Polysaccharide-based materials created by physical processes have received considerable attention for biomedical applications.
- growth factors
- scaffolds
- antimicrobial coatings
1. Introduction
Polysaccharides have hydrophilic functional groups (charged groups, as well as hydrogen bond donors and acceptors) that can stabilize macromolecular assemblies. Polysaccharide assembly can also be achieved via electrostatic crosslinking using small-molecule or metal counterions, and through cooling and freezing–thawing of polysaccharide-based mixtures. These assemblies include polyelectrolyte complexes (PECs), polyelectrolyte multilayers (PEMs), coacervates, and hydrogels. PECs are assemblies mainly formed from the electrostatic complexation in solution of oppositely charged polyelectrolytes. The resulting complexes may remain highly hydrated, and are therefore often characterized as hydrogels or coacervates as well. Coacervates are the result of a liquid–liquid phase separation, resulting in a polysaccharide-rich (liquid) phase that remains hydrated and is suspended in an (aqueous) solution. Coacervates and PECs often have polydisperse size distributions. Hydrogels are hydrophilic condensed (solid) networks of macromolecules, which are capable of absorbing large amounts of water (greater than 90% by weight). Whether formed by coacervation or gelation, the result is often three-dimensionally structured nano- or microparticles. Polysaccharides can also be assembled through various film-forming, fiber-spinning, and phase-separation methods. Films are often obtained by solvent evaporation method or through the layer-by-layer assembly of PEMs [1,2,3][1][2][3]. The formation of polysaccharide PECs, coacervates, hydrogels, fibers, and films is generally achieved at relatively mild conditions; however, processing conditions used for any of these assembly methods (e.g., solution pH, concentration, temperature, and ionic strength) can greatly influence the resulting material structure and properties.
Polysaccharide-based materials have been used as wound dressings, drug delivery systems (DDSs), scaffolds, and coatings for tissue-engineering purposes [4,5][4][5]. Polysaccharides are attractive materials for these applications due to their cytocompatibility, biodegradability, high bioavailability, and natural abundance [5]. Many polysaccharides also exhibit antimicrobial, antimycotic, anti-adhesive, anticoagulant or procoagulant, and wound-healing properties. They have hydrophilic groups (carboxylic acids, amino, hydroxyl, and sulfate groups) in their structures that support bio-adhesion through non-covalent bonds toward biological tissues and growth factors (GFs) [6]. Some polysaccharides naturally occur in the extracellular matrix, and play important roles in binding proteins, cells, and tissues.
2. Principal Polysaccharides Used for Biomedical Materials
Polysaccharides can be chemically stable, pH-responsive, and thermosensitive. These properties, combined with their chemical and biochemical functionality, gelling properties, and structural similarity to extracellular matrix components make them excellent candidate materials for use in biological systems. Here, we highlight the properties of glycosaminoglycans (GAGs) [7], alginate [8], chitosan [9], carrageenans, ulvan, fucoidan [7], and polysaccharide derivatives (especially sulfated materials [10]). Polyanionic polysaccharides (GAGs and marine polysaccharides) have often been used to develop DDSs for cationic GFs [11] and surface coatings. Cationic polysaccharides (chitosan and its derivatives) comprise DDSs for anionic GFs [12], surface coatings, wound dressings, and scaffolds with antimicrobial properties. Our principal focus is on charged polyelectrolytes (polyanionic and polycationic polysaccharides) because these can mainly interact through electrostatic interactions, forming durable assemblies (physical materials) for biomedical applications. Moreover, we focus on cellulose (a neutral polysaccharide), because it is the most abundant polysaccharide in the world. It provides nanocrystalline structures that improve the mechanical properties of polysaccharide-based materials, and bacterial cellulose is attracting significant attention for biomedical applications.
2.1. Glycosaminoglycans (GAGs)
GAGs are linear anionic polysaccharides mainly composed of disaccharide units containing a hexuronic acid (glucuronic acid or iduronic acid) and a hexosamine (glucosamine, or galactosamine). GAGs comprise complicated chemical structures, distinguished by their specific disaccharide repeat sequences, glycosidic bonds, and substituents (O-sulfates, N-sulfonates, and N-acetyl groups). They are present in many human and animal tissues, and are obtained commercially from the tissues of pigs, poultry, sharks, and reptiles. GAGs molecular masses mainly depend on the extraction method and source. They include sulfated polymers, such as heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, and keratan sulfate [7,13,14][7][13][14] (Figure 1). Hyaluronic acid (often called hyaluronan) is the only non-sulfated GAG.
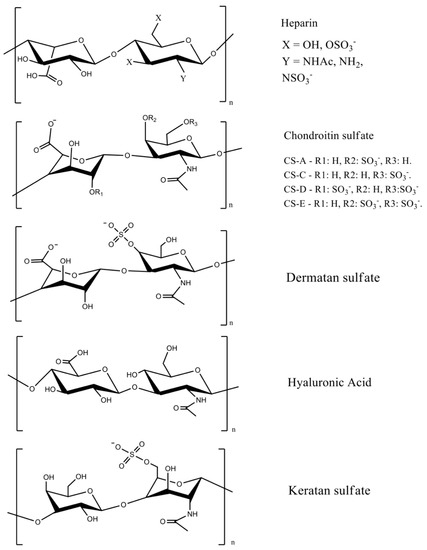
Figure 1. GAG chemical structures. Adapted with permission from [7,15][7][15]; published by Elsevier 2019 and Wiley, 2020. CS = chondroitin sulfate, and the letters A, B, C, D, and E represent different types of chondroitin sulfates.
