects, agitation, seizures, and hyperthermia, while other compounds pertaining to the same class, e.g., meta-chlorophenylpiperazine (m-CPP) and trifluoromethylphenylpiperazine (TFMPP), have low effects on DAT and NET and predominantly act as indirect (transporter inhibitor) and direct serotonergic agonists, resulting in effects such as dysphoria, dizziness, anxiety, and more nausea compared to MDMA [7,116][7][115]. 5-APB has been shown to interact with the dopamine transporter, slowing dopamine reuptake and causing its reverse transport at high doses, and is an agonist at the 5-HT2A and 5-HT2B-receptors in the rat. The interaction with serotoninergic receptors might mediate the hallucinogenic and cardiotoxic effects [152][151].
Stimulants of the thiophene designer drug groups have been shown to interact with 5-HT adrenergic and dopaminergic receptors, as well as N-methyl-D-aspartate (NMDA) and sigma-1 receptors [7]. The locomotor sensitization effect might be mainly mediated by dopaminergic activation, as shown for metathiopropamine (MPA), an NPS of the methamphetamine type, the effect of which is reversed by D2 but not by D1 receptor antagonists [156][155].
Psychedelics and hallucinogen determine alterations in the perception, beside mood and cognition modifications [157][156]. Within this class, tryptamines, e.g., N,N-dimethyltryptamine (DMT) and psilocybin, and “psychedelic amphetamines,” e.g., 2,5-dimethoxy-4-iodoamphetamine (DOI) and N-benzylphenethylamines (NBOMes), are included [7,158][7][157]. Neuropsychological effects of many psychedelics, including the head twitch response, which is used as a behavioral paradigm to distinguish hallucinogenic drugs, are mediated by the activation of 5-HT receptors, for which NBOMes show high affinity [158][157]. Generally, phenethylamines, also called “party pills” [158][157], such as 25B-NBOMe, have high 5-HT2A and 5-HT2C affinity and potency [158][157]. However, many NBOMEs also display affinity for dopaminergic receptors, e.g., D2, and for monoamine transporters, leading to abuse potential and rewarding and reinforcing effects [159,160,161][158][159][160]. Substituted phenethylamines, such as MAL and BOD, also alter the dopaminergic system by interacting with receptors in the nucleus accumbens and dopamine transport [162][161]. In addition, 4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (25I-NBOMe) increases glutamate levels [7]. Although mainly mediating serotoninergic action, most tryptamine bind to 5-HT1A receptors. Moreover, as demonstrated by in vitro studies, they bind on adrenergic, dopaminergic, and histaminergic receptors and transporters. For example, psilocin is a transporter inhibitor, while DMT is a transporter substrate [7].
Another class of NPS, properly of the dissociative type, is represented by derivative of phencyclidine (PCP) and ketamine, which are N-methyl D-aspartate (NMDA) receptor antagonists. Subjective effects associated with the intake of these drugs include dissociative-like effects, with alteration of the mood and thought, and schizophrenia-like effects [163][162].
Antidepressant effects of these compounds, e.g., methoxetamine, as demonstrated by forced swim tests on mice, might be related to the interactions with the glutamatergic system by the activation of the mammalian target of rapamycin, involved in synaptic plasticity, by a modulation of the brain-derived neurotrophic factor (BDNF), or by SERT properties. Moreover, methoxetamine has shown to be a DAT inhibitor and an agonist of muscarinic cholinergic and 5-HT2 receptors, and to produce analgesia [164][163]. Diphenidine and methoxphenidine are also dissociative drugs, acting as NMDA antagonists. Diphenidine further inhibits NET and DAT, while it is a less potent DAT inhibitor, but both do not mediate an efflux of monoamines [137][136]. N-Ethyl-1,2-diphenylethanamine (ephenidine) also acts selectively by blocking NMDA receptors with a higher potency than ketamine, though also interacting with NET and DAT, which might contribute to the behavioral profile of the drug [163][162].
References
- Dignam, G.; Bigham, C. Novel psychoactive substances: A practical approach to dealing with toxicity from legal highs. BJA Educ. 2017, 17, 172–177.
- EMCDDA. New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic—An Update from the EU Early Warning System. Available online: (accessed on 1 April 2021).
- Maurer, H.; Brandt, S.D. New Psychoactive Substances, 1st ed.; Springer Nature Switzerland: Cham, Switzerland, 2018.
- Baumeister, D.; Tojo, L.M.; Tracy, D.K. Legal highs: Staying on top of the flood of novel psychoactive substances. Ther. Adv. Psychopharmacol. 2015, 5, 97–132.
- EMCDDA. Drug European Drug Report 2020: Trends and Developments. Available online: (accessed on 21 March 2021).
- EMCDDA. New Psychoactive Substances in Europe. An Update from the EU Early Warning System (March 2015). Available online: (accessed on 1 April 2021).
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097.
- Vandeputte, M.M.; Cannaert, A.; Stove, C.P. In vitro functional characterization of a panel of non-fentanyl opioid new psychoactive substances. Arch. Toxicol. 2020, 94, 1–12.
- Baumann, M.H.; Kopajtic, T.A.; Madras, B.K. Pharmacological Research as a key component in mitigating the opioid overdose crisis. Trends Pharmacol. Sci. 2018, 39, 995–998.
- Kuczyńska, K.; Grzonkowski, P.; Kacprzak, Ł.; Zawilska, J.B. Abuse of fentanyl: An emerging problem to face. Forensic Sci. Int. 2018, 289, 207–214.
- Giorgetti, A.; Centola, C.; Giorgetti, R. Fentanyl novel derivative-related deaths. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2605.
