Optoacoustic or photoacoustic imaging (OAI/PAI) has the unique ability and scalability to visualize, monitor, and understand the molecular mechanisms of inflammation.
- optoacoustics
- photoacoustics
- imaging inflammation
- MSOT
- RSOM
- PAI
- acute inflammation
- chronic inflammation
- molecular imaging
1. Introduction
Optoacoustic imaging (OAI) encompasses a set of heterogenous optical imaging technologies utilizing the photoacoustic effect. Based on the observation of Alexander Graham Bell that there is formation of sound waves following light absorption [1], the principle has been rediscovered and used to develop imaging approaches [2]. In OAI, optoacoustic contrast arises when the light is absorbed by tissue molecules and converted into acoustic pressure waves, which can be recorded and formed into optoacoustic images [3].
Due to their unique absorption characteristics, endogenous molecules such as deoxyhemoglobin, oxyhemoglobin, collagen, lipids, and melanin enable OAI in the near-infrared (NIR) range [3][4] (Figure 1). In the future, targeted exogenous probes or contrast agents may further enrich the clinical capability of this technology by specifically labeling target molecules or cells for more personalized imaging approaches [1][5][6][7][8]. OAI is able to detect a broad range of endogenous and exogenous molecules within a single imaging modality whilst maintaining high spatial resolution at greater depths compared to optical imaging, due to the detection of ultrasound (US) waves, which scatter less in tissue than light [2][9]. The non-invasive nature of OAI will allow broad application to both experimental and clinical settings without interfering with biological processes.
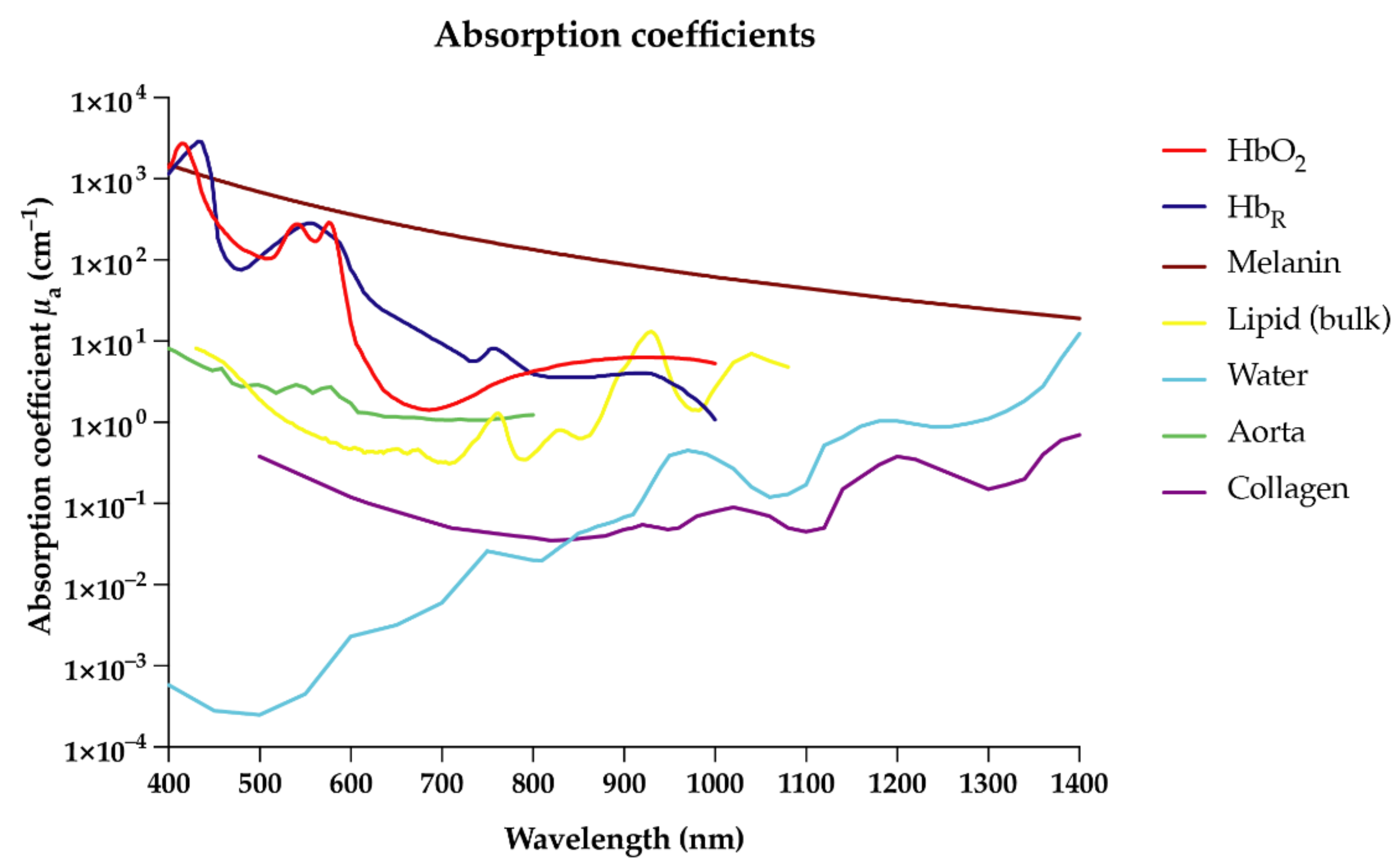
2. Optoacoustic Imaging of Inflammation: Applications
Table 2Table 1 gives an overview of selected studies.
Table 21. Selected inflammatory diseases studied with OAI. ICG = indocyanine green, NET = near-infrared erythrocyte-derived transducers, NP = nanoparticle, VEGF = Vascular endothelial growth factor, TNF α = tumor necrosis factor-α.
| Diseases | Condition | Stage | Target/Contrast | Reference |
|---|---|---|---|---|
| Cardiovascular | Atherosclerosis | Preclinical | Lipid | [18][19][20][21][22][23][24][25][26] |
| Atherosclerosis | Preclinical | CD36 targeted NP | [27] | |
| Atherosclerosis | Preclinical | ICG loaded NETs | [28] | |
| Atherosclerosis | Preclinical | Gold nanoparticles | [29] | |
| Foot vasculature | Clinical | Hemoglobin | [30] | |
| Vascular malformations | Clinical | Hemoglobin | [31] | |
| Carotid arteries | Clinical | Hemoglobin | [32] | |
| Peripheral artery disease | Clinical | Hemoglobin | [33] | |
| Dermatologic | Thermal injuries | Preclinical | Hemoglobin | [34][35][36] |
| LPS-induced wound inflammation | Preclinical | Hemoglobin | [37] | |
| Bacterial wound infection | Preclinical | Targeted sugars | [38][39] | |
| Skin microvasculature, layers | Clinical | Hemoglobin | [40][41] | |