The most important GAGs for biomedical applications are heparin, chondroitin sulfate, and hyaluronic acid, because they are abundant extracellular membrane components. Heparin has a linear chain consisting of an alternating sulfated uronic acid and d-glucosamine units linked by α- and β bonds (1→4). The uronic acid can be l-iduronic or d-glucuronic acid, while the d-glucosamine is N-sulfated or N-acetylated. The l-iduronic acid is sulfated at the C2 position, and the d-glucosamine unit is N- and 6-O sulfated. l-Iduronic acid corresponds to approximately 85% of the uronic acid content, and d-glucuronic acid comprises 15% [7,16][7][16].
Chondroitin sulfate is composed of repeating β-1,3-linked N-acetyl galactosamine and β-1,4-linked d-glucuronic acid disaccharide units [14,17][14][17]. The chemical structure depends on the sulfate groups’ positions on the pyranose ring and sulfation degree. It is often classified as chondroitin sulfate A, C, D, and E (Figure 1). Hyaluronic acid has the highest molecular mass among the GAGs, and it is composed of β-1,4-d-glucuronic acid and β-1,3-N-acetyl-d-glucosamine disaccharide units (Figure 1) [18]. The high molecular mass of hyaluronan imparts viscoelastic and bio-adhesive properties to materials [19,20][19][20]. It is a major component of the extracellular matrix of many tissues, including skin [21].
Sulfated disaccharides on GAGs containing sulfate and carboxylic groups have pKa values between 2.0 and 4.0 [16,22,23][16][22][23]; therefore, all the sulfated GAGs are ionized in water and biological fluids. The ionized sulfates are hydrophilic, making them water-soluble. Sulfated GAGs can strongly bind positively charged proteins [24]. The physicochemical and biochemical properties of GAGs (hydrophilicity, biocompatibility, biodegradability, and chemical cues that regulate major biological processes, including cell growth and differentiation) rely on their chemical structure, specific architecture, sulfation degree, molecular mass, and conformation in solution [21,25,26][21][25][26]. Low-molecular-weight heparin has anti-inflammatory properties and anticoagulant effects, whereas unfractionated heparin has less predictable and controllable biological properties [27].
The sulfated GAGs occur covalently end-grafted to proteins, forming three-dimensional bottlebrush structures called proteoglycans [28]. Proteoglycans are complex macromolecules found in cell membranes, the extracellular milieu, and intracellular granules. These structures can have different amino acid sequences, lengths, and different types and numbers of GAGs attached to their backbones [29]. Proteoglycans and their constituent GAGs are responsible for many biochemical functions of the extracellular matrix and cell membranes, including organizing the nano- and microstructure; enhancing tribological and mechanical properties; regulating the transport of oxygen and nutrients; restoring the structure and function of damaged tissues; providing microenvironments for cell survival [30]; and binding, stabilizing, and activating GFs to control signaling [31].
2.2. Chitin and Chitosan
Chitin is a linear polysaccharide mainly composed of β (1→4) units linked to N-acetyl-2-amino-2-deoxy-d-glucose residues found in fungi cell walls (Aspergillus niger, Penicillium chrysogenum, Penicillium notatum, and others), and in the exoskeletons of crustaceous (shrimps, lobster, krill, goose barnacle, and crabs), insects (cockroach, ladybird, butterfly, and others), algae (Phaeophyceae, Chlorophyceae, and others), and mollusks (cuttlefish, octopus, and squids) [32,33][32][33]. It occurs in different polymorphic forms (α, β, and γ-chitin). The α-chitin arranged in anti-parallel strands is the most stable and abundant form [34]. Chitin is biodegradable and mainly extracted from crustacean wastes that are byproducts of the food fishing industries, comprising an acetylated polymer with aqueous insolubility [33,35][33][35].
Chitin can be formulated into materials (films, beads, hydrogels, and fibers). However, these materials are mainly prepared in volatile organic solvents, ionic liquids, and NaOH/urea mixtures [36]. Residual traces of these solvents are potentially toxic for biomedical applications. Chitin deacetylation in aqueous alkaline solutions at 60–80 °C creates the partially acetylated chitin derivatives [32,35][32][35]. Deacetylation degrees higher than 50% are referred to as chitosan, which are random copolymers of N-acetyl d-glucosamine and 2-amino-2-deoxy-β-d-glucosamine residues (Figure 2).
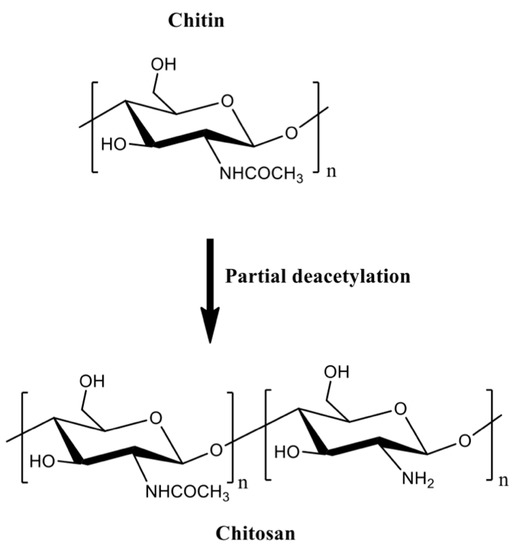
Figure 2.
