Symptomatic treatments are available for Parkinson’s disease and Alzheimer’s disease. An unmet need is cure or disease modification. This review discusses possible reasons for negative clinical study outcomes on disease modification following promising positive findings from experimental research. It scrutinizes current research paradigms for disease modification with antibodies against pathological protein enrichment, such as α-synuclein, amyloid or tau, based on post mortem findings. Instead a more uniform regenerative and reparative therapeutic approach for chronic neurodegenerative disease entities is proposed with stimulation of an endogenously existing repair system, which acts independent of specific disease mechanisms. The repulsive guidance molecule A pathway is involved in the regulation of peripheral and central neuronal restoration. Therapeutic antagonism of repulsive guidance molecule A reverses neurodegeneration according to experimental outcomes in numerous disease models in rodents and monkeys. Antibodies against repulsive guidance molecule A exist. First clinical studies in neurological conditions with an acute onset are under way. Future clinical trials with these antibodies should initially focus on well characterized uniform cohorts of patients. The efficiency of repulsive guidance molecule A antagonism and associated stimulation of neurogenesis should be demonstrated with objective assessment tools to counteract dilution of therapeutic effects by subjectivity and heterogeneity of chronic disease entities. Such a research concept will hopefully enhance clinical test strategies and improve the future therapeutic armamentarium for chronic neurodegeneration
- neurodegeneration
- repulsive guidance molecule A
- neuroprotection
- repair
- oxidative stress
- apoptosis
- neurogenesis
1. Introduction
One of the main causes for disability in humans worldwide is onset of neurological disorders, such as stroke and chronic progressive neurodegenerative brain diseases (PND). The most prevalent PNDs are the idiopathic and genetic Parkinson’s disease entity (PD) and the complex of various dementia syndromes, mainly consisting of Alzheimer’s disease (AD), frontotemporal dementia (FTD), mixed dementia (MD) and vascular dementia (VD) [1][2][3]. They are characterized by a common pathophyiologic mechanism, which is aberrant protein aggregation. Well known neuropathological features are β-amyloid and tau-protein enrichment in AD and accumulation of misfolded a-synuclein in PD [4][5]. Incidence of these PNDs will further rise. As an example, estimates of PD prevalence showed a 2.4 fold rise in the last 30 years. Main reasons are an earlier diagnosis associated with better treatment quality and a general rise of human life expectancy [6]. Increased exposure to endogenous and exogenous toxins contributes to a slowly evolving neurodegeneration in the peripheral and central nervous system and accelerates the overall ageing process in a pathological PND related manner. Typical risk factors are pesticides or herbicides, paraquat, rotenone, various metals (i.e. iron, manganese, lead), gaseous compounds (such as carbon monoxide) and even viruses [7][8]. The rising number of dementia- and PD patients will increase the financial burden for health care systems worldwide. To date, it is far from clear, whether the current SARS-CoV-2 outbreak may cause PND like syndromes in the long run, similar to the observed symptomatic PD forms following the 1918 H5N1 influenza pandemic [9].
1.1. The current situation and unmet needs
Considerable research activities in the past 60 years have focused on symptomatic therapies for alleviation of PD. A success story was the introduction of the dopamine substitution concept. It alleviates motor and to a considerable extent associated non motor symptoms in PD since the 1960s [10][11]. At that time Levodopa (L-dopa) was initially applied in an appropriate dose. The introduction of L-dopa therapy was based on findings, that high dopamine levels exist in the basal ganglia and that the dopamine precursor L-dopa counteracts reserpine induced dopamine decrease and associated impaired motor behavior (for review: [12]). Similar to PD, non motor symptoms also gained more and more interest in recent years in dementia. A considerable overlap exists between mechanisms of disease progression between PD and dementia syndromes. Thus the former focus on the dopamine deficiency in PD, respectively the acetylcholine deficit in AD is superseded by a more wide spread view. It also considers the individual different decline of other neurotransmitter systems, like serotonine (5-HT) or norepinephrine [13][14] Generally, particularly AD and PD are related to each other, i.e. by signs of microglial activation and neuroinflammation, and even in terms of neuropathological abnormalities [15][16][17]. Similar therapeutic approaches are also employed. As an example, acetylcholine esterase inhibiting compounds and glutamate neurotransmission reducing drugs improve cognitive abilities not only in AD, MD and VD but also in PD plus dementia syndromes [18][19][20].
