The defining characteristics of the traditional Sub-Saharan Africa (SSA) cuisine have been the richness in indigenous foods and ingredients, herbs and spices, fermented foods and beverages, and healthy and whole ingredients used. It is crucial to safeguard the recognized benefits of mainstream traditional foods and ingredients, which gradually eroded in the last decades. Notwithstanding poverty, chronic hunger, malnutrition, and undernourishment in the region, traditional eating habits have been related to positive health outcomes and sustainability. The research prevailed dealing with food availability and access rather than the health, nutrition, and diet quality dimensions of food security based on what people consume per country and on the missing data related to nutrient composition of indigenous foods. As countries become more economically developed, they shift to “modern” occidental foods rich in saturated fats, salt, sugar, fizzy beverages, and sweeteners. As a result, there are increased incidences of previously unreported ailments due to an unbalanced diet. Protein-rich foods in dietary guidelines enhance only those of animal or plant sources, while rich protein sources such as mushrooms have been absent in these charts, even in developed countries.
- food insecurity
- mushroom nutrition
- poverty
- health promotion
- health foods
1. Introduction
2. Anti-Inflammatory Role of Mushrooms
3. The Antiviral Role of Mushrooms
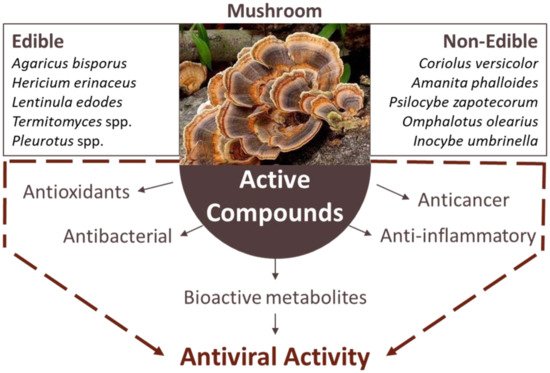
3.1. HIV/AIDS
3.2. Herpes Virus
3.3. Influenza Virus
3.4. Human Papillomaviruses (HPVs)
3.5. The Novel Coronavirus (SARS-CoV-2)
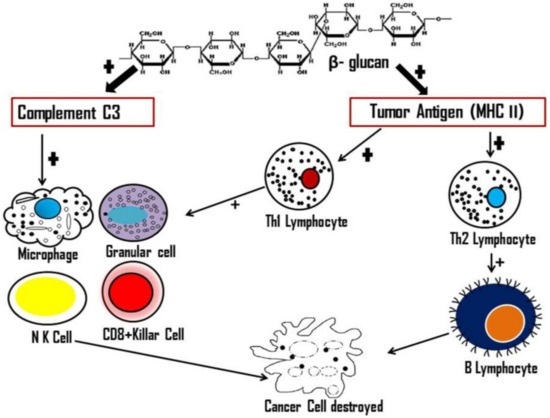
4. Antitumour Activity of Mushrooms
References
- Dodo, K.M. Understanding Africa’s Food Security Challenges. In Food Security in Africa; Mahmoud, B., Ed.; IntechOpen: London, UK, 8 April 2020.
- Raheem, D.; Dayoub, M.; Birech, R.; Nakiyemba, A. The Contribution of Cereal Grains to Food Security and Sustainability in Africa: Potential Application of UAV in Ghana, Nigeria, Uganda, and Namibia. Urban Sci. 2021, 5, 8.
- Gassner, A.; Harris, D.; Mausch, K.; Terheggen, A.; Lopes, C.; Finlayson, R.; Dobie, P. Poverty eradication and food security through agriculture in Africa: Rethinking objectives and entry points. Outlook Agric. 2019, 48, 309–315.
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488.
- Asongu, S.A.; Odhiambo, N.M. Environmental degradation and inclusive human development in sub-Saharan Africa. Sustain. Dev. 2019, 27, 25–34.
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724.
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, December 2020. Available online: (accessed on 14 January 2021).