The partial chitin deacetylation produces chitosans with deacetylation degrees higher than 50%.
Chitosan is a linear cationic polysaccharide with pendent amine groups. The amine moieties on chitosan are protonated at low pH, making chitosan soluble in dilute acidic aqueous solutions [37]. Chitosan advantages over chitin include enzymatic degradation [38], gelling properties [39[39][40],40], pH-responsiveness [41], mucoadhesion, ability to open epithelial tight junctions (due to its cationic behavior that enhances interactions with mucous membrane [42]), and antimicrobial activities [43]. Molar mass and acetylation degree significantly influence the processing of chitosan-based materials [32]. These properties affect the chitosan hydrophobicity, solubility, viscosity, rheological, and gelling features. Chitosan gelation temperature decreases from 75 to 30 and 25 °C when the deacetylation degree is 83, 94, and 96%, respectively. Higher loss modulus (G’’) indicates that the gelation of chitosan solutions forms weak structures, and high deacetylation degrees support stiffer networks due to the effective H-bonds and polymer entanglements [44].
The protonated amino groups on chitosan interact with anionic materials at suitable pH [45]. Chitosan complexes with anions and polyanions have been used to encapsulate proteins. Their pH responsiveness can be used to modulate the release of proteins while protecting them against degradation [46,47,48,49][46][47][48][49]. The anionic materials commonly used to form complexes with chitosan include alginate [50[50][51],51], collagen [52[52][53],53], gelatin [9,54[9][54][55][56],55,56], poly(γ-glutamic acid) [57], β-glycerophosphate [58[58][59][60],59,60], and tripolyphosphate [47,61][47][61]. Chitosan can associate with synthetic polymers (poly(vinyl alcohol) [56[56][62],62], polyethylene glycol [63], and poly(lactic-co-glycolic acid) [64]) and other materials (including clays [65] and graphene oxide [52]) for producing DDSs with enhanced mechanical properties and hydrophilic–hydrophobic balance.
Chitosan generally has low solubility in biological fluids. It can easily be modified to overcome this disadvantage. Chitosan has a reactive amino group at carbon C2 and hydroxyl groups at carbons C3 and C6, in the deacetylated residues. Many reports of chemical modification of chitosan have been published. These chitosan derivatives include graphitized copolymers with poly(ε-caprolactone) [66[66][67],67], and polyethylene glycol [67,68[67][68][69],69], carboxymethyl chitosan [70[70][71][72],71,72], N-succinyl-chitosan [73,74][73][74], hydroxyphenyl acetamide chitosan [73], N,N,N-trimethyl chitosan [75], and chitosan conjugated with mesoporous silica nanoparticles [12,76][12][76] (Figure 3). These chitosan-based materials have been used in biomedical applications.
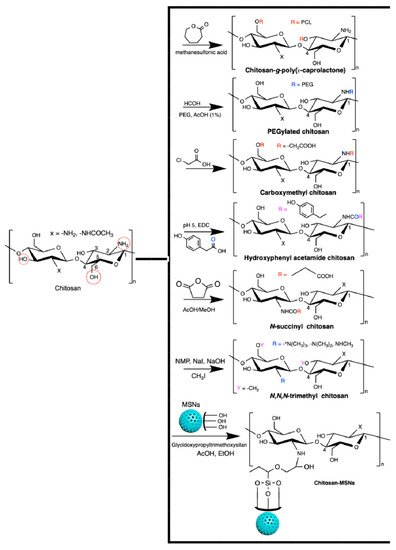
Figure 3.
Chitosan-based materials used in biomedical-engineering applications.
One of the most reported types of chitosan derivatives is carboxymethyl chitosans. These can be prepared from different synthetic pathways [77]. O-Carboxymethyl chitosan is prepared by suspending chitosan in an isopropanol/NaOH mixture and dropping (slowly) monochloroacetic acid in isopropanol in the suspended chitosan at 55 °C. This synthesis occurs using a NaOH excess to prevent the N-carboxymethylation. N-Carboxymethyl chitosan is obtained through the reaction between the free amines on chitosan with glyoxylic acid and sodium borohydride at pH between 3.2 and 4 (60 °C). N,O-Carboxymethyl chitosan is prepared by dissolving chitosan in an isopropanol/sodium hydroxide/chloroacetic acid mixture in a low NaOH concentration at 50 °C. N,N-dicarboxymethyl chitosan is prepared by tuning the chitosan, water, acetic acid, glyoxylic acid, and sodium borohydride contents at pH between 2 and 3. The ratio between amine and glyoxylic moieties should be 1:9. These chitosan derivatives are also used in biomedical applications [77].