2. Pitfalls of translational concepts in clinical research
To date, extensive experimental and neuropathological research provided distinct and better insights and understanding of chronic neuronal and associated glial cell death. The predominant responsible and final mechanism cascades are well identified and described in detail [21]. Based on these findings, i.e. antiapoptotic, neuroprotective or oxidative stress reducing compounds, were successfully tested in experimental chronic neurodegenerative and inflammatory disease models [4][22][23][24] (Figure 1).
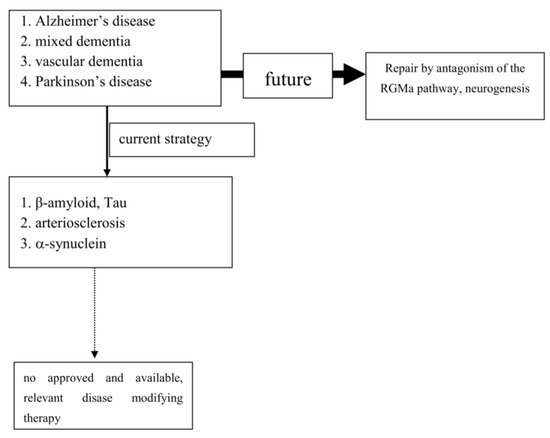
However, translation into positive clinical study results has failed so far, as trials on cure or disease modification in PNDs were more or less negative. Even transplantation of neurons or administration of neuronal growth factors was negative (as examples: [25][26]). Stimulation of growth factor synthesis, gene modification and stem cell applications are still discussed as promising tools [27][28][29][30][31][32][33][34][35]. The unmet need for disease modification, respectively repair regeneration for central nervous system disorders, still exists. The “Neuroplasticity” concept has been suggested to be responsible for the compensation of deleterious metabolic processes and the delayed occurrence of symptoms [36]. Another critical issue is the therapeutic mode of action, which is utilized for disease modification or cure. As an example, antibodies against pathological misfolded proteins were developed based on neuropathological findings. Enrichment of these altered proteins, i.e. in Lewy bodies (LB) or plaques, are looked upon as the main responsible and important, pathological phenomenon in chronic neurodegenerative brain disorders, such as AD or PD [37]. Failures within physiologic activities of protein metabolism may cause protein degradation and misfolding. However it is far from clear, whether these abnormalities represent a specific process, which is responsible for disease onset and progression [38]. This pathologic protein accumulation may also be the result of an unspecific side reaction of the metabolic cascade during chronic neurodegenerative processes. It may hypothetically only represent well wrapped protein garbage as consequence of physiologic defence mechanisms [38]. The extent of compensatory capacity, the triggering causes and the moment of initiation of these misfolded protein enrichments during the disease process are not known in detail. However there is consensus, that an essential clinical precondition for disease modifying therapeutic concepts is an early diagnosis, when the disease caused damage is low. To date, PD and Alzheimer’s disease (AD) are mostly diagnosed relatively late in the disease process due to the compensatory “neuroplasticity” phenomenon in the brain. A treatment allocation following earlier prodromal diagnostic screening will also probably reduce the current abundant missing motivation of PND-at-risk individuals for a testing procedure [39].
3. Conclusion
Cure or modification of progression in dementia, particularly AD, and PD is an important unmet need. Clinical trials, which aimed to translate promising experimental research outcomes into relevant positive results, were negative. No therapy has yet been approved despite well identified final main neuronal and related glial cell death mechanisms mostly on the cellular level. Multifactorial origins, heterogeneity of clinical symptoms, variability of each other complementing disease mechanisms and progression are the mostly likely reasons.
