Although homeostasis is a commonly accepted concept, there is incontrovertible evidence that biological processes and functions are variable, and that variability occurs in cycles. So allostatic model has emerged as the first challenge to homeostasis. Circadian variation is the predominant variation in the body. As there is strong scientific and clinical evidence that blood pressure fluctuations undergo circadian rhythm, there is equally strong evidence that targeted time therapy for hypertension provides a better outcome of the disease. The research has gone even further by ensuring better patients' adherence throughout the development and approval process for the use of pulsatile drug release systems which can be considered as an option for an even more convenient dosage regimen of the medicines needed.
- hypertension
- allostasis
- circadian rhythm
- chronotherapy
Since allostasis involves the achievement of stability through change, including the specific rhythms of various functions in the organism, this entry aimed to briefly introduce the circadian rhythm of blood pressure in health and disease and to present proven possibilities in the treatment of hypertension through the art of pulsatile drug release systems.
1. Circadian Blood Pressure Fluctuations
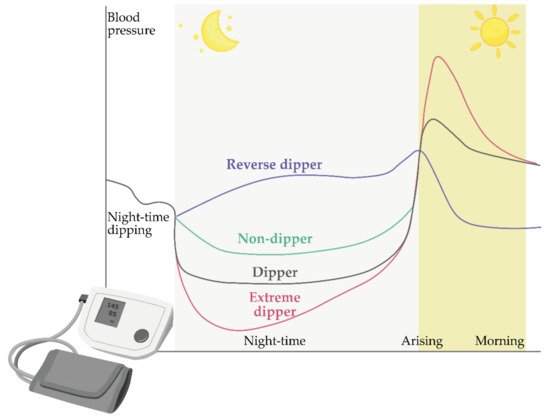
Models of Circadian Blood Pressure Fluctuations
2. Chronotherapy and Chronopharmaceutics
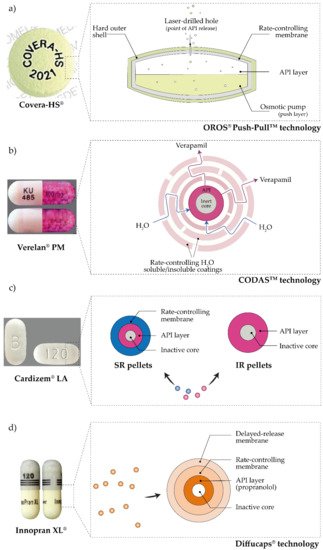
3. Pulsatile Antihypertensives Delivery Systems Approved for Use
References
- Hermida, R.C.; Ayala, D.E.; Portaluppi, F. Circadian variation of blood pressure: The basis for the chronotherapy of hypertension. Adv. Drug Deliv. Rev. 2007, 59, 904–922.
- Casagrande, M.; Favieri, F.; Langher, V.; Guarino, A.; Di Pace, E.; Germanò, G.; Forte, G. The night side of blood pressure: Nocturnal blood pressure dipping and emotional (dys)regulation. Int. J. Environ. Res. Public Health 2020, 17, 1–11.
- Pena-Hernandez, C.; Nugent, K.; Tuncel, M. Twenty-Four-Hour Ambulatory Blood Pressure Monitoring. J. Prim. Care Community Health 2020, 11.
- Rhoads, M.K.; Balagee, V.; Thomas, S.J. Circadian Regulation of Blood Pressure: Of Mice and Men. Curr. Hypertens. Rep. 2020, 22.
- Smolensky, M.H.; Hermida, R.C.; Portaluppi, F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med. Rev. 2017, 33, 4–16.
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; Fisher, S., Reason, J., Eds.; John Wiley & Sons: New York, NY, USA, 1988; pp. 629–649.
- James, G.D. Ambulatory blood pressure variation: Allostasis and adaptation. Auton. Neurosci. Basic Clin. 2013, 177, 87–94.
- James, G.D. Continuous Blood Pressure Variation: Hidden Adaptability. In Biological Measures of Human Experience across the Lifespan: Making Visible the Invisible; Sievert, L.L., Brown, D.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 143–169. ISBN 9783319441030.
- Modesti, P.A.; Morabito, M.; Bertolozzi, I.; Massetti, L.; Panci, G.; Lumachi, C.; Giglio, A.; Bilo, G.; Caldara, G.; Lonati, L.; et al. Weather-related changes in 24-hour blood pressure profile: Effects of age and implications for hypertension management. Hypertension 2006, 47, 155–161.