2.3. Alginates
Alginates are natural polyuronates that have been used to engineer injectable drug delivery devices because of their low-cost of production, cytocompatibility, gelling, mucoadhesive, and pH-responsive properties [78,79,80,81,82][78][79][80][81][82]. Alginates are marine polysaccharides and comprise linear anionic polymers extracted from brown algae (Phaeophyceae, including Laminaria hyperborean, Laminaria japonica, Laminaria digitata, Ascophyllum nodosum, and Macrocystis pyrifera) [83]. Alginate hydrogels established by divalent cations (magnesium, calcium, barium, and strontium) naturally occur in the Phaeophyceae extracellular matrix. The seawater equilibrium influences the counter-ion types found in alginates [84].
The alginate repeat units are composed of (1,4)-linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) residues. The structure of alginate is characterized by homopolymer blocks (MMMM or GGGG) or alternating copolymer blocks (MGMG) [85] (Figure 4A). The relative percentages of M, G, and MG blocks depend upon the seaweed algae source and extraction method, that generally involves (i) acid extraction, (ii) filtration and washing steps, and (iii) filtrate solubilization in an aqueous NaOH solution to create sodium alginate. Further steps of floatation, centrifugation, filtration (to remove impurities and insoluble particles), precipitation (in alcohol), and extraction with barium ions are also carried out. Barium ions have a high affinity to bind to the anionic moieties on alginates, separating them from cytotoxic impurities. Alginates are recovered by precipitation, forming sodium alginates for biomedical materials [83,84][83][84]. These procedures provide purified and water-soluble alginates that are stable alginate gels in mildly acidic conditions [86]. On the other hand, they have instability in alkaline medium. The water solubility depends on the pH, ionic strength and presence of metallic cations in the aqueous solutions [85,87][85][87].
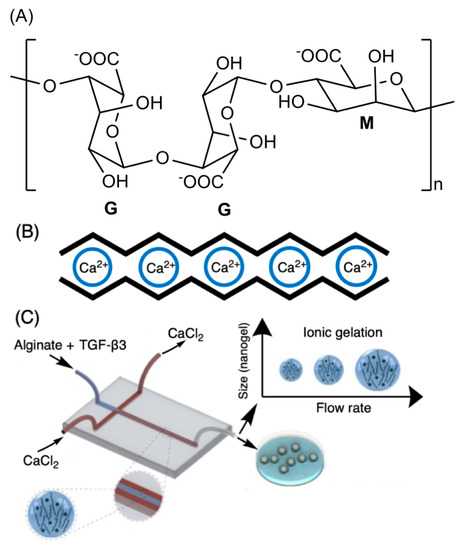
Figure 4. Chemical alginate structure (A), well-established egg-box gelation of alginate with calcium ions (B), and schematic illustration of a microfluidic device for hydrodynamic flow-focusing consisting of one inlet for focusing (core) flow and two separate inlets for the sheath (side) flows. (C) Adapted with permission from [88]; published by Elsevier, 2020.
Higher amounts of G blocks provide stiff alginate-based materials due to axial links and desirable chain conformation to form well-established egg-box structures with metallic cations (especially with calcium ions, Figure 4B) [80,89][80][89]. Both G and GM blocks participate in the egg-box gelation mechanism with divalent cations. High contents of alternating GM sequences increase the aqueous alginate solubility, while the gelation features mainly rely on the alginate molecular mass, G/M ratio, and pH. A higher molecular mass improves the gelling properties of aqueous alginate solutions due to the increase of polymer viscosity, supporting polymer entanglements, and thereby elastic and durable alginate-based materials [90,91,92][90][91][92]. The G/M ratio plays an essential role in the gelation process. At low pH (pH < 2.5), alginates are protonated, making water-insoluble alginic acids [85].
References
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995.
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for Neural Tissue Engineering Applications. Mater. Today Bio 2020, 6, 100043.
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620.
- Borro, B.C.; Malmsten, M. Complexation between Antimicrobial Peptides and Polyelectrolytes. Adv. Colloid Interface Sci. 2019, 270, 251–260.
- Evangelista, T.F.S.; Andrade, G.R.S.; Nascimento, K.N.S.; dos Santos, S.B.; de Fátima Costa Santos, M.; Da Ros Montes D’Oca, C.; dos S. Estevam, C.; Gimenez, I.F.; Almeida, L.E. Supramolecular Polyelectrolyte Complexes Based on Cyclodextrin-Grafted Chitosan and Carrageenan for Controlled Drug Release. Carbohydr. Polym. 2020, 245, 116592.
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-Based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116126.
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-Based Biomaterials for Growth Factor and Cytokine Delivery: Making the Right Choices. J. Control. Release 2019, 313, 131–147.
- Wawrzyńska, E.; Kubies, D. Alginate Matrices for Protein Delivery—A Short Review. Physiol. Res. 2018, 67, S319–S334.
- Azizian, S.; Hadjizadeh, A.; Niknejad, H. Chitosan-Gelatin Porous Scaffold Incorporated with Chitosan Nanoparticles for Growth Factor Delivery in Tissue Engineering. Carbohydr. Polym. 2018, 202, 315–322.
- Cao, L.; Kong, X.; Lin, S.; Zhang, S.; Wang, J.; Liu, C.; Jiang, X. Synergistic Effects of Dual Growth Factor Delivery from Composite Hydrogels Incorporating 2-N,6-O-Sulphated Chitosan on Bone Regeneration. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1–S17.
