Although homeostasis is a commonly accepted concept, there is incontrovertible evidence that biological processes and functions are variable, and that variability occurs in cycles. So allostatic model has emerged as the first challenge to homeostasis. Circadian variation is the predominant variation in the body. As there is strong scientific and clinical evidence that blood pressure fluctuations undergo circadian rhythm, there is equally strong evidence that targeted time therapy for hypertension provides a better outcome of the disease. The research has gone even further by ensuring better patients' adherence throughout the development and approval process for the use of pulsatile drug release systems which can be considered as an option for an even more convenient dosage regimen of the medicines needed.
Since allostasis involves the achievement of stability through change, including the specific rhythms of various functions in the organism, this entry aimed to briefly introduce the circadian rhythm of blood pressure in health and disease and to present proven possibilities in the treatment of hypertension through the art of pulsatile drug release systems.
1. Circadian Blood Pressure Fluctuations
Until fifteen years ago, the conventional homeostatic assumption was valid that blood pressure and heart rate have a constant value for 24 h unless the body is exposed to exercise, stress, or other environmental influences. However, studies involving outpatient all-day blood pressure measurement
[1][56] indicated the existence of significant fluctuations in blood pressure during the day in both normotensive and hypertensive patients
[2][3][4][5][57,58,59,60].
When it was introduced, allostasis was explained by physiological fluctuations in blood pressure, as the fluctuation of blood pressure within 24 h is indeed the perfect example of allostatic control
[6][3]. On a 24-h basis, blood pressure changes dramatically and continuously to adapt individuals to everyday environmental conditions. Therefore, there is not only one “homeostatic” blood pressure, but many stable blood pressure states
[7][61], which is precisely the property of blood pressure that defines its hidden adaptability
[8][62]. Blood pressure fluctuations are seamless and are influenced by temperature
[9][63], temporary physicochemical stressors, such as work requirements and childcare
[10][64], or when people think of emotionally charged memories
[11][65]. Changes in blood pressure also occur in response to the intake of salt
[12][66], alcohol, nicotine, and caffeine
[13][67].
Models of Circadian Blood Pressure Fluctuations
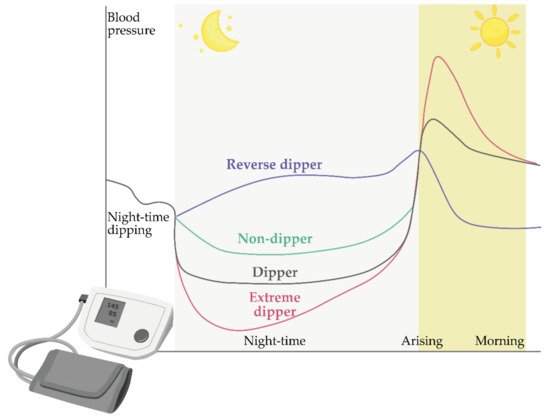
With the development and application of ambulatory blood pressure measurement, the presence of significant fluctuations in blood pressure levels during the day was detected. Furthermore, it was observed that in normotensive patients, but also in hypertensive patients, both systolic and diastolic blood pressure decreases during the night
[4][5][14][59,60,68]. Blood pressure drop values range from 10% to 20% and are estimated at an average of 15%. In relation to this phenomenon, all patients can be divided into two groups, i.e., their so-called dipping status is determined ()
[4][5][7][8][59,60,61,62].
Figure 15. Schematic representation of the dipping status of patients during the night, including dippers (black), non-dippers (green), extreme dippers (purple), and reverse dippers/risers (violet). The image was created with the Adobe Illustrator CC (Version 23.0.1.; Adobe Inc., 2019)
[15][10].
The first group consists of patients whose blood pressure drops by more than 10% at night, and this group of patients is collectively referred to as “dippers”. On the other hand, some patients have no pressure drop at night or who have a pressure drop below 10%; these patients are referred to as “non-dippers”. More recently, the classification has been extended to include two further groups: “extreme-dippers” who have a nocturnal blood pressure drop of more than 20% and “rising/inverse dippers” in which systolic blood pressure is higher at night than during the day
[16][69]. A schematic representation of blood pressure fluctuations in these categories is given in .