- James, G.D.; Gates, E.M.; Pickering, T.G.; Laragh, J.H. Parity and Perceived Job Stress Elevate Blood Pressure in Young Normotensive Working Women. Am. J. Hypertens. 1989, 2, 637–639.
- Gerin, W.; James, G.D. Psychosocial determinants of hypertension: Laboratory and field models. Blood Press. Monit. 2010, 15, 93–99.
- Van Berge-Landry, H.; James, G.D. Serum electrolyte, serum protein, serum fat and renal responses to a dietary sodium challenge: Allostasis and allostatic load. Ann. Hum. Biol. 2004, 31, 477–487.
- James, G.D.; Brown, D.E. The Biological Stress Response and Lifestyle: Catecholamines and Blood Pressure. Annu. Rev. Anthropol. 1997, 26, 313–335.
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Mojón, A.; Smolensky, M.H.; Fabbian, F.; Portaluppi, F. Administration-Time Differences in Effects of Hypertension Medications on Ambulatory Blood Pressure Regulation. Chronobiol. Int. 2013, 30, 280–314.
- Adobe Inc. Adobe Illustrator. Available online: (accessed on 11 March 2021).
- Chugh, A.R.; Loughran, J.H.; Slaughter, M.S. Circadian variations in blood pressure in health and disease: Implications for patient management. ChronoPhysiology Ther. 2011, 1, 17–31.
- Smolensky, M.H.; Siegel, R.A.; Haus, E.; Hermida, R.; Portaluppi, F. Biological rhythms, drug delivery, and chronotherapeutics. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, NY, USA, 2012; pp. 359–443. ISBN 9781461408819.
- Gowthami, B.; Krishna, S.V.G.; Rao, D.S. Application of coating technology to chronotherapeutic drug delivery systems: Recent publications and patents. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100015.
- Youan, B.B.C. Chronopharmaceutics; Youan, B.B.C., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470498392.
- Lemmer, B. The importance of biological rhythms in drug treatment of hypertension and sex-dependent modifications. ChronoPhysiology Ther. 2012, 2, 9.
- Hermida, R.C.; Ayala, D.E.; Smolensky, M.H.; Portaluppi, F. Chronotherapy in hypertensive patients: Administration-time dependent effects of treatment on blood pressure regulation. Expert Rev. Cardiovasc. Ther. 2007, 5, 463–475.
- Myburgh, D.P.; Verho, M.; Botes, J.H.; Erasmus, T.P.; Luus, H.G. 24-hour blood pressure control with ramipril: Comparison of once-daily morning and evening administration. Curr. Ther. Res. 1995, 56, 1298–1306.
- Palatini, P.; Racioppa, A.; Raule, G.; Zaninotto, M.; Penzo, M.; Pessina, A.C. Effect of timing of administration on the plasma ACE inhibitory activity and the antihypertensive effect of quinapril. Clin. Pharmacol. Ther. 1992, 52, 378–383.
- Witte, K.; Weisser, K.; Neubeck, M.; Mutschler, E.; Lehmann, K.; Hopf, R.; Lemmer, B. Cardiovascular effects, pharmacokinetics, and converting enzyme inhibition of enalapril after morning versus evening administration. Clin. Pharmacol. Ther. 1993, 54, 177–186.
- Hermida, R.C.; Ayala, D.E.; Calvo, C.; Portaluppi, F.; Smolensky, M.H. Chronotherapy of hypertension: Administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv. Drug Deliv. Rev. 2007, 59, 923–939.
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Fernández, J.R.; Covelo, M.; Mojón, A.; López, J.E. Treatment of non-dipper hypertension with bedtime administration of valsartan. J. Hypertens. 2005, 23, 1913–1922.
- Smolensky, M.H.; Hermida, R.C.; Portaluppi, F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiol. Int. 2007, 24, 171–181.
- Fukuda, M.; Yamanaka, T.; Mizuno, M.; Motokawa, M.; Shirasawa, Y.; Miyagi, S.; Nishio, T.; Yoshida, A.; Kimura, G. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J. Hypertens. 2008, 26, 583–588.