- Jooybar, E.; Abdekhodaie, M.J.; Karperien, M.; Mousavi, A.; Alvi, M.; Dijkstra, P.J. Developing Hyaluronic Acid Microgels for Sustained Delivery of Platelet Lysate for Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 144, 837–846.
- Yao, Q.; Liu, Y.; Selvaratnam, B.; Koodali, R.T.; Sun, H. Mesoporous Silicate Nanoparticles/3D Nanofibrous Scaffold-Mediated Dual-Drug Delivery for Bone Tissue Engineering. J. Control. Release 2018, 279, 69–78.
- Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R. Perspective on Computational Simulations of Glycosaminoglycans. Wires Comput. Mol. Sci. 2019, 9, e1388.
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent Advance in Delivery System and Tissue Engineering Applications of Chondroitin Sulfate. Carbohydr. Polym. 2020, 230, 115650.
- Sabino, R.M.; Kauk, K.; Madruga, L.Y.C.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Enhanced Hemocompatibility and Antibacterial Activity on Titania Nanotubes with Tanfloc/Heparin Polyelectrolyte Multilayers. J. Biomed. Mater. Res. 2020, 108, 992–1005.
- Wang, H.M.; Loganathan, D.; Linhardt, R.J. Determination of the PKa of Glucuronic Acid and the Carboxy Groups of Heparin by 13C-Nuclear-Magnetic-Resonance Spectroscopy. Biochem. J. 1991, 278, 689–695.
- Winter, W.T.; Arnott, S.; Isaac, D.H.; Atkins, E.D.T. Chondroitin 4-sulfate: The structure of a sulfated glycosaminoglycan. J. Mol. Biol. 1978, 125, 1–19.
- Tavsanli, B.; Okay, O. Mechanically Strong Hyaluronic Acid Hydrogels with an Interpenetrating Network Structure. Eur. Polym. J. 2017, 94, 185–195.
- Behrendt, P.; Ladner, Y.; Stoddart, M.J.; Lippross, S.; Alini, M.; Eglin, D.; Armiento, A.R. Articular Joint-Simulating Mechanical Load Activates Endogenous TGF-β in a Highly Cellularized Bioadhesive Hydrogel for Cartilage Repair. Am. J. Sports Med. 2020, 48, 210–221.
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive Functional Hydrogels: Controlled Release of Catechol Species with Antioxidant and Antiinflammatory Behavior. Mater. Sci. Eng. C 2019, 105, 110040.
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364.
- Eldridge, S.L.; Higgins, L.A.; Dickey, B.J.; Larive, C.K. Insights into the Capillary Electrophoresis Separation of Heparin Disaccharides from Nuclear Magnetic Resonance, pKa, and Electrophoretic Mobility Measurements. Anal. Chem. 2009, 81, 7406–7415.
- Larsson, B.; Nilsson, M.; Tjälve, H. The Binding of Inorganic and Organic Cations and H+ to Cartilage In Vitro. Biochem. Pharmacol. 1981, 30, 2963–2970.
- Shi, J.; Fan, C.; Zhuang, Y.; Sun, J.; Hou, X.; Chen, B.; Xiao, Z.; Chen, Y.; Zhan, Z.; Zhao, Y.; et al. Heparan Sulfate Proteoglycan Promotes Fibroblast Growth Factor-2 Function for Ischemic Heart Repair. Biomater. Sci. 2019, 7, 5438–5450.
- Corti, F.; Wang, Y.; Rhodes, J.M.; Atri, D.; Archer-Hartmann, S.; Zhang, J.; Zhuang, Z.W.; Chen, D.; Wang, T.; Wang, Z.; et al. N-Terminal Syndecan-2 Domain Selectively Enhances 6-O Heparan Sulfate Chains Sulfation and Promotes VEGFA165-Dependent Neovascularization. Nat. Commun. 2019, 10, 1562.
- Silva, J.C.; Carvalho, M.S.; Han, X.; Xia, K.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Compositional and Structural Analysis of Glycosaminoglycans in Cell-Derived Extracellular Matrices. Glycoconj. J. 2019, 36, 141–154.
- Yan, Y.; Ji, Y.; Su, N.; Mei, X.; Wang, Y.; Du, S.; Zhu, W.; Zhang, C.; Lu, Y.; Xing, X.-H. Non-Anticoagulant Effects of Low Molecular Weight Heparins in Inflammatory Disorders: A Review. Carbohydr. Polym. 2017, 160, 71–81.
- Prudnikova, K.; Lightfoot Vidal, S.E.; Sarkar, S.; Yu, T.; Yucha, R.W.; Ganesh, N.; Penn, L.S.; Han, L.; Schauer, C.L.; Vresilovic, E.J.; et al. Aggrecan-like Biomimetic Proteoglycans (BPGs) Composed of Natural Chondroitin Sulfate Bristles Grafted onto a Poly(Acrylic Acid) Core for Molecular Engineering of the Extracellular Matrix. Acta Biomater. 2018, 75, 93–104.
- Rajarathnam, K.; Desai, U.R. Structural Insights Into How Proteoglycans Determine Chemokine-CXCR1/CXCR2 Interactions: Progress and Challenges. Front. Immunol. 2020, 11, 660.