4. Outlook
Experimental research convincingly described a number of cellular pathways leading to chronic neuronal degeneration and death in PNDs. Examples are mitochondriopathy, dysfunction of the ubiquitin/proteasome system, oxidative and nitrosative stress, dysregulation of heat shock response, altered iron metabolism and vesicular transport systems, apoptosis, necrosis, autophagy, microglial activation combined with neuroinflammation [16][40][41]. Therapeutic concepts were and are developed based on findings, such as that the pro-inflammatory TNF-alpha cytokine is able to modify neuronal plasticity, maturation, and function of human cholinergic neurons also by epigenetic mechanisms [42]. All of these disease related alterations and their possible therapies will consequently change neurotransmission pathways [43]. However to date, preventive or PND progression delaying therapeutic strategies failed following translation into clinical study programs (as an example: [44]). Even the current clinical testing of specific antibodies against certain proteins, which accumulate in the various sporadic PNDs subtypes, may probably fail as shown in AD. This may suggest that the enrichment of these proteins is not specific. Their accumulation overlaps between various clinical PND pictures. This protein enrichment in LB may hypothetically only represent a defence mechanisms against the disease process itself, but do not cause it (Table 1; [45]). To date all clinical studies, which aimed to demonstrate neuroprotection or disease modification, i.e. in PD and AD, showed that research on a specific pathological disease mechanism does not lead to an essential therapeutic innovation in terms of disease course modification (Figure 1). Therefore, the underlying research method is worth to be considered for a modification. As an alternative to this misconception, one may consider the stimulation of an existing repair system in the peripheral and central nervous system as a more promising research paradigm [46][47][48][49][50][51]. Therapeutic strategies, which antagonize the repulsive guidance molecule A (RGMa) pathway, are worth for further development in clinical trials. A RGMa increase in the substantia nigra was found by in situ hybridization and immunohistochemistry in neuromelanin-positive neurons in post-mortem tissue of treated PD patients [47]. It may also be related to L-dopa administration and associated oxidative stress generation to a certain extent [52][53]. Extracellular RGMa inhibits axon regeneration and therefore may accelerate demise of neurons [54][55][56]. However targeting the RGMa pathway with antibodies or neutralisation, respectively antagonism of the neogenin receptor activity, may start regeneration not only in acute, but also in chronic inflammatory and neurodegenerative disorders [36][57][58][59][60] (Figure 1). It is well known, that considerable metabolic similarities exist both in the peripheral and central nervous system. Therefore, it is hypothesized that other syndromes than PD and AD, may also respond to this approach [49][51][55][61][62]. It may restore neuronal function in the long term as a general concept for repair and may weaken efficiency of toxin exposure [36][47][48][57][58][63][64][65]. Well designed clinical long term trials with RGMa antagonizing approaches are urgently needed in multiple sclerosis, PD, dementia syndromes, stroke, or spinal cord injury. Neuropathies (NP), diabetic retinopathy, Guillian Barre syndrome and amyotrophic lateral sclerosis are particularly suitable disorders. They allow testing of this approach in rather homogenous, well defined study cohorts with objective assessment tools, such as visual function and visual evoked potentials in retinopathy, or sensory or motor nerve conduction assessment in NP. RGMa antagonism may probably also help to counteract heterogeneous neurological deficits as consequence from severe viral infections, including SARS-CoV-2. Currently two different neutralizing RGMa antibodies (ABT-555; MT-3921) are in phase 2 clinical trials in spinal cord injury. In addition ABT-555 is in phase 2 clinical trials in progressive and relapse-remitting multiple sclerosis and in ischemic stroke. A positive outcome of these clinical trials will support this strategy for regeneration and repair in the damaged human nervous system. A further important pathological mechanism-of-action in PNDs is the potential inhibition of neurogenesis by the RGMa-neogenin pathway also in PD and dementia syndromes [46][49][51][66]. Neurogenesis also occurs in the adult human brain, i.e. in the dentate gyrus or the subventricular zone. RGMa blocks neurogenesis in these areas [55][67]. As shown in the hippocampal dentate gyrus, blocking of RGMa promoted formation of new neurons [46]. Targeting RGMa by antibodies may promote neurogenesis in the adult human brain of PND patients. An increased neurogenesis may also improve motor symptoms in PD or cognitive deficits in dementia. This is an alternative to cell replacement and stem cell concepts. Both have a focus on specific cell types only in contrast to the potential of RGMa antagonism in chronic neurodegeneration [68][28][69][70][71][72][73].
References
- Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018 Nov;17(11):939-53.
- Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019 Jan;18(1):88-106.
- Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020 Oct;5(10):e551-e567.
- Chaplot K, Jarvela TS, Lindberg I. Secreted Chaperones in Neurodegeneration. Front Aging Neurosci 2020;12:268.
- Gracia P, Camino JD, Volpicelli-Daley L, Cremades N. Multiplicity of alpha-Synuclein Aggregated Species and Their Possible Roles in Disease. Int J Mol Sci 2020 Oct 28;21(21).
- Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020 Feb 11;323(6):548-60.
- Ahmed H, Abushouk AI, Gabr M, Negida A, bdel-Daim MM. Parkinson's disease and pesticides: A meta-analysis of disease connection and genetic alterations. Biomed Pharmacother 2017 Jun;90:638-49.