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Domínguez, M.J.; Covelo, M.; Fernández, J.R.; Fontao, M.J.; López, J.E. Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol. Int. 2004, 21, 277–296.
- Koga, H.; Hayashi, J.; Yamamoto, M.; Kitamoto, K. Prevention of morning surge of hypertension by the evening administration of carvedilol. Jpn. Med. Assoc. J. 2005, 48, 398–403.
- Hermida, R.C.; Calvo, C.; Ayala, D.; Rodríguez, M.; Chayán, L.; López, J. Administration time-dependent effects of nebivolol on the diurnal/nocturnal blood pressure ratio in hypertensive patients. J. Hypertens. 2006, 24, S89.
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Lopez, J.; Rodriguez, M.; Covelo, M. Administration time-dependent effects of amlodipine on ambulatory blood pressure in patients with essential hypertension. Am. J. Hypertens. 2005, 18, A61.
- Kitahara, Y.; Saito, F.; Akao, M.; Fujita, H.; Takahashi, A.; Taguchi, H.; Hino, T.; Otsuka, Y.; Kushiro, T.; Kanmatsuse, K. Effect of Morning and Bedtime Dosing with Cilnidipine on Blood Pressure, Heart Rate, and Sympathetic Nervous Activity in Essential Hypertensive Patients. J. Cardiovasc. Pharmacol. 2004, 43, 68–73.
- Kohno, I.; Iwasaki, H.; Okutani, M.; Mochizuki, Y.; Sano, S.; Satoh, Y.; Ishihara, T.; Ishii, H.; Mukaiyama, S.; Ijiri, H.; et al. Administration-time-dependent effects of diltiazem on the 24-hour blood pressure profile of essential hypertension patients. Chronobiol. Int. 1997, 14, 71–84.
- Portaluppi, F.; Vergnani, L.; Manfredini, R.; Degli Uberti, E.C.; Fersini, C. Time-dependent effect of isradipine on the nocturnal hypertension in chronic renal failure. Am. J. Hypertens. 1995, 8, 719–726.
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Alonso, I.; Fernández, J.R. Reduction of morning blood pressure surge after treatment with nifedipine GITS at bedtime, but not upon awakening, in essential hypertension. Blood Press. Monit. 2009, 14, 152–159.
- White, W.B.; Mansoor, G.A.; Pickering, T.G.; Vidt, D.G.; Hutchinson, H.G.; Johnson, R.B.; Noveck, R. Differential effects of morning and evening dosing of nisoldipine ER on circadian blood pressure and heart rate. Am. J. Hypertens. 1999, 12, 806–814.
- Meilhac, B.; Mallion, J.M.; Carre, A.; Chanudet, X.; Poggi, L.; Gosse, P.; Dallocchio, M. Study of the influence of the time of administration on the antihypertensive effect and nitrendipine tolerance in mild to moderate essential hypertensive patients. Value of ambulatory recording of blood pressure on 24 hours. Therapie 1992, 47, 205–210.
- Cutler, N.R.; Anders, R.J.; Jhee, S.S.; Sramek, J.J.; Awan, N.A.; Bultas, J.; Lahiri, A.; Woroszylska, M. Placebo-controlled evaluation of three doses of a controlled-onset, extended-release formulation of verapamil in the treatment of stable angina pectoris. Am. J. Cardiol. 1995, 75, 1102–1106.
- Frishman, W.H.; Glasser, S.; Stone, P.; Deedwania, P.C.; Johnson, M.; Fakouhi, T.D. Comparison of controlled-onset, extended-release verapamil with amlodipine and amlodipine plus atenolol on exercise performance and ambulatory ischemia in patients with chronic stable angina pectoris. Am. J. Cardiol. 1999, 83, 507–514.
- FDA; CDER. COVERA-HS ® (Verapamil Hydrochloride) Extended-Release Tablets Controlled-Onset; FDA: Silver Spring, MA, USA, 2011.
- Maroni, A.; Foppoli, A.; Palugan, L.; Gazzaniga, A. Drug Delivery: Pulsatile Systems. In Encyclopedia of Pharmaceutical Science and Technology; Swarbrick, J., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1173–1182.
- Davar, N.; Ghosh, S. Oral Controlled Release-Based Products for Life Cycle Management. In Oral Controlled Release Formulation Design and Drug Delivery; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 305–320.