- Ullah, S.; Chen, X. Fabrication, Applications and Challenges of Natural Biomaterials in Tissue Engineering. Appl. Mater. Today 2020, 20, 100656.
- Lim, J.J.; Temenoff, J.S. The Effect of Desulfation of Chondroitin Sulfate on Interactions with Positively Charged Growth Factors and Upregulation of Cartilaginous Markers in Encapsulated MSCs. Biomaterials 2013, 34, 5007–5018.
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan: An Undisputed Bio-Fabrication Material for Tissue Engineering and Bio-Sensing Applications. Int. J. Biol. Macromol. 2018, 110, 110–123.
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50.
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and Chitosan Based Polyurethanes: A Review of Recent Advances and Prospective Biomedical Applications. Int. J. Biol. Macromol. 2016, 86, 630–645.
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457.
- Martins, A.F.; Vlcek, J.; Wigmosta, T.; Hedayati, M.; Reynolds, M.M.; Popat, K.C.; Kipper, M.J. Chitosan/Iota-Carrageenan and Chitosan/Pectin Polyelectrolyte Multilayer Scaffolds with Antiadhesive and Bactericidal Properties. Appl. Surf. Sci. 2020, 502, 144282.
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural Biomacromolecule Based Composite Scaffolds from Silk Fibroin, Gelatin and Chitosan toward Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294.
- Alishahi, A.; Aïder, M. Applications of Chitosan in the Seafood Industry and Aquaculture: A Review. Food Bioprocess Technol. 2012, 5, 817–830.
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.-L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373.
- Huang, T.-W.; Ho, Y.-C.; Tsai, T.-N.; Tseng, C.-L.; Lin, C.; Mi, F.-L. Enhancement of the Permeability and Activities of Epigallocatechin Gallate by Quaternary Ammonium Chitosan/Fucoidan Nanoparticles. Carbohydr. Polym. 2020, 242, 116312.
- Shafabakhsh, R.; Yousefi, B.; Asemi, Z.; Nikfar, B.; Mansournia, M.A.; Hallajzadeh, J. Chitosan: A Compound for Drug Delivery System in Gastric Cancer-a Review. Carbohydr. Polym. 2020, 242, 116403.
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832.
- Tavares, L.; Esparza Flores, E.E.; Rodrigues, R.C.; Hertz, P.F.; Noreña, C.P.Z. Effect of Deacetylation Degree of Chitosan on Rheological Properties and Physical Chemical Characteristics of Genipin-Crosslinked Chitosan Beads. Food Hydrocoll. 2020, 106, 105876.
- Kumar, P.V.; Maki, M.A.A.; Takahje, M.L.; Wei, Y.S.; Tatt, L.M.; Majeed, A.B.B.A. Detection of Formation of Recombinant Human Keratinocyte Growth Factor Loaded Chitosan Nanoparticles Based on Its Optical Properties. Curr. Nanosci. 2018, 14, 127–135. Available online: (accessed on 9 June 2020).
- Anouz, R.; Repanas, A.; Schwarz, E.; Groth, T. Novel Surface Coatings Using Oxidized Glycosaminoglycans as Delivery Systems of Bone Morphogenetic Protein 2 (BMP-2) for Bone Regeneration. Macromol. Biosci. 2018, 18, 1800283.
- Dehkordi, N.K.; Minaiyan, M.; Talebi, A.; Akbari, V.; Taheri, A. Nanocrystalline Cellulose–Hyaluronic Acid Composite Enriched with GM-CSF Loaded Chitosan Nanoparticles for Enhanced Wound Healing. Biomed. Mater. 2019, 14, 035003.
- Pan, Y.; Xiao, C.; Tan, H.; Yuan, G.; Li, J.; Li, S.; Jia, Y.; Xiong, D.; Hu, X.; Niu, X. Covalently Injectable Chitosan/Chondroitin Sulfate Hydrogel Integrated Gelatin/Heparin Microspheres for Soft Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 70, 149–157.
- Wang, X.; Zhang, J.; Cui, W.; Fang, Y.; Li, L.; Ji, S.; Mao, D.; Ke, T.; Yao, X.; Ding, D.; et al. Composite Hydrogel Modified by IGF-1C Domain Improves Stem Cell Therapy for Limb Ischemia. ACS Appl. Mater. Interfaces 2018, 10, 4481–4493.
- Parchen, G.P.; Jacumazo, J.; Koop, H.S.; Biscaia, S.M.P.; Trindade, E.S.; Silveira, J.L.M.; de Freitas, R.A. Modulation of Epidermal Growth Factor Release by Biopolymer-Coated Liposomes. J. Pharm. Sci. 2020, 109, 2294–2301.
- Reed, S.; Wu, B.M. Biological and Mechanical Characterization of Chitosan-Alginate Scaffolds for Growth Factor Delivery and Chondrogenesis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 272–282.
- Liu, T.; Dan, W.; Dan, N.; Liu, X.; Liu, X.; Peng, X. A Novel Grapheme Oxide-Modified Collagen-Chitosan Bio-Film for Controlled Growth Factor Release in Wound Healing Applications. Mater. Sci. Eng. C 2017, 77, 202–211.