- Prekupec, M.P.; Mansky, P.A.; Baumann, M.H. Misuse of novel synthetic opioids. J. Addict. Med. 2017, 11, 256–265.
- Solimini, R.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Giorgetti, R. Pharmacotoxicology of non-fentanyl derived new synthetic opioids. Front. Pharmacol. 2018, 9, 654.
- Vo, Q.; Mahinthichaichan, P.; Shen, J.; Ellis, C. How mu-opioid receptor recognizes fentanyl. Nat. Commun. 2021, 12, 984.
- Cannaert, A.; Ambach, L.; Blanckaert, P.; Stove, C.P. Activity-based detection and bioanalytical confirmation of a fatal carfentanil intoxication. Front. Pharmacol. 2018, 9, 486.
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381.
- Charbogne, P.; Kieffer, B.L.; Befort, K. 15 Years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 2014, 76, 204–217.
- Naji, A.; Ramsingh, D. Oral Transmucosal Fentanyl. Available online: (accessed on 1 April 2021).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197.
- Baumann, M.H.; Majumdar, S.; Rouzic, V.L.; Hunkele, A.; Uprety, R.; Huang, X.P.; Xu, J.; Roth, B.L.; Pan, Y.-X.; Pasternak, G.W. Pharmacological characterization of Novel Synthetic Opioids (NSO) found in the recreational drug marketplace. Neuropharmacology 2018, 134, 101–107.
- Wilde, M.; Pichini, S.; Pacifici, R.; Tagliabracci, A.; Busardò, F.P.; Auwärter, V.; Solimini, R. Metabolic pathways and potencies of new fentanyl analogs. Front. Pharmacol. 2019, 10, 238.
- Sharma, K.K.; Hales, T.G.; Rao, V.J.; NicDaeid, N.; McKenzie, C. The Search for the “next” euphoric non-fentanil novel synthetic opioids on the illicit drugs market: Current status and horizon scanning. Forensic Toxicol. 2019, 37, 1–16.
- Vasudevan, L.; Vandeputte, M.; Deventer, M.; Wouters, E.; Cannaert, A.; Stove, C.P. Assessment of structure-activity relationships and biased agonism at the mu opioid receptor of novel synthetic opioids using a novel, stable bio-assay platform. Biochem. Pharmacol. 2020, 177, 113910.
- Kolesnikova, T.O.; Shevyrin, V.A.; Eltsov, O.S.; Khatsko, S.L.; Demin, K.A.; Galstyan, D.S.; de Abreu, M.S.; Kalueff, A.V. Psychopharmacological characterization of an emerging drug of abuse, a synthetic opioid U-47700, in adult zebrafish. Brain Res. Bull. 2021, 167, 48–55.
- Bilel, S.; Azevedo, N.; Arfè, R.; Tirri, M.; Gregori, A.; Serpelloni, G.; De-Giorgio, F.; Frisoni, P.; Neri, M.; Calò, G.; et al. In Vitro and in vivo pharmacological characterization of the synthetic opioid MT-45. Neuropharmacology 2020, 171, 108110.
- Baumann, M.H.; Tocco, G.; Papsun, D.M.; Mohr, A.L.; Fogarty, M.F.; Krotulski, A.J. U-47700 and its analogs: Non-fentanyl synthetic opioids impacting the recreational drug market. Brain Sci. 2020, 10, 895.
- Armenian, P.; Vo, K.T.; Barr-Walker, J.; Lynch, K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 2018, 134, 121–132.
- Hsu, T.; Mallareddy, J.R.; Yoshida, K.; Bustamante, V.; Lee, T.; Krstenansky, J.L.; Zambon, A.C. Synthesis and pharmacological characterization of ethylenediamine synthetic opioids in human Μ-opiate Receptor 1 (OPRM1) expressing cells. Pharmacol Res. Perspect. 2019, 7, e00511.
- Blanckaert, P.; Cannaert, A.; Uytfanghe, K.V.; Hulpia, F.; Deconinck, E.; Calenbergh, S.V.; Stove, C. Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene. Drug Test Anal. 2020, 12, 422–430.
- Katselou, M.; Papoutsis, I.; Nikolaou, P.; Spiliopoulou, C.; Athanaselis, S. AH-7921: The list of new psychoactive opioids is expanded. Forensic Toxicol. 2015, 33, 195–201.
- Schaaf, T.; Lyutenska, M.; Urban, B.W.; Wittmann, M. Direct effects of morphine but not of fentanyl-type opioids on human 5-HT3A receptors in outside-out patch-clamp studies. Eur. J. Pain 2014, 18, 1165–1172.
- Baldo, B.A. Opioid analgesic drugs and serotonin toxicity (syndrome): Mechanisms, animal models, and links to clinical effects. Arch. Toxicol. 2018, 92, 2457–2473.
- Scott, P.J.H.; Koeppe, R.A.; Shao, X.; Rodnick, M.E.; Sowa, A.R.; Henderson, B.D.; Stauff, J.; Sherman, P.S.; Arteaga, J.; Carlo, D.J.; et al. The effects of intramuscular naloxone dose on Mu receptor displacement of Carfentanil in Rhesus monkeys. Molecules 2020, 25, 1360.
- Rickli, A.; Liakoni, E.; Hoener, M.C.; Liechti, M.E. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: Link to clinical reports of serotonin syndrome. Brit. J. Pharmacol. 2018, 175, 532–543.
- Barann, M.; Stamer, U.M.; Lyutenska, M.; Stüber, F.; Bönisch, H.; Urban, B. Effects of opioids on human serotonin transporters. Naunyn Schmiedeberg’s Arch. Pharmacol. 2015, 388, 43–49.