| Psoriasis | Clinical | Hemoglobin | [42][43] | |
| Atopic dermatitis | Clinical | Hemoglobin | [44][45] | |
| Gastrointestinal | Acute liver damage | Preclinical | ICG Perfusion | [46] |
| Acute liver damage | Preclinical | Probes, NO, H2S and leucine aminopeptidase | [47][48][49] | |
| Liver fibrosis | Preclinical | Collagen | [50] | |
| Intestinal strictures | Preclinical | Collagen | [51][52] | |
| Intestinal inflammation | Preclinical | Hemoglobin | [53][54] | |
| Image-guided surgery | Preclinical | Hemoglobin | [53] | |
| Intestinal vasculature and lymphatic vessels | Preclinical | Hemoglobin, Evans blue dye | [54] | |
| Intestinal inflammation | Clinical | Hemoglobin | [55][56] | |
| Musculoskeletal | Arthritis | Preclinical | NP: targeting L-selectin/P-selectin, TNFα, VEGF | [57][58][59] |
| Rheumatoid arthritis | Clinical | Hemoglobin | [60] | |
| Enthesitis | Clinical | Hemoglobin | [61] | |
| Systemic sclerosis | Clinical | Hemoglobin | [31][62] | |
| Neurodegenerative | Alzheimer’s diseases | Preclinical | CDnir7 | [63] |
| Cerebrovascular damage | Preclinical | Hemoglobin | [64] | |
| Muscular dystrophy | Clinical | Collagen | [65] | |
| Kidney | Organ perfusion | Preclinical | ICG Perfusion | [66] |
| Acute injury | Preclinical | NP | [67] | |
| Organ transplant | Clinical | Collagen | [68] | |
| Gynecologic | Preeclampsia | Preclinical | Hemoglobin | [69][70] |
| Preeclampsia | Preclinical | ICG targeting FRα | [71] |
Limitations of OAI
Despite its remarkable potential to image inflammation in a clinical setting, OAI has several limitations. Until now, penetration depths have still been limited to a few centimeters (up to ~7 cm), which limits the imaging of organs at depth, especially in patients with a high body mass index (BMI). However, not only increased body fat, but also the natural heterogenous fat distribution in different genders is a limiting factor [72]. OA endoscopy approaches may bypass this depth limitation in some scenarios. In addition, patients with increased hair or darker skin (skin type 4 to 6 on the Fitzpatrick scale) present a challenge due to the high level of light absorption by melanin, which will significantly reduce the light energy (fluence) reaching deeper tissue. Comparable problems are seen in animals with melanin expression (e.g., C57BL6 and others), limiting options for in vivo animal models with intact immune systems. The problem of wavelength- and depth-dependent decreases in light fluence distribution becomes more pronounced in deep tissues, causing errors in estimating blood oxygen saturation differences [73] in particular. Correcting for spectral distortions of illumination light as it passes through tissue would allow the absolute quantification of optoacoustic molecules, but it remains a significant challenge to apply in vivo and hence is an active area of research in the field [73][74].