2. Chronotherapy and Chronopharmaceutics
Chronotherapy can be defined as the targeted administration of drugs at a given time, regardless of whether they are drugs with the time-modified or immediate release of the active pharmaceutical ingredient (API). The timing of administration of such drugs is adjusted so that the concentrations of their API in serum and tissues are in accordance with the known circadian rhythm of the disease or the symptoms for which they are intended. In this way, it is possible to increase the effectiveness and to reduce or eliminate the side effects of the drug
[17][70].
Chronotherapeutics or chronotherapeutic drug delivery systems (CDDSs) can release the required amount of API at the appropriate site of action and at an exact time according to chronobiology and inherent mechanisms. CDDSs are primarily formulated for bedtime administration since diseases affected by circadian rhythms are usually worse in the middle of the night or early morning. CDDSs release API following sigmoid release profile with a specific lag time adapted to the condition
[18][71]. An ideal chronotherapeutic dosage form should have an integrated, time-controlled, and site-specific drug delivery system, regardless of the site of administration
[19][12].
Chronotherapy of hypertension should be adjusted to the specific circadian rhythm of the patient’s hypertension. Particular attention should be paid to the treatment of a sudden rise in blood pressure in the morning to try to normalize high blood pressure during the day and at night, and to correct the “non-dipper” status to dipper status, as the latter is associated with a reduced risk of severe hypertension on peripheral organs. Numerous studies have been conducted on the efficacy of various groups of antihypertensives at certain times of the day. Angiotensin-converting enzyme (ACE) inhibitors include benazepril, captopril, enalapril, imidapril, lisinopril, perindopril, quinapril, trandolapril, and zofenopril and have a greater effect on blood pressure during sleep than during waking. Furthermore, this group of antihypertensives normalizes circadian blood pressure rhythm by influencing the normalization of the patient’s dipping status when administered instead of the morning before bedtime
[20][14][21][22][23][24][18,68,72,73,74,75]. Better efficacy in lowering blood pressure when administered at bedtime instead of in the morning has also been shown for angiotensin-II receptor blockers, of which irbesartan, olmesartan, telmisartan, and valsartan have been investigated so far
[14][25][26][27][28][68,76,77,78,79]. Similar results have been shown for α-adrenergic receptor antagonists
[29][80]. When it comes to β-adrenoreceptor antagonists, findings were similar, but it has also been shown that a decrease of a sudden rise in blood pressure in the morning may occur when an additional dose of carvedilol is administered in the evening. The same effect was not observed when an additional dose of this antihypertensive was administered in the morning
[30][81]. A similar result was found for nebivolol
[31][82].
In contrast to other groups of antihypertensives, calcium channel blockers (amlodipine
[32][83], cilnidipine
[33][84], diltiazem
[34][85], isradipine
[35][86], nifedipine
[36][87], nisoldipine
[37][88], and nitrendipine
[38][89]) are the only ones that reduce blood pressure equally, regardless of the time of administration.
CDDSs can be one of the solutions for the chronotherapy of hypertension apart from the application of conventional dosage forms at a specific time of day. They enable more uniform control of blood pressure for 24 h. In addition, the administration of these drugs once a day has a particular advantage in terms of the convenience of administration to patients. Chronotherapeutics are designed to regulate blood pressure over 24 h and to harmonize the circadian rhythm of hypertension with the circadian rhythm of normal blood pressure in humans.
In addition to these advantages, the chronopharmaceutical approach to the treatment of hypertension, but also of other diseases, has certain disadvantages. The most common deficiency is the unpredictable and decreased bioavailability, the possibility of lack of action due to technological errors in the development of these forms, as well as higher economic costs in their development and production
[19][12].
When formulating dosage forms, several approaches ensure the release of the API in accordance with the chronotherapeutic requirements. Systems with the pulsatile release of the API are particularly suitable for use in chronotherapy.