- Torralva, R.; Eshleman, A.J.; Swanson, T.L.; Schmachtenberg, J.L.; Schutzer, W.E.; Bloom, S.H.; Wolfrum, K.M.; Reed, J.F.; Janowsky, A. Fentanyl but not morphine interacts with non-opioid recombinant human neurotransmitter receptors and transporters. J. Pharmacol. Exp. Ther. 2020, 374, 376–391.
- Berry-Cabán, C.S.; Kleinschmidt, P.E.; Rao, D.S.; Jenkins, J. Synthetic cannabinoid and cathinone use among US soldiers. US Army Med. Dep. J. 2012, 19–24.
- Every-Palmer, S. Synthetic Cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depen. 2011, 117, 152–157.
- Wiley, J.; Marusich, J.; Huffman, J.W.; Balster, R.L.; Thomas, B. Hijacking of basic research: The case of synthetic Cannabinoids. Methods Rep. RTi. Press 2011, 2011, 17971.
- Sachdev, S.; Vemuri, K.; Banister, S.D.; Longworth, M.; Kassiou, M.; Santiago, M.; Makriyannis, A.; Connor, M. In vitro determination of the efficacy of illicit synthetic cannabinoids at CB1 receptors. Brit. J. Pharmacol. 2019, 176, 4653–4665.
- Noble, C.; Cannaert, A.; Linnet, K.; Stove, C.P. Application of an activity-based receptor bioassay to investigate the in vitro activity of selected indole- and indazole-3-carboxamide-based synthetic Cannabinoids at CB1 and CB2 receptors. Drug Test Anal. 2019, 11, 501–511.
- Gamage, T.F.; Farquhar, C.E.; Lefever, T.W.; Marusich, J.A.; Kevin, R.C.; McGregor, I.S.; Wiley, J.L.; Thomas, B.F. Molecular and behavioral pharmacological characterization of abused synthetic cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J. Pharmacol. Exp. Ther. 2018, 365, 437–446.
- Angerer, V.; Mogler, L.; Steitz, J.; Bisel, P.; Hess, C.; Schoeder, C.T.; Müller, C.E.; Huppertz, L.M.; Westphal, F.; Schäper, J.; et al. Structural characterization and pharmacological evaluation of the new synthetic cannabinoid CUMYL-PEGACLONE. Drug Test Anal. 2018, 10, 597–603.
- Ford, B.M.; Tai, S.; Fantegrossi, W.E.; Prather, P.L. Synthetic pot: Not your grandfather’s marijuana. Trends Pharmacol. Sci. 2017, 38, 257–276.
- Tai, S.; Fantegrossi, W.E. Neuropharmacology of New Psychoactive Substances (NPS), the science behind the headlines. Curr. Top. Behav. Neurosci. 2016, 249–262.
- Cannaert, A.; Storme, J.; Franz, F.; Auwärter, V.; Stove, C.P. Detection and activity profiling of synthetic Cannabinoids and their metabolites with a newly developed bioassay. Anal. Chem. 2016, 88, 11476–11485.
- Wiley, J.L.; Lefever, T.W.; Marusich, J.A.; Grabenauer, M.; Moore, K.N.; Huffman, J.W.; Thomas, B.F. Evaluation of first generation synthetic Cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacology 2016, 110, 143–153.
- Hess, C.; Schoeder, C.T.; Pillaiyar, T.; Madea, B.; Müller, C.E. Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice. Forensic Toxicol. 2016, 34, 329–343.
- Brunt, T.M.; Bossong, M.G. The neuropharmacology of cannabinoid receptor ligands in central signaling pathways. Eur. J. Neurosci. 2020, 1–13.
- Walsh, K.B.; Andersen, H.K. Molecular pharmacology of synthetic cannabinoids: Delineating CB1 receptor-mediated cell signaling. Int. J. Mol. Sci. 2020, 21, 6115.
- Spanagel, R. Cannabinoids and the endocannabinoid system in reward processing and addiction: From mechanisms to interventions. Dialogues Clin. Neurosci. 2020, 22, 241–250.
- Basavarajappa, B.S.; Subbanna, S. Potential mechanisms underlying the deleterious effects of synthetic Cannabinoids found in spice/K2 products. Brain Sci. 2019, 9, 14.
- Dong, C.; Chen, J.; Harrington, A.; Vinod, K.Y.; Hegde, M.L.; Hegde, V.L. Cannabinoid exposure during pregnancy and its impact on immune function. Cell. Mol. Life Sci. 2019, 76, 729–743.
- Canazza, I.; Ossato, A.; Trapella, C.; Fantinati, A.; Luca, M.A.D.; Margiani, G.; Vincenzi, F.; Rimondo, C.; Rosa, F.D.; Gregori, A.; et al. Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology 2016, 233, 3685–3709.
- Wilson, C.D.; Tai, S.; Ewing, L.; Crane, J.; Lockhart, T.; Yarbrough, A.L.; Fujiwara, R.; Radominska-Pandya, A.; Fantegrossi, W.E. Convulsant effects of abused synthetic cannabinoids JWH-018 and 5F-AB-PINACA are mediated by agonist actions at CB1 receptors in mice. J. Pharmacol. Exp. Ther. 2018, 368, 146–156.
- Schindler, C.W.; Gramling, B.R.; Justinova, Z.; Thorndike, E.B.; Baumann, M.H. Synthetic cannabinoids found in “spice” products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depen. 2017, 179, 387–394.
- Grim, T.W.; Morales, A.J.; Gonek, M.M.; Wiley, J.L.; Thomas, B.F.; Endres, G.W.; Sim-Selley, L.J.; Selley, D.E.; Negus, S.S.; Lichtman, A.H. Stratification of Cannabinoid 1 Receptor (CB1R) agonist efficacy: Manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J. Pharmacol. Exp. Ther. 2016, 359, 329–339.