A topic currently under investigation is the standardization of OAI measurements, especially in quantitative measurements for clinical applications [75][76]. Even though there is evidence for repeatable and stable OAI measurements over time [72], their use is still far from clinical “routine”. To aid clinical translation, the clinician would also require more intuitive approaches to interpret optoacoustic images, preferably together with sufficient familiar anatomical information. Therefore, a hybrid imaging approach together with co-registration of (high-resolution) B-mode US signals is highly important [77][78]. The use of exogenous contrast agents to specifically label target molecules should be conducted with caution, owing to the possible toxicities associated with their administration [79].
Finally, most of the clinical studies conducted to date have been observational and/or conducted on a limited number of patients. The studies have not impacted the clinical decision-making of the patient. Therefore, the true impact and benefits of using OAI in clinical inflammation imaging need to be assessed in multi-center prospective studies, of which there is one study currently underway in Europe (https://euphoria2020.eu, last access: 9 April 2021, ClinicalTrials.gov Identifier: NCT04456400).
3. Conclusions
OAI is an emerging non-invasive imaging modality which has already had significant impact on unravelling the pathophysiology of inflammation in preclinical and clinical scenarios. OAI provides a multi-scale perspective of inflammatory processes, monitoring both structural and functional aspects at various anatomical locations, resolutions, and imaging depths. Besides visualizing strong absorbing hemoglobin, and therefore vascular structure, future approaches will focus on novel endogenous, as well as exogenous, OA compounds.
OAI may be sensitive enough to detect even slight inflammatory changes in tissues, therefore enabling early or even preventive treatment strategies. Despite the first preclinical studies, OAI awaits the translation of novel contrast agents for imaging inflammatory cells or microorganisms [80]. This approach could also enable the targeted delivery of functionalized multimodal and theranostic compounds to abnormal tissues while sparing the other organ systems [81][82][83]. At the same time, a high signal intensity and a high degree of biodegradation is required when administered in humans [84].
References
- Manohar, S.; Razansky, D. Photoacoustics: A historical review. Adv. Opt. Photon. 2016, 8, 586–617.
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462.
- Ntziachristos, V.; Razansky, D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794.
- Tzoumas, S.; Deliolanis, N.; Morscher, S.; Ntziachristos, V. Unmixing Molecular Agents From Absorbing Tissue in Multispectral Optoacoustic Tomography. IEEE Trans. Med. Imaging 2014, 33, 48–60.
- Zeng, L.; Ma, G.; Lin, J.; Huang, P. Photoacoustic Probes for Molecular Detection: Recent Advances and Perspectives. Small 2018, 14, e1800782.