3. Pulsatile Antihypertensives Delivery Systems Approved for Use
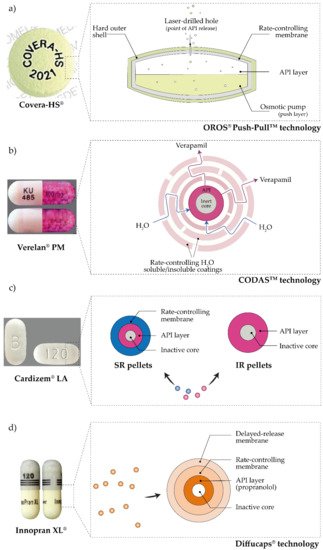
There are currently four pulsatile release antihypertensives approved for use by the Food and Drug Administration (FDA). These are COVERA-HS
® and Verelan
® PM (containing verapamil), Cardizem
® LA (containing diltiazem), and Innopran XL
® (containing propranolol)
[19][12]. The first approved system for the chronotherapeutic treatment of hypertension and stable angina pectoris with the pulsatile release was COVERA-HS
® (Pfizer Inc., New York, NY, USA)
[39][40][90,91] (a). It contains verapamil, a calcium channel blocker, as an API. The FDA approved this drug in 1996. Release of an API is delayed and occurs 4–5 h after ingestion and is recommended to be taken in the evening, before bedtime. The drug release phase is prolonged with the peak plasma concentration (Cmax) occurring approximately 11 h after administration, with the lowest concentrations occurring approximately 4 h after bedtime dosing while the patient is sleeping. Steady-state pharmacokinetics determined in healthy volunteers is reached by the third or fourth day of dosing. Consumption of a high-fat meal just prior to dosing at night has no effect on the pharmacokinetics of COVERA-HS. The pharmacokinetics were also not affected by whether the volunteers were supine or ambulatory for the 8 h following dosing
[41][92]. This is a formulation that works on the principle of an osmotic pump. This formulation is based on OROS
® Push-Pull
TM technology (ALZA Corporation, Mountain View, CA, USA). The osmotic formulation consists of a two-part tablet core, one containing a “pushing” polymer and the other an API. The tablet core is completely coated with a semipermeable membrane, containing tiny openings made by a laser, that connects the core to an external medium. A hydrophilic polymer coating the core, located below the semipermeable membrane, helps to prolong the lag time. As water penetrates, the API dissolves, and the “push” compartment swells. Consequently, the drug solution is pumped at a constant rate through the openings of the semipermeable membrane, as shown in a
[42][93].
Figure 26. A schematic representation of approved pulsatile antihypertensives drug systems: (
a) COVERA-HS
®, (
b) Verelan
® PM, (
c) Cardizem
® LA, and (
d) Innopran XL
® (API—active pharmaceutical ingredient, SR—sustained-release, IR—immediate-release). The image was created with the Adobe Illustrator CC (Version 23.0.1.; Adobe Inc., 2019)
[15][10].
Verelan
® PM (Lannett Company Inc., Philadelphia, PA, USA) is another pulsatile release system of verapamil (b). The FDA approved the use of Verelan
® PM in 1999
[25][76]. This formulation releases verapamil after 4–5 h but uses CODAS
TM technology (Elan Drug Technologies, Athlone, Ireland). This dosage form consists of capsules filled with pellets, which are coated with polymers to control the release of the API. The coating consists of a combination of water-soluble and water-insoluble polymers. Whereas the hydrosoluble polymer forms a channel system after dissolution through which the drug is released, the hydrophobic polymer represents a release barrier and thus controls it
[19][12].
Cardizem
® LA (Bausch Health US LLC, Bridgewater, NJ, USA) (c) is a system with a pulsatile release of diltiazem. Diltiazem is also a calcium channel blocker. Cardizem
® LA was approved for use by the FDA in 2003. It is dosed once a day, either morning or evening
[25][76]. This system consists of two types of pellets. Some are uncoated and allow immediate release of diltiazem, while others are coated and achieve delayed release of diltiazem. The pellets are coated with a polymer mixture of Eudragit
® S100 and Eudragit
® L100 (Evonik Industries AG, Darmstadt, Germany). The pellets are then compressed into a tablet that releases diltiazem with a lag period, recording the maximum plasma concentration 11–18 h after administration
[43][94].
Innopran XL
® (ANI Pharmaceuticals, Inc., Baudette, MN, USA) (d) contains propranolol, a non-selective β-adrenergic receptor blocker. This drug was approved by the FDA in 2003
[25][76]. Diffucaps
® technology (Adare Pharmaceuticals, Inc., Vandalia, OH, USA) was used to make this system. Innopran XL
® consists of a capsule filled with pellets. The pellets consist of an inert core onto which a layer of API has been applied. Then, two coatings are applied to the API layer. The outer coating delays the release of propranolol, while the inner coating controls the release of this drug. This chronotherapeutic approach allows the plasma drug concentrations to vary throughout the day according to physiological needs, mimicking circadian rhythms and reaching maximum concentrations when disease symptoms are most pronounced and most dangerous
[19][12].