- Liu X, Ma T, Qu B, Ji Y, Liu Z. Pesticide-induced gene mutations and Parkinson disease risk: a meta-analysis. Genet Test Mol Biomarkers 2013 Nov;17(11):826-32.
- Riederer P, Ter Meulen V. Coronaviruses: a challenge of today and a call for extended human postmortem brain analyses. J Neural Transm (Vienna ) 2020 Sep;127(9):1217-28.
- Birkmayer W, Hornykiewicz O. [The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. Wien Klin Wochenschr 1961 Nov 10;73:787-8.
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med 1969 Feb 13;280(7):337-45.
- Carlsson A. Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol Scand Suppl 1972;51:11-42.
- Gannon M, Che P, Chen Y, Jiao K, Roberson ED, Wang Q. Noradrenergic dysfunction in Alzheimer's disease. Front Neurosci 2015;9:220.
- Moll G, Gsell W, Wichart I, Jellinger K, Riederer P. Cholinergic and monoaminergic neuromediator systems in DAT. Neuropathological and neurochemical findings. In: Maurer K, Riederer P, Beckmann H, editors. Alzheimer's Disease. Epidemiology, Neuropathology, Neurochemistry, and Clinics. Vienna: Springer; 1990. p. 235-43.
- Gilhus NE, Deuschl G. Neuroinflammation - a common thread in neurological disorders. Nat Rev Neurol 2019 Aug;15(8):429-30.
- Hirsch EC, Standaert DG. Ten Unsolved Questions About Neuroinflammation in Parkinson's Disease. Mov Disord 2021 Jan;36(1):16-24.
- Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol 2018 Jan;25(1):59-70.
- Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer's disease. Neuropharmacology 2020 Oct 6;108352.
- Koola MM. Galantamine-Memantine combination in the treatment of Alzheimer's disease and beyond. Psychiatry Res 2020 Nov;293:113409.
- Petrazzuoli F, Vinker S, Palmqvist S, Midlov P, Lepeleire J, Pirani A, et al. Unburdening dementia - a basic social process grounded theory based on a primary care physician survey from 25 countries. Scand J Prim Health Care 2020 Sep;38(3):253-64.
- Toricelli M, Pereira AAR, Souza AG, Malerba HN, Maia J, Buck HS, et al. Mechanisms of neuroplasticity and brain degeneration: strategies for protection during the aging process. Neural Regen Res 2021 Jan;16(1):58-67.
- Boonman Z, Isacson O. Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp Neurol 1999 Mar;156(1):1-15.
- Demicheva E, Cui YF, Bardwell P, Barghorn S, Kron M, Meyer AH, et al. Targeting repulsive guidance molecule A to promote regeneration and neuroprotection in multiple sclerosis. Cell Rep 2015 Mar 24;10(11):1887-98.
- Saitoh Y, Takahashi Y. Riluzole for the treatment of amyotrophic lateral sclerosis. Neurodegener Dis Manag 2020 Dec;10(6):343-55.
- Gross RE, Watts RL, Hauser RA, Bakay RA, Reichmann H, von KR, et al. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol 2011 Jun;10(6):509-19.
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 2006 Mar;59(3):459-66.
- Guarnieri G, Sarchielli E, Vannelli GB, Morelli A. Cell-based therapy in Alzheimer's disease: Can human fetal cholinergic neurons "untangle the skein"? Neural Regen Res 2018 Dec;13(12):2105-7.
- Liu Z, Cheung HH. Stem Cell-Based Therapies for Parkinson Disease. Int J Mol Sci 2020 Oct 29;21(21).
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 2013 May;138(2):155-75.
- Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer's and Parkinson's diseases: implications for pathogenesis and therapy. Neural Regen Res 2017 Apr;12(4):549-57.
- Barker RA, Mason SL, Harrower TP, Swain RA, Ho AK, Sahakian BJ, et al. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington's disease. J Neurol Neurosurg Psychiatry 2013 Jun;84(6):657-65.
- Lige L, Zengmin T. Transplantation of Neural Precursor Cells in the Treatment of Parkinson Disease: An Efficacy and Safety Analysis. Turk Neurosurg 2016;26(3):378-83.
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003 Sep;54(3):403-14.
- Reddy AP, Ravichandran J, Carkaci-Salli N. Neural regeneration therapies for Alzheimer's and Parkinson's disease-related disorders. Biochim Biophys Acta Mol Basis Dis 2020 Apr 1;1866(4):165506.