- Hansen, M.E.B.; Rubel, M.A.; Bailey, A.G.; Ranciaro, A.; Thompson, S.R.; Campbell, M.C.; Beggs, W.; Dave, J.R.; Mokone, G.G.; Mpoloka, S.W.; et al. Population structure of human gut bacteria in a diverse cohort from rural Tanzania and Botswana. Genome Biol. 2019, 20, 16.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218.
- Punchard, N.A.; Whelan, C.J.; Adcock, I. The Journal of Inflammation. J. Inflamm. 2004, 1.
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012.
- Yu, S.; Weaver, V.; Martin, K.; Cantorna, M.T. The effects of whole mushrooms during inflammation. BMC Immunol. 2009, 10, 12.
- Zhang, P.; Sutheerawattananonda, M. Kinetic Models for Glucosamine Production by Acid Hydrolysis of Chitin in Five Mushrooms. Int. J. Chem. Eng. 2020, 2020, 1–8.
- Salazar, J.; Bello, L.; Chávez, M.; Añez, R.; Rojas, J.; Bermúdez, V. Glucosamine for Osteoarthritis: Biological Effects, Clinical Efficacy, and Safety on Glucose Metabolism. Arthritis 2014, 2014, 1–13.
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2019, 9, 184–198.
- Elkhateeb, W.; Daba, G.; Thomas, P.; Wen, T.-C. Medicinal mushrooms as a new source of natural therapeutic bioactive compounds. Egypt. Pharm. J. 2019, 18, 88–101.
- Nie, A.; Chao, Y.; Zhang, X.; Al, E. Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A. Wolf) Ryvarden & Gilb. Front. Pharmacol. 2020, 11, 505249.
- Staniszewska, J.; Szymański, M.; Ignatowicz, E. Antitumor and immunomodulatory activity of Inonotus obliquus. Herba Pol. 2017, 63, 48–58.
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Al, E. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634.
- Dicks, L.; Ellinger, S. Effect of the Intake of Oyster Mushrooms (Pleurotus ostreatus) on Cardiometabolic Parameters—A Systematic Review of Clinical Trials. Nutrients 2020, 12, 1134.
- Baeva, E.; Bleha, R.; Lavrova, E.; Al, E. Polysaccharides from Basidiocarps of Cultivating Mushroom Pleurotus ostreatus: Isolation and Structural Characterization. Molecules 2019, 24, 2740.
- Xiao, Z.; Zhou, W.; Zhang, Y. Fungal polysaccharides. Adv. Pharmacol. 2020, 87, 277–299.
- Vetvicka, V.; Vannucci, L.; Sima, P.; Al, E. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251.
- Huang, Q.; Li, L.; Chen, H.; Liu, Q.; Wang, Z. GPP (Composition of Ganoderma Lucidum Poly-saccharides and Polyporus Umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice. Nutrients 2019, 11, 1480.
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135.
- Ferreiro, E.; Pita, I.R.; Mota, S.I.; Valero, J.; Ferreira, N.R.; Fernandes, T.; Calabrese, V.; Fontes-Ribeiro, C.A.; Pereira, F.C.; Rego, A.C. Coriolus versicolor biomass increases dendritic arborization of newly-generated neurons in mouse hippocampal dentate gyrus. Oncotarget 2018, 9, 32929–32942.
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219.
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396.
- Cheah, I.K.; Halliwell, B. Could Ergothioneine Aid in the Treatment of Coronavirus Patients? Antioxidants 2020, 9, 595.
- Borodina, I.; Kenny, L.; McCarthy, C.; Al, E. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217.
- Seo, D.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350.
- Suwannarach, N.; Kumla, J.; Sujarit, K.; Pattananandecha, T.; Saenjum, C.; Lumyong, S. Natural Bioactive Compounds from Fungi as Potential Candidates for Protease Inhibitors and Immunomodulators to Apply for Coronaviruses. Molecules 2020, 25, 1800.
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. 2020, 63, 383–385.
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393.
- Roy, B.G. Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir. Chem. Chemother. 2017, 25, 20–52.
- Geller, A.; Yan, J. Could the Induction of Trained Immunity by β-Glucan Serve as a Defense Against COVID-19? Front. Immunol. 2020, 11.