- Grim, T.W.; Wiebelhaus, J.M.; Morales, A.J.; Negus, S.S.; Lichtman, A.H. Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice. Drug Alcohol Depen. 2015, 150, 31–37.
- Worob, A.; Wenthur, C. DARK classics in chemical neuroscience: Synthetic Cannabinoids (Spice/K2). ACS Chem. Neurosci. 2019, 11, 3881–3892.
- Ito, S.; Deyama, S.; Domoto, M.; Zhang, T.; Sasase, H.; Fukao, A.; Esaki, H.; Hinoi, E.; Kaneko, S.; Kaneda, K. Effects of the synthetic Cannabinoid 5F-AMB on anxiety and recognition memory in mice. Psychopharmacology 2019, 236, 2235–2242.
- Fantegrossi, W.; Moran, J. Distinct pharmacology and metabolism of K2 synthetic Cannabinoids compared to Δ9-THC: Mechanism underlying greater toxicity? Life Sci. 2014, 97, 45–54.
- Domoto, M.; Sasase, H.; Wada, S.; Ito, S.; Deyama, S.; Hinoi, E.; Kaneko, S.; Kaneda, K. The synthetic Cannabinoid 5F-AMB changes the balance between excitation and inhibition of layer V pyramidal neurons in the mouse medial prefrontal cortex. Psychopharmacology 2018, 235, 2367–2376.
- Gatch, M.B.; Forster, M.J. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology 2016, 233, 1901–1910.
- Kevin, R.C.; Anderson, L.; McGregor, I.S.; Boyd, R.; Manning, J.J.; Glass, M.; Connor, M.; Banister, S.D. CUMYL-4CN-BINACA is an efficacious and potent pro-convulsant synthetic cannabinoid receptor agonist. Front. Pharmacol. 2019, 10, 595.
- Ożarowski, M.; Karpiński, T.M.; Zielińska, A.; Souto, E.B.; Wielgus, K. Cannabidiol in neurological and neoplastic diseases: Latest developments on the molecular mechanism of action. Int. J. Mol. Sci. 2021, 22, 4294.
- Musa, A.; Simola, N.; Piras, G.; Caria, F.; Onaivi, E.S.; Luca, M.A.D. Neurochemical and behavioral characterization after acute and repeated exposure to novel synthetic cannabinoid agonist 5-MDMB-PICA. Brain Sci. 2020, 10, 1011.
- Alexandre, J.; Carmo, H.; Carvalho, F.; Silva, J.P. Synthetic Cannabinoids and their impact on neurodevelopmental processes. Addict. Biol. 2020, 25, e12824.
- Canazza, I.; Ossato, A.; Vincenzi, F.; Gregori, A.; Rosa, F.D.; Nigro, F.; Rimessi, A.; Pinton, P.; Varani, K.; Borea, P.A.; et al. Pharmaco-toxicological effects of the novel third-generation fluorinate synthetic cannabinoids, 5F-ADBINACA, AB-FUBINACA, and STS-135 in mice. In vitro and in vivo studies. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2601.
- Hruba, L.; McMahon, L.R. Apparent affinity estimates and reversal of the effects of synthetic Cannabinoids AM-2201, CP-47,497, JWH-122, and JWH-250 by Rimonabant in Rhesus Monkeys. J. Pharmacol. Exp. Ther. 2017, 362, 278–286.
- Almada, M.; Costa, L.; Fonseca, B.M.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. The synthetic cannabinoid WIN-55,212 induced-apoptosis in cytotrophoblasts cells by a mechanism dependent on CB1 receptor. Toxicology 2017, 385, 67–73.
- Tomiyama, K.; Funada, M. Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: The involvement of Cannabinoid CB1 receptors and apoptotic cell death. Toxicol. Appl. Pharm. 2014, 274, 17–23.
- Tomiyama, K.; Funada, M. Synthetic Cannabinoid CP-55,940 induces apoptosis in a human skeletal muscle model via regulation of CB1 receptors and l-Type Ca2+ channels. Arch. Toxicol. 2021, 95, 617–630.
- Koller, V.J.; Zlabinger, G.J.; Auwärter, V.; Fuchs, S.; Knasmueller, S. Toxicological profiles of selected synthetic Cannabinoids showing high binding affinities to the cannabinoid receptor subtype CB1. Arch. Toxicol. 2013, 87, 1287–1297.
- Koller, V.J.; Auwärter, V.; Grummt, T.; Moosmann, B.; Mišík, M.; Knasmüller, S. Investigation of the in vitro toxicological properties of the synthetic cannabimimetic drug CP-47,497-C8. Toxicol. Appl. Pharm. 2014, 277, 164–171.
- Funada, M.; Takebayashi-Ohsawa, M.; Tomiyama, K. Synthetic Cannabinoids enhanced ethanol-induced motor impairments through reduction of central glutamate neurotransmission. Toxicol. Appl. Pharm. 2020, 408, 115283.
- Chen, X.; Cowan, A.; Inan, S.; Geller, E.B.; Meissler, J.J.; Rawls, S.M.; Tallarida, R.J.; Tallarida, C.S.; Watson, M.N.; Adler, M.W.; et al. Opioid-sparing effects of Cannabinoids on morphine analgesia: Participation of CB1 and CB2 receptors. Brit. J. Pharmacol. 2019, 176, 3378–3389.
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Müller, C.E. Pharmacological evaluation of new constituents of “spice”: Synthetic Cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol. 2018, 36, 385–403.
- Banister, S.D.; Olson, A.; Winchester, M.; Stuart, J.; Edington, A.R.; Kevin, R.C.; Longworth, M.; Herrera, M.; Connor, M.; McGregor, I.S.; et al. The chemistry and pharmacology of synthetic Cannabinoid SDB-006 and its regioisomeric fluorinated and methoxylated analogs. Drug Test Anal. 2018, 10, 1099–1109.