- Yin, J.; Qiu, S.; Shi, B.; Xu, X.; Zhao, Y.; Gao, J.; Zhao, S.; Min, S. Controlled Release of FGF-2 and BMP-2 in Tissue Engineered Periosteum Promotes Bone Repair in Rats. Biomed. Mater. 2018, 13, 025001.
- Chi, H.; Song, X.; Song, C.; Zhao, W.; Chen, G.; Jiang, A.; Wang, X.; Yu, T.; Zheng, L.; Yan, J. Chitosan-Gelatin Scaffolds Incorporating Decellularized Platelet-Rich Fibrin Promote Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5305–5315.
- Huang, H.; Hu, X.; Zhang, X.; Duan, X.; Zhang, J.; Fu, X.; Dai, L.; Yuan, L.; Zhou, C.; Ao, Y. Codelivery of Synovium-Derived Mesenchymal Stem Cells and TGF-β by a Hybrid Scaffold for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 805–816.
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated Full-Thickness Wound Healing via Sustained BFGF Delivery Based on a PVA/Chitosan/Gelatin Hydrogel Incorporating PCL Microspheres. Int. J. Pharm. 2018, 537, 278–289.
- Kim, W.; Kim, M.; Tae, G. Injectable System and Its Potential Application for the Delivery of Biomolecules by Using Thermosensitive Poly(γ-Glutamic Acid)-Based Physical Hydrogel. Int. J. Biol. Macromol. 2018, 110, 457–464.
- Alinejad, Y.; Bitar, C.M.E.; Martinez Villegas, K.; Perignon, S.; Hoesli, C.A.; Lerouge, S. Chitosan Microbeads Produced by One-Step Scalable Stirred Emulsification: A Promising Process for Cell Therapy Applications. ACS Biomater. Sci. Eng. 2020, 6, 288–297.
- Min, Q.; Liu, J.; Yu, X.; Zhang, Y.; Wu, J.; Wan, Y. Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels. Mar. Drugs 2019, 17, 365.
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of Chitosan Hydrogel for Sustained Delivery of VEGF for Odontogenic Differentiation of Dental Pulp Stem Cells. Stem Cells Int. 2019, 2019, 1515040. Available online: (accessed on 9 June 2020).
- Mili, B.; Das, K.; Kumar, A.; Saxena, A.C.; Singh, P.; Ghosh, S.; Bag, S. Preparation of NGF Encapsulated Chitosan Nanoparticles and Its Evaluation on Neuronal Differentiation Potentiality of Canine Mesenchymal Stem Cells. J. Mater. Sci. Mater. Med. 2017, 29, 4.
- Jimi, S.; Jaguparov, A.; Nurkesh, A.; Sultankulov, B.; Saparov, A. Sequential Delivery of Cryogel Released Growth Factors and Cytokines Accelerates Wound Healing and Improves Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 345.
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-Chitosan Hydrogels for Controlled Antibiotic/Protein Delivery. ACS Appl. Biol. Mater. 2019, 2, 5313–5322.
- Chen, M.-M.; Cao, H.; Liu, Y.-Y.; Liu, Y.; Song, F.-F.; Chen, J.-D.; Zhang, Q.-Q.; Yang, W.-Z. Sequential Delivery of Chlorhexidine Acetate and BFGF from PLGA-Glycol Chitosan Core-Shell Microspheres. Colloids Surf. B Biointerfaces 2017, 151, 189–195.
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned Microchannel Polymer-Nanotube Composites for Peripheral Nerve Regeneration: Small Molecule Drug Delivery. J. Control. Release 2019, 296, 54–67.
- de Cassan, D.; Sydow, S.; Schmidt, N.; Behrens, P.; Roger, Y.; Hoffmann, A.; Hoheisel, A.L.; Glasmacher, B.; Hänsch, R.; Menzel, H. Attachment of Nanoparticulate Drug-Release Systems on Poly(ε-Caprolactone) Nanofibers via a Graftpolymer as Interlayer. Colloids Surf. B Biointerfaces 2018, 163, 309–320.
- Wen, Y.; Li, F.; Li, C.; Yin, Y.; Li, J. High Mechanical Strength Chitosan-Based Hydrogels Cross-Linked with Poly(Ethylene Glycol)/Polycaprolactone Micelles for the Controlled Release of Drugs/Growth Factors. J. Mater. Chem. B 2017, 5, 961–971.
- Vijayan, A.; Sabareeswaran, A.; Vinod Kumar, G.S. PEG Grafted Chitosan Scaffold for Dual Growth Factor Delivery for Enhanced Wound Healing. Sci. Rep. 2019, 9, 19165.
- Park, K.M.; Lee, H.J.; Koo, K.-T.; Ben Amara, H.; Leesungbok, R.; Noh, K.; Lee, S.C.; Lee, S.W. Oral Soft Tissue Regeneration Using Nano Controlled System Inducing Sequential Release of Trichloroacetic Acid and Epidermal Growth Factor. Tissue Eng. Regen. Med. 2020, 17, 91–103.
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel In Situ Forming Hydrogel Based on Xanthan and Chitosan Re-Gelifying in Liquids for Local Drug Delivery. Carbohydr. Polym. 2018, 186, 54–63.