- Bayer, C.L.; Joshi, P.P.; Emelianov, S.Y. Photoacoustic imaging: A potential tool to detect early indicators of metastasis. Expert Rev. Med. Devices 2013, 10, 125–134.
- Zhang, C.; Kimura, R.; Abou-Elkacem, L.; Levi, J.; Xu, L.; Gambhir, S.S. A Cystine Knot Peptide Targeting Integrin alphavbeta6 for Photoacoustic and Fluorescence Imaging of Tumors in Living Subjects. J. Nucl. Med. 2016, 57, 1629–1634.
- De la Zerda, A.; Bodapati, S.; Teed, R.; May, S.Y.; Tabakman, S.M.; Liu, Z.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano 2012, 6, 4694–4701.
- Zackrisson, S.; van de Ven, S.; Gambhir, S.S. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014, 74, 979–1004.
- Jacques, S.L.; McAuliffe, D.J. The melanosome: Threshold temperature for explosive vaporization and internal absorption coefficient during pulsed laser irradiation. Photochem. Photobiol. 1991, 53, 769–775.
- Jacques, S.; Glickman, R.; Schwartz, J. Internal Absorption Coefficient and Threshold for Pulsed Laser Disruption of Melanosomes Isolated from Retinal Pigment Epithelium; SPIE: Bellingham, WA, USA, 1996; Volume 2681.
- Sliney, D.H.; Palmisano, W.A. The evaluation of laser hazards. Am. Ind. Hyg. Assoc. J. 1968, 29, 425–431.
- Goldman, L. The skin. Arch. Environ. Health 1969, 18, 434–436.
- Van Veen, R.L.P.; Sterenborg, H.J.C.M.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Determination of VIS-NIR absorption coefficients of mammalian fat, with time- and spatially resolved diffuse reflectance and transmission spectroscopy. In Proceedings of the Biomedical Topical Meeting, Miami Beach, FL, USA, 14 April 2004; p. SF4.
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-microm Wavelength Region. Appl. Opt. 1973, 12, 555–563.
- Oraevsky, A.A.; Jacques, S.L.; Pettit, G.H.; Saidi, I.S.; Tittel, F.K.; Henry, P.D. XeCl laser ablation of atherosclerotic aorta: Optical properties and energy pathways. Lasers Surg. Med. 1992, 12, 585–597.
- Sekar, S.K.; Bargigia, I.; Mora, A.D.; Taroni, P.; Ruggeri, A.; Tosi, A.; Pifferi, A.; Farina, A. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J. Biomed. Opt. 2017, 22, 15006.
- Wang, L.; Lei, P.; Wen, X.; Zhang, P.; Yang, S. Tapered fiber-based intravascular photoacoustic endoscopy for high-resolution and deep-penetration imaging of lipid-rich plaque. Opt. Express 2019, 27, 12832–12840.
- Iskander-Rizk, S.; Wu, M.; Springeling, G.; van Beusekom, H.M.M.; Mastik, F.; Te Lintel Hekkert, M.; Beurskens, R.; Hoogendoorn, A.; Hartman, E.M.J.; van der Steen, A.F.W.; et al. In vivo intravascular photoacoustic imaging of plaque lipid in coronary atherosclerosis. EuroIntervention 2019, 15, 452–456.
- Hui, J.; Cao, Y.; Zhang, Y.; Kole, A.; Wang, P.; Yu, G.; Eakins, G.; Sturek, M.; Chen, W.; Cheng, J.X. Real-time intravascular photoacoustic-ultrasound imaging of lipid-laden plaque in human coronary artery at 16 frames per second. Sci. Rep. 2017, 7, 1417.
- Cao, Y.; Hui, J.; Kole, A.; Wang, P.; Yu, Q.; Chen, W.; Sturek, M.; Cheng, J.X. High-sensitivity intravascular photoacoustic imaging of lipid-laden plaque with a collinear catheter design. Sci. Rep. 2016, 6, 25236.
- Sangha, G.S.; Phillips, E.H.; Goergen, C.J. In vivo photoacoustic lipid imaging in mice using the second near-infrared window. Biomed. Opt. Express 2017, 8, 736–742.
- Sangha, G.S.; Goergen, C.J. Label-free photoacoustic and ultrasound imaging for murine atherosclerosis characterization. APL Bioeng. 2020, 4, 026102.