- Banister, S.D.; Longworth, M.; Kevin, R.; Sachdev, S.; Santiago, M.; Stuart, J.; Mack, J.B.C.; Glass, M.; McGregor, I.S.; Connor, M.; et al. Pharmacology of valinate and tert-leucinate synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem. Neurosci. 2016, 7, 1241–1254.
- Haschimi, B.; Giorgetti, A.; Mogler, L.; Nagy, T.Z.; Kramer, S.; Halter, S.; Boros, S.; Dobos, A.; Hidvégi, E.; Auwärter, V. The novel psychoactive substance Cumyl-CH-MEGACLONE: Human phase-i metabolism, basic pharmacological characterization and comparison to other synthetic cannabinoid receptor agonists with a γ-Carboline-1-one core. J. Anal. Toxicol. 2020, 45, 277–290.
- Banister, S.D.; Adams, A.; Kevin, R.C.; Macdonald, C.; Glass, M.; Boyd, R.; Connor, M.; McGregor, I.S.; Havel, C.M.; Bright, S.J.; et al. Synthesis and pharmacology of new psychoactive substance 5F-CUMYL-P7AICA, a scaffold-hopping analog of synthetic cannabinoid receptor agonists 5F-CUMYL-PICA and 5F-CUMYL-PINACA. Drug Test Anal. 2019, 11, 279–291.
- Vigolo, A.; Ossato, A.; Trapella, C.; Vincenzi, F.; Rimondo, C.; Seri, C.; Varani, K.; Serpelloni, G.; Marti, M. Novel halogenated derivates of JWH-018: Behavioral and binding studies in mice. Neuropharmacology 2015, 95, 68–82.
- Ametovski, A.; Macdonald, C.; Manning, J.J.; Haneef, S.A.S.; Santiago, M.; Martin, L.; Sparkes, E.; Reckers, A.; Gerona, R.R.; Connor, M.; et al. Exploring stereochemical and conformational requirements at cannabinoid receptors for synthetic cannabinoids related to SDB-006, 5F-SDB-006, CUMYL-PICA, and 5F-CUMYL-PICA. ACS Chem. Neurosci. 2020, 11, 3672–3682.
- Doi, T.; Tagami, T.; Takeda, A.; Asada, A.; Sawabe, Y. Evaluation of carboxamide-type synthetic Cannabinoids as CB1/CB2 receptor agonists: Difference between the enantiomers. Forensic Toxicol. 2018, 36, 51–60.
- Giorgetti, A.; Busardò, F.P.; Tittarelli, R.; Auwärter, V.; Giorgetti, R. Post-mortem toxicology: A systematic review of death cases involving synthetic cannabinoid receptor agonists. Front. Psychiatry 2020, 11, 464.
- Yun, J.; Yoon, K.S.; Lee, T.-H.; Lee, H.; Gu, S.M.; Song, Y.J.; Cha, H.J.; Han, K.M.; Seo, H.; Shin, J.; et al. Synthetic Cannabinoid, JWH-030, induces QT prolongation through HERG channel inhibition. Toxicol. Res. 2016, 5, 1663–1671.
- López-Dyck, E.; Andrade-Urzúa, F.; Elizalde, A.; Ferrer-Villada, T.; Dagnino-Acosta, A.; Huerta, M.; Osuna-Calleros, Z.; Rangel-Sandoval, C.; Sánchez-Pastor, E. ACPA and JWH-133 modulate the vascular tone of superior mesenteric arteries through cannabinoid receptors, BKCa channels, and nitric oxide dependent mechanisms. Pharmacol. Rep. 2017, 69, 1131–1139.
- Maggo, S.; Ashton, J.C. Effect of cannabinoid receptor agonists on isolated rat atria. J. Cardiovasc. Pharm. 2018, 72, 191–194.
- Ozturk, H.M.; Yetkin, E.; Ozturk, S. Synthetic cannabinoids and cardiac arrhythmia risk: Review of the literature. Cardiovasc. Toxicol. 2019, 19, 191–197.
- Pinson, A.; Yarbrough, A.L.; Bush, J.M.; Cabanlong, C.V.; Shoeib, A.; Jackson, B.K.; Fukuda, S.; Gogoi, J.; Fantegrossi, W.E.; McCain, K.; et al. Metabolism, CB1 cannabinoid receptor binding and in vivo activity of synthetic cannabinoid 5f-akb48: Implications for toxicity. Pharmacol. Biochem. Behav. 2020, 195, 172949.
- Hutchison, R.D.; Ford, B.M.; Franks, L.N.; Wilson, C.D.; Yarbrough, A.L.; Fujiwara, R.; Su, M.K.; Fernandez, D.; James, L.P.; Moran, J.H.; et al. Atypical pharmacodynamic properties and metabolic profile of the abused synthetic cannabinoid AB-PINACA: Potential contribution to pronounced adverse effects relative to Δ9-THC. Front. Pharmacol. 2018, 9, 1084.
- Longworth, M.; Connor, M.; Banister, S.D.; Kassiou, M. Synthesis and pharmacological profiling of the metabolites of synthetic cannabinoid drugs APICA, STS-135, ADB-PINACA, and 5F-ADB-PINACA. ACS Chem. Neurosci. 2017, 8, 1673–1680.
- Rajasekaran, M.; Brents, L.K.; Franks, L.N.; Moran, J.H.; Prather, P.L. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol. Appl. Pharm. 2013, 269, 100–108.