- Nguyen, C.-T.; Nguyen, T.-T.; Nguyen, T.-T.; Nguyen, P.P.T.; Nguyen, A.D.; Tran, L.T.; Tran-Van, H. Preparation and In Vitro Evaluation of FGF-2 Incorporated Carboxymethyl Chitosan Nanoparticles. Carbohydr. Polym. 2017, 173, 114–120.
- Yao, Y.; Wang, T.; Liu, Y.; Zhang, N. Co-Delivery of Sorafenib and VEGF-SiRNA via PH-Sensitive Liposomes for the Synergistic Treatment of Hepatocellular Carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1374–1383.
- Linh, N.T.B.; Abueva, C.D.G.; Lee, B.-T. Enzymatic In Situ Formed Hydrogel from Gelatin–Tyramine and Chitosan-4-Hydroxylphenyl Acetamide for the Co-Delivery of Human Adipose-Derived Stem Cells and Platelet-Derived Growth Factor towards Vascularization. Biomed. Mater. 2017, 12, 015026.
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K.; Khan, A.A. N-Succinyl Chitosan Preparation, Characterization, Properties and Biomedical Applications: A State of the Art Review. Rev. Chem. Eng. 2015, 31, 563–597.
- Place, L.W.; Sekyi, M.; Kipper, M.J. Aggrecan-Mimetic, Glycosaminoglycan-Containing Nanoparticles for Growth Factor Stabilization and Delivery. Biomacromolecules 2014, 15, 680–689.
- Sun, P.; Zhang, Q.; Nie, W.; Zhou, X.; Chen, L.; Du, H.; Yang, S.; You, Z.; He, J.; He, C. Biodegradable Mesoporous Silica Nanocarrier Bearing Angiogenic QK Peptide and Dexamethasone for Accelerating Angiogenesis in Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 6766–6778.
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical Applications of Carboxymethyl Chitosans. Carbohydr. Polym. 2013, 91, 452–466.
- Gombotz, W. Protein Release from Alginate Matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285.
- Natsheh, H.; Touitou, E. Phospholipid Magnesome—A Nasal Vesicular Carrier for Delivery of Drugs to Brain. Drug Deliv. Transl. Res. 2018, 8, 806–819.
- Raimondo, T.M.; Li, H.; Kwee, B.J.; Kinsley, S.; Budina, E.; Anderson, E.M.; Doherty, E.J.; Talbot, S.G.; Mooney, D.J. Combined Delivery of VEGF and IGF-1 Promotes Functional Innervation in Mice and Improves Muscle Transplantation in Rabbits. Biomaterials 2019, 216, 119246.
- Rao, S.S.; Venkatesan, J.; Prabhu, A.; Rekha, P.D. Natural Polymeric Biomaterials in Growth Factor Delivery for Treating Diabetic Foot Ulcers. J. Drug Deliv. Sci. Technol. 2020, 55, 101385.
- Shi, M.; Zhang, H.; Song, T.; Liu, X.; Gao, Y.; Zhou, J.; Li, Y. Sustainable Dual Release of Antibiotic and Growth Factor from PH-Responsive Uniform Alginate Composite Microparticles to Enhance Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 22730–22744.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305.
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389.
- Karpov, A.A.; Puzanov, M.V.; Ivkin, D.Y.; Krasnova, M.V.; Anikin, N.A.; Docshin, P.M.; Moiseeva, O.M.; Galagudza, M.M. Non-inferiority of Microencapsulated Mesenchymal Stem Cells to Free Cells in Cardiac Repair after Myocardial Infarction: A Rationale for Using Paracrine Factor(s) Instead of Cells. Int. J. Exp. Path. 2019, 100, 102–113.
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New Magnetic Chitosan/Alginate/Fe3O4@SiO2 Hydrogel Composites Applied for Removal of Pb(II) Ions from Aqueous Systems. Chem. Eng. J. 2018, 337, 595–608.
- Mahmoudi, Z.; Mohammadnejad, J.; Razavi Bazaz, S.; Abouei Mehrizi, A.; Saidijam, M.; Dinarvand, R.; Ebrahimi Warkiani, M.; Soleimani, M. Promoted Chondrogenesis of HMCSs with Controlled Release of TGF-Β3 via Microfluidics Synthesized Alginate Nanogels. Carbohydr. Polym. 2020, 229, 115551.
- Farrelly, J.S.; Bianchi, A.H.; Ricciardi, A.S.; Buzzelli, G.L.; Ahle, S.L.; Freedman-Weiss, M.R.; Luks, V.L.; Saltzman, W.M.; Stitelman, D.H. Alginate Microparticles Loaded with Basic Fibroblast Growth Factor Induce Tissue Coverage in a Rat Model of Myelomeningocele. J. Pediatric Surg. 2019, 54, 80–85.
- Madrigal, J.L.; Sharma, S.N.; Campbell, K.T.; Stilhano, R.S.; Gijsbers, R.; Silva, E.A. Microgels Produced Using Microfluidic On-Chip Polymer Blending for Controlled Released of VEGF Encoding Lentivectors. Acta Biomater. 2018, 69, 265–276.
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120.
- Page, D.J.; Clarkin, C.E.; Mani, R.; Khan, N.A.; Dawson, J.I.; Evans, N.D. Injectable Nanoclay Gels for Angiogenesis. Acta Biomater. 2019, 100, 378–387.