- Sethuraman, S.; Amirian, J.H.; Litovsky, S.H.; Smalling, R.W.; Emelianov, S.Y. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques. Opt. Express 2008, 16, 3362–3367.
- Wang, B.; Karpiouk, A.; Yeager, D.; Amirian, J.; Litovsky, S.; Smalling, R.; Emelianov, S. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Opt. Lett. 2012, 37, 1244–1246.
- Wang, B.; Karpiouk, A.; Yeager, D.; Amirian, J.; Litovsky, S.; Smalling, R.; Emelianov, S. In vivo intravascular ultrasound-guided photoacoustic imaging of lipid in plaques using an animal model of atherosclerosis. Ultrasound Med. Biol. 2012, 38, 2098–2103.
- Xie, Z.; Yang, Y.; He, Y.; Shu, C.; Chen, D.; Zhang, J.; Chen, J.; Liu, C.; Sheng, Z.; Liu, H.; et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics 2020, 10, 4694–4704.
- Liu, Y.; Hanley, T.; Chen, H.; Long, S.R.; Gambhir, S.S.; Cheng, Z.; Wu, J.C.; Fakhri, G.E.; Anvari, B.; Zaman, R.T. Non-Invasive Photoacoustic Imaging of In Vivo Mice with Erythrocyte Derived Optical Nanoparticles to Detect CAD/MI. Sci. Rep. 2020, 10, 5983.
- Wang, B.; Yantsen, E.; Larson, T.; Karpiouk, A.B.; Sethuraman, S.; Su, J.L.; Sokolov, K.; Emelianov, S.Y. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2009, 9, 2212–2217.
- Taruttis, A.; Timmermans, A.C.; Wouters, P.C.; Kacprowicz, M.; van Dam, G.M.; Ntziachristos, V. Optoacoustic Imaging of Human Vasculature: Feasibility by Using a Handheld Probe. Radiology 2016, 281, 256–263.
- Masthoff, M.; Helfen, A.; Claussen, J.; Roll, W.; Karlas, A.; Becker, H.; Gabriels, G.; Riess, J.; Heindel, W.; Schafers, M.; et al. Multispectral optoacoustic tomography of systemic sclerosis. J. Biophotonics 2018, 11, e201800155.
- Ivankovic, I.; Mercep, E.; Schmedt, C.G.; Dean-Ben, X.L.; Razansky, D. Real-time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 181325.
- Karlas, A.; Fasoula, N.A.; Paul-Yuan, K.; Reber, J.; Kallmayer, M.; Bozhko, D.; Seeger, M.; Eckstein, H.H.; Wildgruber, M.; Ntziachristos, V. Cardiovascular optoacoustics: From mice to men—A review. Photoacoustics 2019, 14, 19–30.
- Ida, T.; Iwazaki, H.; Kawaguchi, Y.; Kawauchi, S.; Ohkura, T.; Iwaya, K.; Tsuda, H.; Saitoh, D.; Sato, S.; Iwai, T. Burn depth assessments by photoacoustic imaging and laser Doppler imaging. Wound Repair Regen. 2016, 24, 349–355.
- Vionnet, L.; Gateau, J.; Schwarz, M.; Buehler, A.; Ermolayev, V.; Ntziachristos, V. 24-MHz scanner for optoacoustic imaging of skin and burn. IEEE Trans. Med. Imaging 2014, 33, 535–545.
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Imaging acute thermal burns by photoacoustic microscopy. J. Biomed. Opt. 2006, 11, 054033.
- Guo, Z.; Li, Z.; Deng, Y.; Chen, S.L. Photoacoustic microscopy for evaluating a lipopolysaccharide-induced inflammation model in mice. J. Biophotonics 2019, 12, e201800251.
- Ning, X.; Lee, S.; Wang, Z.; Kim, D.; Stubblefield, B.; Gilbert, E.; Murthy, N. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat. Mater. 2011, 10, 602–607.
- Zlitni, A.; Gowrishankar, G.; Steinberg, I.; Haywood, T.; Sam Gambhir, S. Maltotriose-based probes for fluorescence and photoacoustic imaging of bacterial infections. Nat. Commun. 2020, 11, 1250.