- Couceiro, J.; Bandarra, S.; Sultan, H.; Bell, S.; Constantino, S.; Quintas, A. Toxicological Impact of JWH-018 and its phase I metabolite N-(3-Hydroxypentyl) on human cell lines. Forensic Sci. Int. 2016, 264, 100–105.
- Robinson, L.; Goonawardena, A.V.; Pertwee, R.; Hampson, R.E.; Platt, B.; Riedel, G. WIN55,212-2 induced deficits in spatial learning are mediated by cholinergic hypofunction. Behav. Brain Res. 2010, 208, 584–592.
- Ossato, A.; Uccelli, L.; Bilel, S.; Canazza, I.; Domenico, G.D.; Pasquali, M.; Pupillo, G.; Luca, M.A.D.; Boschi, A.; Vincenzi, F.; et al. Psychostimulant effect of the synthetic cannabinoid JWH-018 and AKB48: Behavioral, neurochemical, and dopamine transporter scan imaging studies in mice. Front. Psychiatry 2017, 8, 130.
- Irie, T.; Kikura-Hanajiri, R.; Usami, M.; Uchiyama, N.; Goda, Y.; Sekino, Y. MAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar purkinje cells via activation of presynaptic CB1 receptors. Neuropharmacology 2015, 95, 479–491.
- Grassin-Delyle, S.; Naline, E.; Buenestado, A.; Faisy, C.; Alvarez, J.; Salvator, H.; Abrial, C.; Advenier, C.; Zemoura, L.; Devillier, P. Cannabinoids inhibit cholinergic contraction in human airways through prejunctional CB1 receptors. Brit. J. Pharmacol. 2014, 171, 2767–2777.
- Fantegrossi, W.E.; Wilson, C.D.; Berquist, M.D. Pro-psychotic effects of synthetic cannabinoids: Interactions with central dopamine, serotonin, and glutamate systems. Drug Metab. Rev. 2018, 50, 1–9.
- Singh, N.; Hroudová, J.; Fišar, Z. Cannabinoid-induced changes in the activity of electron transport chain complexes of brain mitochondria. J. Mol. Neurosci. 2015, 56, 926–931.
- Petrocellis, L.D.; Marzo, V.D. Non-CB1, Non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: Focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharm. 2010, 5, 103–121.
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2019, 11, 487.
- Yano, H.; Adhikari, P.; Naing, S.; Hoffman, A.F.; Baumann, M.H.; Lupica, C.R.; Shi, L. Positive allosteric modulation of the 5-HT 1A receptor by indole-based synthetic cannabinoids abused by humans. ACS Chem. Neurosci. 2020, 11, 1400–1405.
- Elmore, J.S.; Baumann, M.H. Repeated exposure to the “spice” cannabinoid JWH-018 induces tolerance and enhances responsiveness to 5-HT1A receptor stimulation in male rats. Front. Psychiatry 2018, 9, 55.
- Shi, B.; Yang, R.; Wang, X.; Liu, H.; Zou, L.; Hu, X.; Wu, J.; Zou, A.; Liu, L. Inhibition of 5-HT3 receptors-activated currents by cannabinoids in rat trigeminal ganglion neurons. J. Huazhong Univ. Sci. Technol. Med Sci. 2012, 32, 265–271.
- Linsen, F.; Koning, R.P.J.; Laar, M.; Niesink, R.J.M.; Koeter, M.W.; Brunt, T.M. 4-Fluoroamphetamine in The Netherlands: More than a one-night stand. Addiction 2015, 110, 1138–1143.
- Nelson, M.E.; Bryant, S.M.; Aks, S.E. Emerging drugs of abuse. Emerg. Med. Clin. N. Am. 2014, 32, 1–28.
- Costa, J.L.; Cunha, K.F.; Lanaro, R.; Cunha, R.L.; Walther, D.; Baumann, M.H. Analytical quantification, intoxication case series, and pharmacological mechanism of action for N-ethylnorpentylone (N-ethylpentylone or Ephylone). Drug Test Anal. 2019, 11, 461–471.
- Karch, S. Cathinone neurotoxicity (“The “3Ms”). Curr. Neuropharmacol. 2015, 13, 21–25.
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath Salts and Synthetic Cathinones: An Emerging Designer Drug Phenomenon. Life Sci. 2014, 97, 2–8.
- Sitte, H.H.; Freissmuth, M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol. Sci. 2015, 36, 41–50.
- Simmons, S.J.; Gregg, R.A.; Tran, F.H.; Mo, L.; Weltin, E.; Barker, D.J.; Gentile, T.A.; Watterson, L.R.; Rawls, S.M.; Muschamp, J.W. Comparing rewarding and reinforcing properties between ‘Bath Salt’ 3,4-methylenedioxypyrovalerone (MDPV) and Cocaine using ultrasonic vocalizations in rats. Addict. Biol. 2018, 23, 102–110.
- De-Giorgio, F.; Bilel, S.; Ossato, A.; Tirri, M.; Arfè, R.; Foti, F.; Serpelloni, G.; Frisoni, P.; Neri, M.; Marti, M. Acute and repeated administration of MDPV increases aggressive behavior in mice: Forensic implications. Int J. Leg. Med. 2019, 133, 1797–1808.
- Altun, B.; Çok, İ. Psychoactive bath salts and neurotoxicity risk. Turk. J. Pharm Sci. 2020, 17, 235–241.
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem. Pharmacol. 2014, 88, 237–244.
- Liechti, M. Novel psychoactive substances (designer drugs): Overview and pharmacology of modulators of monoamine signaling. Swiss Med. Wkly. 2015, 145, w14043.
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of synthetic cathinones. Handb. Exp. Pharmacol. 2018, 113–142.