- Lin, B.; Li, S. Cordyceps as an Herbal Drug. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011.
- Guidry, C.A.; Mansfield, S.A.; Sawyer, R.G.; Cook, C.H. Resistant Pathogens, Fungi, and Viruses. Surg. Clin. N. Am. 2014, 94, 1195–1218.
- Wang, S.; Welte, T.; Fang, H.; Chang, G.-J.J.; Born, W.K.; O’Brien, R.L.; Sun, B.; Fujii, H.; Kosuna, K.-I.; Wang, T. Oral Administration of Active Hexose Correlated Compound Enhances Host Resistance to West Nile Encephalitis in Mice. J. Nutr. 2009, 139, 598–602.
- Teplyakova, T.V.; Kosogova, T.A. Antiviral Effect of Agaricomycetes Mushrooms (Review). Int. J. Med. Mushrooms 2016, 18, 375–386.
- Chunchao, H.; Guo, J. A Hypothesis: Supplementation with Mushroom-Derived Active Compound Modulates Immunity and Increases Survival in Response to Influenza Virus (H1N1) Infection. Evid. Based Complement. Altern. Med. 2011, 2011, 1–3.
- He, M.; Su, D.; Liu, Q.; Gao, W.; Kang, Y. Mushroom lectin overcomes hepatitis B virus tolerance via TLR6 signaling. Sci. Rep. 2017, 7, 5814.
- Rodríguez-Valentín, M.; López, S.; Rivera, M.; Ríos-Olivares, E.; Cubano, L.; Boukli, N.M. Naturally Derived Anti-HIV Polysaccharide Peptide (PSP) Triggers a Toll-Like Receptor 4-Dependent Antiviral Immune Response. J. Immunol. Res. 2018, 2018, 1–14.
- Teplyakova, T.V.; Psurtseva, N.V.; Kosogova, T.A.; Mazurkova, N.A.; Khanin, V.A.; Vlasenko, V.A. Antiviral Activity of Polyporoid Mushrooms (Higher Basidiomycetes) from Altai Mountains (Russia). Int. J. Med. Mushrooms 2012, 14, 37–45.
- Foster, M.; Samman, S. Zinc and Regulation of Inflammatory Cytokines: Implications for Cardiometabolic Disease. Nutrients 2012, 4, 676–694.
- Jang, I.-S.; Ko, Y.-H.; Moon, Y.-S.; Sohn, S.-H. Effects of Vitamin C or E on the Pro-inflammatory Cytokines, Heat Shock Protein 70 and Antioxidant Status in Broiler Chicks under Summer Conditions. Asian Australas. J. Anim. Sci. 2014, 27, 749–756.
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778.
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five major mushrooms: Application to integrative oncology. Integr. Med. 2014, 13, 32–44.
- Fernandes, T.; Chaquisse, E.; Ferrão, J. HIV and the Antiviral Role of Mushroom Nutraceuticals. Adv. Image Video Process. 2020, 8, 64–100.
- World Health Organization. Essential Prevention and Care Interventions for Adults and Adolescents Living with HIV in Resource-Limited Settings/Coordinated by Kevin O’Reilly; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-159670-1. Available online: (accessed on 17 January 2021).
- Rose, A.M.; Hall, C.S.; Martinez-Alier, N. Aetiology and management of malnutrition in HIV-positive children. Arch. Dis. Child. 2014, 99, 546–551.
- Maldonado, S.; Fitzgerald-Bocarsly, P. Antifungal Activity of Plasmacytoid Dendritic Cells and the Impact of Chronic HIV Infection. Front. Immunol. 2017, 8.
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.-D. The Pharmacological Potential of Mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299.
- Guo, W.-L.; Guo, J.-B.; Liu, B.-Y.; Lu, J.-Q.; Chen, M.; Liu, B.; Bai, W.-D.; Rao, P.-F.; Ni, L.; Lv, X.-C. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833.
- Cao, F.-R.; Feng, L.; Ye, L.-H.; Wang, L.-S.; Xiao, B.-X.; Tao, X.; Chang, Q. Ganoderic Acid A Metabolites and Their Metabolic Kinetics. Front. Pharmacol. 2017, 8.