- Russ K, Flores J, Brudek T, Doudet D. Neonatal human retinal pigment epithelial cells secrete limited trophic factors in vitro and in vivo following striatal implantation in parkinsonian rats. J Neural Transm (Vienna ) 2016 Mar;123(3):167-77.
- Mothe AJ, Coelho M, Huang L, Monnier PP, Cui YF, Mueller BK, et al. Delayed administration of the human anti-RGMa monoclonal antibody elezanumab promotes functional recovery including spontaneous voiding after spinal cord injury in rats. Neurobiol Dis 2020 Sep;143:104995.
- Emamzadeh FN, Surguchov A. Parkinson's Disease: Biomarkers, Treatment, and Risk Factors. Front Neurosci 2018;12:612.
- Sian-Hulsmann J, Monoranu C, Strobel S, Riederer P. Lewy Bodies: A Spectator or Salient Killer? CNS Neurol Disord Drug Targets 2015;14(7):947-55.
- Muller T. Investigational agents for the management of Huntington's disease. Expert Opin Investig Drugs 2017 Feb;26(2):175-85.
- Kreisl WC, Kim MJ, Coughlin JM, Henter ID, Owen DR, Innis RB. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol 2020 Nov;19(11):940-50.
- Kuhn W, Muller T, Nastos I, Poehlau D. The neuroimmune hypothesis in Parkinson's disease. Rev Neurosci 1997 Jan;8(1):29-34.
- Guarnieri G, Sarchielli E, Comeglio P, Herrera-Puerta E, Piaceri I, Nacmias B, et al. Tumor Necrosis Factor alpha Influences Phenotypic Plasticity and Promotes Epigenetic Changes in Human Basal Forebrain Cholinergic Neuroblasts. Int J Mol Sci 2020 Aug 25;21(17).
- Sian-Hulsmann J, Riederer P. The role of alpha-synuclein as ferrireductase in neurodegeneration associated with Parkinson's disease. J Neural Transm (Vienna ) 2020 May;127(5):749-54.
- Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 2014 May;71(5):543-52.
- Jellinger KA. Interaction between alpha-synuclein and other proteins in neurodegenerative disorders. ScientificWorldJournal 2011;11:1893-907.
- Isaksen TJ, Yamashita T. Repulsive Guidance Molecule A Regulates Adult Neurogenesis Via the Neogenin Receptor. Neurosci Insights 2020;15:2633105520948481.
- Korecka JA, Moloney EB, Eggers R, Hobo B, Scheffer S, Ras-Verloop N, et al. Repulsive Guidance Molecule a (RGMa) Induces Neuropathological and Behavioral Changes That Closely Resemble Parkinson's Disease. J Neurosci 2017 Sep 27;37(39):9361-79.
- Muller T, Barghorn S, Lutge S, Haas T, Mueller R, Gerlach B, et al. Decreased levels of repulsive guidance molecule A in association with beneficial effects of repeated intrathecal triamcinolone acetonide application in progressive multiple sclerosis patients. J Neural Transm (Vienna ) 2015 Jun;122(6):841-8.
- Oda W, Fujita Y, Baba K, Moshizuki H, Niwa H, Yamashita T. Inhibition of repulsive guidance molecule-a protects dopaminergic neurons in a mouse model of Parkinson's disease. Neurobiol Dis. In press 2021.
- Robinson RA, Griffiths SC, van de Haar LL, Malinauskas T, van Battum EY, Zelina P, et al. Simultaneous binding of Guidance Cues NET1 and RGM blocks extracellular NEO1 signaling. Cell 2021 Mar 11.
- Satoh J, Tabunoki H, Ishida T, Saito Y, Arima K. Accumulation of a repulsive axonal guidance molecule RGMa in amyloid plaques: a possible hallmark of regenerative failure in Alzheimer's disease brains. Neuropathol Appl Neurobiol 2013 Feb;39(2):109-20.
- Liedhegner EA, Steller KM, Mieyal JJ. Levodopa activates apoptosis signaling kinase 1 (ASK1) and promotes apoptosis in a neuronal model: implications for the treatment of Parkinson's disease. Chem Res Toxicol 2011 Oct 17;24(10):1644-52.
- Muller T, Trommer I, Muhlack S, Mueller BK. Levodopa increases oxidative stress and repulsive guidance molecule A levels: a pilot study in patients with Parkinson's disease. J Neural Transm (Vienna ) 2016 Feb 15;123(4):401-6.