- Aguirre, J.; Hindelang, B.; Berezhnoi, A.; Darsow, U.; Lauffer, F.; Eyerich, K.; Biedermann, T.; Ntziachristos, V. Assessing nailfold microvascular structure with ultra-wideband raster-scan optoacoustic mesoscopy. Photoacoustics 2018, 10, 31–37.
- Aguirre, J.; Schwarz, M.; Soliman, D.; Buehler, A.; Omar, M.; Ntziachristos, V. Broadband mesoscopic optoacoustic tomography reveals skin layers. Opt. Lett. 2014, 39, 6297–6300.
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994.
- Aguirre, J.; Schwarz, M.; Garzorz, N.; Omar, M.; Buehler, A.; Eyerich, K.; Ntziachristos, V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017, 1, 0068.
- Yew, Y.W.; Dinish, U.S.; Yu Kuan, A.H.; Li, X.; Dev, K.; Ebrahim Attia, A.B.; Bi, R.; Moothanchery, M.; Balasundaram, G.; Aguirre, J.; et al. Raster-scanning optoacoustic mesoscopy (RSOM) imaging as an objective disease severity tool in atopic dermatitis patients. J. Am. Acad. Dermatol. 2020, 84, 1121–1123.
- Yew, Y.W.; Dinish, U.S.; Choi, E.C.E.; Bi, R.; Ho, C.J.H.; Dev, K.; Li, X.; Attia, A.B.E.; Wong, M.K.W.; Balasundaram, G.; et al. Investigation of morphological, vascular and biochemical changes in the skin of an atopic dermatitis (AD) patient in response to dupilumab using raster scanning optoacoustic mesoscopy (RSOM) and handheld confocal Raman spectroscopy (CRS). J. Dermatol. Sci. 2019, 95, 123–125.
- Brillant, N.; Elmasry, M.; Burton, N.C.; Rodriguez, J.M.; Sharkey, J.W.; Fenwick, S.; Poptani, H.; Kitteringham, N.R.; Goldring, C.E.; Kipar, A.; et al. Dynamic and accurate assessment of acetaminophen-induced hepatotoxicity by integrated photoacoustic imaging and mechanistic biomarkers in vivo. Toxicol. Appl. Pharm. 2017, 332, 64–74.
- Wu, Y.; Sun, L.; Zeng, F.; Wu, S. A conjugated-polymer-based ratiometric nanoprobe for evaluating in-vivo hepatotoxicity induced by herbal medicine via MSOT imaging. Photoacoustics 2019, 13, 6–17.
- Sun, L.; Wu, Y.; Chen, J.; Zhong, J.; Zeng, F.; Wu, S. A Turn-On Optoacoustic Probe for Imaging Metformin-Induced Upregulation of Hepatic Hydrogen Sulfide and Subsequent Liver Injury. Theranostics 2019, 9, 77–89.
- Huang, Y.; Qi, Y.; Zhan, C.; Zeng, F.; Wu, S. Diagnosing Drug-Induced Liver Injury by Multispectral Optoacoustic Tomography and Fluorescence Imaging Using a Leucine-Aminopeptidase-Activated Probe. Anal. Chem. 2019, 91, 8085–8092.
- Van den Berg, P.J.; Bansal, R.; Daoudi, K.; Steenbergen, W.; Prakash, J. Preclinical detection of liver fibrosis using dual-modality photoacoustic/ultrasound system. Biomed. Opt. Express 2016, 7, 5081–5091.
- Zhu, Y.; Johnson, L.A.; Huang, Z.; Rubin, J.M.; Yuan, J.; Lei, H.; Ni, J.; Wang, X.; Higgins, P.D.R.; Xu, G. Identifying intestinal fibrosis and inflammation by spectroscopic photoacoustic imaging: An animal study. Biomed. Opt. Express 2018, 9, 1590–1600.
- Lei, H.; Johnson, L.A.; Liu, S.; Moons, D.S.; Ma, T.; Zhou, Q.; Rice, M.D.; Ni, J.; Wang, X.; Higgins, P.D.; et al. Characterizing intestinal inflammation and fibrosis in Crohn’s disease by photoacoustic imaging: Feasibility study. Biomed. Opt. Express 2016, 7, 2837–2848.