- Aarde, S.M.; Taffe, M.A. Predicting the abuse liability of entactogen-class, new and emerging psychoactive substances via preclinical models of drug self-administration. Curr. Top. Behav. Neurosci. 2017, 145–164.
- Gannon, B.M.; Galindo, K.I.; Mesmin, M.P.; Sulima, A.; Rice, K.C.; Collins, G.T. Relative reinforcing effects of second-generation synthetic Cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 2018, 134, 28–35.
- Javadi-Paydar, M.; Nguyen, J.D.; Vandewater, S.A.; Dickerson, T.J.; Taffe, M.A. Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology 2018, 134, 57–64.
- Wee, S.; Anderson, K.G.; Baumann, M.H.; Rothman, R.B.; Blough, B.E.; Woolverton, W.L. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J. Pharmacol. Exp. Ther. 2005, 313, 848–854.
- Wee, S.; Woolverton, W.L. Self-Administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol. Biochem. Behav. 2006, 84, 337–343.
- Suyama, J.A.; Banks, M.L.; Negus, S.S. Effects of repeated treatment with methcathinone, mephedrone, and fenfluramine on intracranial self-stimulation in rats. Psychopharmacology 2019, 236, 1057–1066.
- Rickli, A.; Kolaczynska, K.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of the aminorex analogs 4-MAR, 4,4′-DMAR, and 3,4-DMAR. Neurotoxicology 2019, 72, 95–100.
- Zona, L.C.; Grecco, G.G.; Sprague, J.E. Cooling down the bath salts: Carvedilol attenuation of methylone and mephedrone mediated hyperthermia. Toxicol. Lett. 2016, 263, 11–15.
- Silva, B.; Fernandes, C.; de Pinho, P.G.; Remião, F. Chiral resolution and enantioselectivity of synthetic cathinones: A brief review. J. Anal. Toxicol. 2017, 42, 17–24.
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.; Huwyler, J.; Chaboz, S.; Hoener, M.; Liechti, M. Pharmacological characterization of designer cathinones in vitro. Brit. J. Pharmacol. 2013, 168, 458–470.
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160.
- Fleckenstein, A.E.; Volz, T.J.; Riddle, E.L.; Gibb, J.W.; Hanson, G.R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698.
- Partilla, J.S.; Dempsey, A.G.; Nagpal, A.S.; Blough, B.E.; Baumann, M.H.; Rothman, R.B. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 2006, 319, 237–246.
- Dolder, P.C.; Perna, E.B.S.F.; Mason, N.L.; Hutten, N.R.P.W.; Toennes, S.W.; Theunissen, E.L.; Ramaekers, J.G.; Kuypers, K.P.C. Independent elevation of peripheral oxytocin concentrations and reduction in cognitive empathy during 4-fluoroamphetamine intoxication. Hum. Psychopharmacol. Clin. Exp. 2018, 33, e2680.
- Brandt, S.D.; Baumann, M.H.; Partilla, J.S.; Kavanagh, P.V.; Power, J.D.; Talbot, B.; Twamley, B.; Mahony, O.; O’Brien, J.; Elliott, S.P.; et al. Characterization of a novel and potentially lethal designer drug (±)-cis-para-methyl-4-methylaminorex (4,4’-DMAR, or ‘Serotoni’). Drug Test Anal. 2014, 6, 684–695.
- McLaughlin, G.; Morris, N.; Kavanagh, P.V.; Power, J.D.; Dowling, G.; Twamley, B.; O’Brien, J.; Talbot, B.; Walther, D.; Partilla, J.S.; et al. Synthesis, characterization and monoamine transporter activity of the new psychoactive substance mexedrone and Its N-methoxy Positional Isomer, N-methoxymephedrone. Drug Test Anal. 2017, 9, 358–368.
- Davidson, C.; Raby, C.A.R.; Barrese, V.; Ramsey, J. In vitro neurochemical assessment of methylphenidate and its “legal high” analogs 3,4-CTMP and ethylphenidate in rat nucleus accumbens and bed nucleus of the stria terminalis. Front. Psychiatry 2018, 9, 149.
- Deutsch, H.M.; Shi, Q.; Gruszecka-Kowalik, E.; Schweri, M.M. Synthesis and pharmacology of potential cocaine antagonists. 2. structure−activity relationship studies of aromatic ring-substituted methylphenidate analogs. J. Med. Chem. 1996, 39, 1201–1209.
- Luethi, D.; Hoener, M.C.; Liechti, M.E. Effects of the new psychoactive substances diclofensine, diphenidine, and methoxphenidine on monoaminergic systems. Eur. J. Pharmacol. 2018, 819, 242–247.
- Dybdal-Hargreaves, N.F.; Holder, N.D.; Ottoson, P.E.; Sweeney, M.D.; Williams, T. Mephedrone: Public health risk, mechanisms of action, and behavioral effects. Eur. J. Pharmacol. 2013, 714, 32–40.
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013, 85, 1803–1815.
- Niello, M.; Cintulova, D.; Hellsberg, E.; Jäntsch, K.; Holy, M.; Ayatollahi, L.H.; Cozzi, N.V.; Freissmuth, M.; Sandtner, W.; Ecker, G.F.; et al. Para-trifluoromethyl-methcathinone is an allosteric modulator of the serotonin transporter. Neuropharmacology 2019, 161, 107615.
- Shokry, I.M.; Sinha, V.; Silva, G.D.; Park, S.; Callanan, J.J.; Tao, R. Comparison of Electroencephalogram (EEG) response to MDPV versus the hallucinogenic drugs MK-801 and ketamine in rats. Exp. Neurol. 2019, 313, 26–36.