- Babitt JL, Zhang Y, Samad TA, Xia Y, Tang J, Campagna JA, et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem 2005 Aug 19;280(33):29820-7.
- Key B, Lah GJ. Repulsive guidance molecule A (RGMa): a molecule for all seasons. Cell Adh Migr 2012 Mar;6(2):85-90.
- Malinauskas T, Peer TV, Bishop B, Mueller TD, Siebold C. Repulsive guidance molecules lock growth differentiation factor 5 in an inhibitory complex. Proc Natl Acad Sci U S A 2020 Jul 7;117(27):15620-31.
- Kubo T, Tokita S, Yamashita T. Repulsive guidance molecule-a and demyelination: implications for multiple sclerosis. J Neuroimmune Pharmacol 2012 Sep;7(3):524-8.
- Mothe AJ, Tassew NG, Shabanzadeh AP, Penheiro R, Vigouroux RJ, Huang L, et al. RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci Rep 2017 Sep 5;7(1):10529.
- Charish J, Shabanzadeh AP, Chen D, Mehlen P, Sethuramanujam S, Harada H, et al. Neogenin neutralization prevents photoreceptor loss in inherited retinal degeneration. J Clin Invest 2020 Apr 1;130(4):2054-68.
- Shabanzadeh AP, Tassew NG, Szydlowska K, Tymianski M, Banerjee P, Vigouroux RJ, et al. Uncoupling Neogenin association with lipid rafts promotes neuronal survival and functional recovery after stroke. Cell Death Dis 2015 May 7;6:e1744.
- Song M, Tian F, Xia H, Xie Y. Repulsive guidance molecule a suppresses seizures and mossy fiber sprouting via the FAKp120RasGAPRas signaling pathway. Mol Med Rep 2019 Apr;19(4):3255-62.
- Tanabe S, Yamashita T. Repulsive guidance molecule-a is involved in Th17-cell-induced neurodegeneration in autoimmune encephalomyelitis. Cell Rep 2014 Nov 20;9(4):1459-70.
- Chen J, Shifman MI. Inhibition of neogenin promotes neuronal survival and improved behavior recovery after spinal cord injury. Neuroscience 2019 Jun 1;408:430-47.
- Nakagawa H, Ninomiya T, Yamashita T, Takada M. Treatment With the Neutralizing Antibody Against Repulsive Guidance Molecule-a Promotes Recovery From Impaired Manual Dexterity in a Primate Model of Spinal Cord Injury. Cereb Cortex 2019 Feb 1;29(2):561-72.
- Yang W, Sun P. Promoting functions of microRNA-29a/199B in neurological recovery in rats with spinal cord injury through inhibition of the RGMA/STAT3 axis. J Orthop Surg Res 2020 Sep 18;15(1):427.
- Isaksen TJ, Fujita Y, Yamashita T. Repulsive Guidance Molecule A Suppresses Adult Neurogenesis. Stem Cell Reports 2020 Apr 14;14(4):677-91.
- Tian C, Shi H, Xiong S, Hu F, Xiong WC, Liu J. The neogenin/DCC homolog UNC-40 promotes BMP signaling via the RGM protein DRAG-1 in C. elegans. Development 2013 Oct;140(19):4070-80.
- Guarnieri G, Sarchielli E, Vannelli GB, Morelli A. Cell-based therapy in Alzheimer's disease: Can human fetal cholinergic neurons "untangle the skein"? Neural Regen Res 2018 Dec;13(12):2105-7.
- Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 2003 Jul;54(1):93-101.
- Schweyer K, Ruschoff-Steiner C, rias-Carrion O, Oertel WH, Rosler TW, Hoglinger GU. Neuronal precursor cells with dopaminergic commitment in the rostral migratory stream of the mouse. Sci Rep 2019 Sep 16;9(1):13359.
- Desplats P, Spencer B, Crews L, Pathel P, Morvinski-Friedmann D, Kosberg K, et al. alpha-Synuclein induces alterations in adult neurogenesis in Parkinson disease models via p53-mediated repression of Notch1. J Biol Chem 2012 Sep 14;287(38):31691-702.
- Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, et al. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci 2012 Nov 21;32(47):16906-16.
- Winner B, Marchetto MC, Winkler J, Gage FH. Human-induced pluripotent stem cells pave the road for a better understanding of motor neuron disease. Hum Mol Genet 2014 Sep 15;23(R1):R27-R34.