- Zhang, L.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19.
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645.
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526.
- Magden, J.; Kääriäinen, L.; Ahola, T. Inhibitors of virus replication: Recent developments and prospects. Appl. Microbiol. Biotechnol. 2005, 66, 612–621.
- Weber, F. Antiviral Innate Immunity: Introduction. Encycl. Virol. 2021, 577–583.
- Ellan, K.; Thayan, R.; Raman, J.; Hidari, K.I.P.J.; Ismail, N.; Sabaratnam, V. Anti-viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: An in-vitro study. BMC Complement. Altern. Med. 2019, 19, 260.
- Lederman, M.M.; Margolis, L. The lymph node in HIV pathogenesis. Semin. Immunol. 2008, 20, 187–195.
- Jeong, Y.-U.; Park, Y.-J. Ergosterol Peroxide from the Medicinal Mushroom Ganoderma lucidum Inhibits Differentiation and Lipid Accumulation of 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2020, 21, 460.
- Cagno, V.; Tintori, C.; Civra, A.; Cavalli, R.; Tiberi, M.; Botta, L.; Brai, A.; Poli, G.; Tapparel, C.; Lembo, D.; et al. Novel broad spectrum virucidal molecules against enveloped viruses. PLoS ONE 2018, 13, e0208333.
- Raut, J.K. Mushroom: A potent source of natural antiviral drugs. Appl. Sci. Technol. Ann. 2020, 1, 81–91.
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.E.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329.
- Looker, K.J.; Welton, N.J.; Sabin, K.M.; Dalal, S.; Vickerman, P.; Turner, K.M.E.; Boily, M.-C.; Gottlieb, S.L. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: A population attributable fraction analysis using published epidemiological data. Lancet Infect. Dis. 2020, 20, 240–249.
- Mangold, C.A.; Szpara, M.L. Persistent Infection with Herpes Simplex Virus 1 and Alzheimer’s Disease—A Call to Study How Variability in Both Virus and Host may Impact Disease. Viruses 2019, 11, 966.
- Duarte, L.F.; Farías, M.A.; Álvarez, D.M.; Bueno, S.M.; Riedel, C.A.; González, P.A. Herpes Simplex Virus Type 1 Infection of the Central Nervous System: Insights Into Proposed Interrelationships With Neurodegenerative Disorders. Front. Cell. Neurosci. 2019, 13.
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59.
- Vlasenko, V.; Vlasenko, A. Antiviral activity of fungi of the Novosibirsk Region: Pleurotus ostreatus and P. pulmonarius (Review). BIO Web Conf. 2018, 11, 00044.
- Elkhateeb, W.; Elnahas, M.; Thomas, P. Fomes fomentarius and Polyporus squamosus Models of Marvel Medicinal Mushrooms. Biomed. Res. Rev. 2020, 3, 119.
- Eguchi, N.; Fujino, K.; Thanasut, K.; Taharaguchi, M.; Motoi, M.; Motoi, A.; Taharaguchi, S. In vitro Anti-Influenza Virus Activity of Agaricus brasiliensis KA21. Biocontrol Sci. 2017, 22, 171–174.
- Couto, J.S.; Silva, D.P. Coriolus versicolor supplementation in HPV patients. In Proceedings of the 20th European Congress of Obstetrics and Gynaecology, Lisbon, Portugal, 4–8 March 2008; Available online: (accessed on 15 February 2021).
- Smith, J.A.; Mathew, L.; Gaikwad, A.; Rech, B.; Burney, M.N.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. From Bench to Bedside: Evaluation of AHCC Supplementation to Modulate the Host Immunity to Clear High-Risk Human Papillomavirus Infections. Front. Oncol. 2019, 9.
- Gincheva, D.; Gincheva, V.; Konova, E. Effect of combined therapy polyhexamethylene biguanide and Coriolus-MRL on human papilloma virus (HPV) cervical and vulvar-related lesions. IJMDAT 2020, 3, e220.
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12, 2573.