- Schiavi, S.; Melancia, F.; Carbone, E.; Buzzelli, V.; Manduca, A.; Peinado, P.J.; Zwergel, C.; Mai, A.; Campolongo, P.; Vanderschuren, L.J.M.J.; et al. Detrimental effects of the ‘bath salt’ methylenedioxypyrovalerone on social play behavior in male rats. Neuropsychopharmacology 2020, 45, 2012–2019.
- Marusich, J.A.; Antonazzo, K.R.; Wiley, J.L.; Blough, B.E.; Partilla, J.S.; Baumann, M.H. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 2014, 87, 206–213.
- Wojcieszak, J.; Kuczyńska, K.; Zawilska, J.B. Four Synthetic Cathinones: 3-Chloromethcathinone, 4-chloromethcathinone, 4-fluoro-α-pyrrolidinopentiophenone, and 4-methoxy-α-pyrrolidinopentiophenone produce changes in the spontaneous locomotor activity and motor performance in mice with varied profiles. Neurotox. Res. 2020, 38, 536–551.
- Dolan, S.B.; Chen, Z.; Huang, R.; Gatch, M.B. “Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology 2018, 133, 171–180.
- Valente, M.J.; Bastos, M.d.L.; Fernandes, E.; Carvalho, F.; de Pinho, P.G.; Carvalho, M. Neurotoxicity of β-keto amphetamines: Deathly mechanisms elicited by methylone and MDPV in human dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859.
- Glennon, R.A.; Young, R. Neurobiology of 3,4-Methylenedioxypyrovalerone (MDPV) and α-Pyrrolidinovalerophenone (α-PVP). Brain Res. Bull. 2016, 126, 111–126.
- Huskinson, S.L.; Naylor, J.E.; Townsend, E.A.; Rowlett, J.K.; Blough, B.E.; Freeman, K.B. Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 2017, 234, 589–598.
- Chen, Y.; Canal, C.E. Structure–activity relationship study of psychostimulant synthetic cathinones reveals nanomolar antagonist potency of α-pyrrolidinohexiophenone at human muscarinic M 2 receptors. ACS Chem. Neurosci. 2020, 11, 960–968.
- Chen, Y.; Blough, B.E.; Murnane, K.S.; Canal, C.E. The synthetic cathinone psychostimulant A-PPP antagonizes serotonin 5-HT2A receptors: In vitro and in vivo evidence. Drug Test Anal. 2019, 11, 990–998.
- Rickli, A.; Kopf, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of novel psychoactive benzofurans. Br. J. Pharmacol. 2015, 172, 3412–3425.
- Dawson, P.; Opacka-Juffry, J.; Moffatt, J.D.; Daniju, Y.; Dutta, N.; Ramsey, J.; Davidson, C. The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2014, 48, 57–63.
- Iversen, L.; Gibbons, S.; Treble, R.; Setola, V.; Huang, X.-P.; Roth, B.L. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013, 700, 147–151.
- Marusich, J.A.; Antonazzo, K.R.; Blough, B.E.; Brandt, S.D.; Kavanagh, P.V.; Partilla, J.S.; Baumann, M.H. The new psychoactive substances 5-(2-Aminopropyl)Indole (5-IT) and 6-(2-Aminopropyl)Indole (6-IT) interact with monoamine transporters in brain tissue. Neuropharmacology 2016, 101, 68–75.
- Herraiz, T.; Brandt, S.D. 5-(2-Aminopropyl)Indole (5-IT): A psychoactive substance used for recreational purposes is an inhibitor of human monoamine oxidase (MAO). Drug Test Anal. 2014, 6, 607–613.
- Yoon, H.S.; Cai, W.T.; Lee, Y.H.; Park, K.T.; Lee, Y.S.; Kim, J.-H. The expression of methiopropamine-induced locomotor sensitization requires dopamine D2, but not D1, receptor activation in the rat. Behav. Brain Res. 2016, 311, 403–407.
- Halberstadt, A.L. Recent Advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015, 277, 99–120.
- Poulie, C.B.M.; Jensen, A.A.; Halberstadt, A.L.; Kristensen, J.L. DARK classics in chemical neuroscience: NBOMes. ACS Chem. Neurosci. 2019, 11, 3860–3869.
- Hur, K.-H.; Kim, S.-E.; Lee, B.-R.; Ko, Y.-H.; Seo, J.-Y.; Kim, S.-K.; Ma, S.-X.; Kim, Y.-J.; Jeong, Y.; Pham, D.T.; et al. 25C-NBF, a new psychoactive substance, has addictive and neurotoxic potential in rodents. Arch. Toxicol. 2020, 94, 2505–2516.
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; You, K.Y.; Kim, M.; Lee, H.J.; Yoo, S.Y.; Lee, K.W.; Lee, Y.S.; et al. 25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential. Addict. Biol. 2020, 25, e12850.
- Seo, J.-Y.; Hur, K.-H.; Ko, Y.-H.; Kim, K.; Lee, B.-R.; Kim, Y.-J.; Kim, S.-K.; Kim, S.-E.; Lee, Y.-S.; Kim, H.-C.; et al. A novel designer drug, 25N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents. Brain Res. Bull. 2019, 152, 19–26.
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; Kim, M.; Lee, H.J.; Ryu, H.W.; Lee, Y.S.; Kim, H.J.; Cheong, J.H. Two newly-emerging substituted phenethylamines MAL and BOD induce differential psychopharmacological effects in rodents. J. Psychopharmacol. 2020, 34, 1056–1067.
- Kang, H.; Park, P.; Bortolotto, Z.A.; Brandt, S.D.; Colestock, T.; Wallach, J.; Collingridge, G.L.; Lodge, D. Ephenidine: A new psychoactive agent with ketamine-like NMDA receptor antagonist properties. Neuropharmacology 2017, 112, 144–149.
More