- Kempski, K.M.; Wiacek, A.; Graham, M.; Gonzalez, E.; Goodson, B.; Allman, D.; Palmer, J.; Hou, H.; Beck, S.; He, J.; et al. In vivo photoacoustic imaging of major blood vessels in the pancreas and liver during surgery. J. Biomed. Opt. 2019, 24, 1–12.
- Yang, J.M.; Favazza, C.; Chen, R.; Yao, J.; Cai, X.; Maslov, K.; Zhou, Q.; Shung, K.K.; Wang, L.V. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 2012, 18, 1297–1302.
- Knieling, F.; Neufert, C.; Hartmann, A.; Claussen, J.; Urich, A.; Egger, C.; Vetter, M.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity. N. Engl. J. Med. 2017, 376, 1292–1294.
- Waldner, M.J.; Knieling, F.; Egger, C.; Morscher, S.; Claussen, J.; Vetter, M.; Kielisch, C.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity. Gastroenterology 2016, 151, 238–240.
- Beziere, N.; von Schacky, C.; Kosanke, Y.; Kimm, M.; Nunes, A.; Licha, K.; Aichler, M.; Walch, A.; Rummeny, E.J.; Ntziachristos, V.; et al. Optoacoustic imaging and staging of inflammation in a murine model of arthritis. Arthritis Rheumatol. 2014, 66, 2071–2078.
- Fournelle, M.; Bost, W.; Tarner, I.H.; Lehmberg, T.; Weiß, E.; Lemor, R.; Dinser, R. Antitumor necrosis factor-α antibody-coupled gold nanorods as nanoprobes for molecular optoacoustic imaging in arthritis. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 346–354.
- Zhao, C.; Zhang, R.; Luo, Y.; Liu, S.; Tang, T.; Yang, F.; Zhu, L.; He, X.; Yang, M.; Jiang, Y. Multimodal VEGF-Targeted Contrast-Enhanced Ultrasound and Photoacoustic Imaging of Rats with Inflammatory Arthritis: Using Dye-VEGF-Antibody-Loaded Microbubbles. Ultrasound Med. Biol. 2020, 46, 2400–2411.
- Hallasch, S.; Giese, N.; Stoffels, I.; Klode, J.; Sondermann, W. Multispectral optoacoustic tomography might be a helpful tool for noninvasive early diagnosis of psoriatic arthritis. Photoacoustics 2021, 21, 100225.
- Jo, J.; Xu, G.; Schiopu, E.; Chamberland, D.; Gandikota, G.; Wang, X. Imaging of enthesitis by an LED-based photoacoustic system. J. Biomed. Opt. 2020, 25.
- Daoudi, K.; Kersten, B.E.; van den Ende, C.H.M.; van den Hoogen, F.H.J.; Vonk, M.C.; de Korte, C.L. Photoacoustic and high-frequency ultrasound imaging of systemic sclerosis patients. Arthritis Res. 2021, 23, 22.
- Park, S.J.; Ho, C.J.H.; Arai, S.; Samanta, A.; Olivo, M.; Chang, Y.T. Visualizing Alzheimer’s Disease Mouse Brain with Multispectral Optoacoustic Tomography using a Fluorescent probe, CDnir7. Sci. Rep. 2019, 9, 12052.
- Ichkova, A.; Rodriguez-Grande, B.; Zub, E.; Saudi, A.; Fournier, M.L.; Aussudre, J.; Sicard, P.; Obenaus, A.; Marchi, N.; Badaut, J. Early cerebrovascular and long-term neurological modifications ensue following juvenile mild traumatic brain injury in male mice. Neurobiol. Dis. 2020, 141, 104952.
- Regensburger, A.P.; Fonteyne, L.M.; Jungert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 1905–1915.
- Buehler, A.; Herzog, E.; Razansky, D.; Ntziachristos, V. Video rate optoacoustic tomography of mouse kidney perfusion. Opt. Lett. 2010, 35, 2475–2477.
- Pan, W.; Peng, W.; Ning, F.; Zhang, Y.; Wang, Y.; Xie, W.; Zhang, J.; Xin, H.; Li, C.; Zhang, X. Non-invasive detection of the early phase of kidney injury by photoacoustic/computed tomography imaging. Nanotechnology 2018, 29, 265101.