- Kaymakci, M.; Guler, E. Promising Potential Pharmaceuticals from the Genus Cordyceps for COVID-19 Treatment: A Review Study. Bezmialem Sci. 2020, 8, 140–144.
- Hetland, G.; Johnson, E.; Bernardshaw, S.; Grinde, B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID–19 and its pneumonic superinfection and complicating inflammation? Scand J. Immunol. 2021, 93, e12937.
- Al-jumaili, M.; Al-dulaimi, F.; Ajeel, M. The Role of Ganoderma lucidum Uptake on Some Hematological and Immunological Response in Patients with Coronavirus (COVID-19). Sys. Rev. Pharm. 2020, 11, 537–541.
- USA Unsubstantiated Claims for Coronavirus Prevention and Treatment—Carlin Creative Concepts LLC. Federal Trade Commission. Published 2020. Available online: (accessed on 23 November 2020).
- Lam, C.; Cheng, L.; Zhou, L.; Al, E. Herb-drug interactions between the medicinal mushrooms Lingzhi and Yunzhi and cytotoxic anticancer drugs: A systematic review. Chin. Med. 2020, 15, 75.
- Ngai, P.H.K.; Ng, T.B. A hemolysin from the mushroom Pleurotus eryngii. Appl. Microbiol. Biotechnol. 2006, 72, 1185–1191.
- Matuszewska, A.; Stefaniuk, D.; Jaszek, M.; Pięt, M.; Zając, A.; Matuszewski, Ł.; Cios, I.; Grąz, M.; Paduch, R.; Bancerz, R. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci. Rep. 2019, 9, 1975.
- Chan, W.Y.; Ng, T.B.; Lam, J.S.Y.; Wong, J.H.; Chu, K.T.; Ngai, P.H.K.; Lam, S.K.; Wang, H.X. The mushroom ribosome-inactivating protein lyophyllin exerts deleterious effects on mouse embryonic development in vitro. Appl. Microbiol. Biotechnol. 2010, 85, 985–993.
- Ivanova, T.S.; Krupodorova, T.A.; Barshteyn, V.Y.; Artamonova, A.B.; Shlyakhovenko, V.A. Anticancer substances of mushroom origin. Exp. Oncol. 2014, 36, 58–66.
- Cappadocia, L.; Lima, C.D. Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism. Chem. Rev. 2018, 118, 889–918.
- Roca-Lema, D.; Martinez-Iglesias, O.; Portela, C.F.d.A.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In Vitro Anti-proliferative and Anti-invasive Effect of Polysaccharide-rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 2019, 16, 231–240.
- Jakopovic, B.; Oršoli’c, N.; Paveli´c, S. Antitumor, Immunomodulatory and Antiangiogenic Efficacy of Medicinal Mushroom Extract Mixtures in Advanced Colorectal Cancer Animal Model. Molecules 2020, 25, 5005.
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008.
- Yamanaka, D.; Takatsu, K.; Kimura, M.; Swamydas, M.; Ohnishi, H.; Umeyama, T.; Oyama, F.; Lionakis, M.S.; Ohno, N. Development of a novel β-1,6-glucan–specific detection system using functionally-modified recombinant endo-β-1,6-glucanase. J. Biol. Chem. 2020, 295, 5362–5376.
- Han, B.; Baruah, K.; Cox, E.; Al, E. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658.
- Jameel, G.H.; AL-Ezzy, A.I.A.; Mohammed, I.H. Immunomodulatory, Apoptosis Induction and Antitumor Activities of Aqueous and Methanolic Extract of Calvatia Craniiformis in Mice Transfected with Murine Hepatocellular Carcinoma Cells. Open Access Maced. J. Med. Sci. 2018, 6, 1206–1214.
- Bryant, J.M.; Bouchard, M.; Haque, A. Anticancer Activity of Ganoderic Acid DM: Current Status and Future Perspective. J. Clin. Cell. Immunol. 2017, 8.
- Wang, X.-Y.; Zhang, D.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59, S96–S115.
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274.
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703.
- Del Cornò, M.; Gessani, S.; Conti, L. Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers 2020, 12, 155.