- Hysi, E.; He, X.; Fadhel, M.N.; Zhang, T.; Krizova, A.; Ordon, M.; Farcas, M.; Pace, K.T.; Mintsopoulos, V.; Lee, W.L.; et al. Photoacoustic imaging of kidney fibrosis for assessing pretransplant organ quality. JCI Insight 2020, 5.
- Lawrence, D.J.; Escott, M.E.; Myers, L.; Intapad, S.; Lindsey, S.H.; Bayer, C.L. Spectral photoacoustic imaging to estimate in vivo placental oxygenation during preeclampsia. Sci. Rep. 2019, 9, 558.
- Yamaleyeva, L.M.; Brosnihan, K.B.; Smith, L.M.; Sun, Y. Preclinical Ultrasound-Guided Photoacoustic Imaging of the Placenta in Normal and Pathologic Pregnancy. Mol. Imaging 2018, 17, 1536012118802721.
- Huda, K.; Wu, C.; Sider, J.G.; Bayer, C.L. Spherical-view photoacoustic tomography for monitoring in vivo placental function. Photoacoustics 2020, 20, 100209.
- Wagner, A.L.; Danko, V.; Federle, A.; Klett, D.; Simon, D.; Heiss, R.; Jungert, J.; Uder, M.; Schett, G.; Neurath, M.F.; et al. Precision of handheld multispectral optoacoustic tomography for muscle imaging. Photoacoustics 2021, 21, 100220.
- Cox, B.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 061202.
- Brochu, F.M.; Brunker, J.; Joseph, J.; Tomaszewski, M.R.; Morscher, S.; Bohndiek, S.E. Towards Quantitative Evaluation of Tissue Absorption Coefficients Using Light Fluence Correction in Optoacoustic Tomography. IEEE Trans. Med. Imaging 2017, 36, 322–331.
- Joseph, J.; Tomaszewski, M.R.; Quiros-Gonzalez, I.; Weber, J.; Brunker, J.; Bohndiek, S.E. Evaluation of Precision in Optoacoustic Tomography for Preclinical Imaging in Living Subjects. J. Nucl. Med. 2017, 58, 807–814.
- Bohndiek, S. Addressing photoacoustics standards. Nat. Photonics 2019, 13, 298.
- Mercep, E.; Jeng, G.; Morscher, S.; Li, P.C.; Razansky, D. Hybrid optoacoustic tomography and pulse-echo ultrasonography using concave arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1651–1661.
- Mercep, E.; Dean-Ben, X.L.; Razansky, D. Combined Pulse-Echo Ultrasound and Multispectral Optoacoustic Tomography With a Multi-Segment Detector Array. IEEE Trans. Med. Imaging 2017, 36, 2129–2137.
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376.
- Conjusteau, A.; Liopo, A.; Tsyboulski, D.; Ermilov, S.; Elliott, W.; Barsalou, N.; Maswadi, S.; Glickman, R.; Oraevsky, A. Optoacoustic Sensor for Nanoparticle Linked Immunosorbent Assay (NanoLISA); SPIE: Bellingham, WA, USA, 2011; Volume 7899.
- Longo, D.L.; Stefania, R.; Aime, S.; Oraevsky, A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. Int. J. Mol. Sci. 2017, 18, 1719.
- Liopo, A.; Oraevsky, A. Nanoparticles as Contrast Agents for Optoacoustic Imaging. In Nanotechnology for Biomedical Imaging and Diagnostics: From Nanoparticle Design to Clinical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 111–149.
- Hannah, A.; Luke, G.; Wilson, K.; Homan, K.; Emelianov, S. Indocyanine green-loaded photoacoustic nanodroplets: Dual contrast nanoconstructs for enhanced photoacoustic and ultrasound imaging. ACS Nano 2014, 8, 250–259.
- Jokerst, J.V.; Van de Sompel, D.; Bohndiek, S.E.; Gambhir, S.S. Cellulose Nanoparticles are a Biodegradable Photoacoustic Contrast Agent for Use in Living Mice. Photoacoustics 2014, 2, 119–127.
