Flavonoids as the largest group of natural phytochemical compounds have received significant attention, as demonstrated by clinical trials, due to their chemotherapeutic and/or pharmacological effects against non-small-cell lung cancer (NSCLC) and asthma. Scutellaria baicalensis (S. baicalensis), known as one of the most popular medicinal plants and used in several countries, contains natural active flavone constituents, with the major compounds of the roots being baicalein, baicalin, wogonin, wogonoside and oroxylin A. S. baicalensis and their compounds are proven to have inhibitory effects on NSCLC cells when used at different concentrations. However, the exact mechanisms by which these compounds exert their therapeutic effects against asthma remain unexplored. Indeed, the mechanisms by which S. baicalensis and its flavone compounds exert a protective effect against nicotine-induced NSCLC and asthma are not yet fully understood.
- Scutellaria baicalensis
- flavone compounds
- nicotine
- NSCLC
- asthma
1. Introduction
Scutellaria baicalensis Georgi (S. baicalensis), also known as Chinese skullcap or Huang-Qin, is a traditional Chinese medicinal plant that belongs to the family Lamiaceae and is widely used in East Asian countries, Russia, Europe and North America as an adjuvant to chemotherapy for many diseases [1][2][3][37,38,39]. Flavonoids and their glycosides are the major bioactive chemical compounds of S. baicalensis, with the main constituents of the root-specific 4′-deoxyflavones (known as Scutellariae radix, S. radix) being baicalein, baicalin, wogonin, wogonoside and oroxylin A [2][3][38,39]. These flavones exert anti-tumor functions against various types of cancer, including brain, prostate and oral squamous cell carcinoma (SCC) [4][40], and have been recorded in Chinese and European Pharmacopoeia as being medicinal drugs used to treat several diseases [3][39]. Previous studies are still insufficient to understand the effect of flavone compounds in NSCLC treatment in vivo. Studies in mice showed that A549 and H-460 cells treated with S. baicalensis compounds (baicalin, baicalein and wogonin) result in inhibition of cell proliferation and angiogenesis by downregulating expressions of inhibitors of differentiation 1 (Id1) protein, EMT-related molecules (N-cadherin, vimentin), vascular endothelial growth factor A (VEGF-A) and fibroblast growth factor receptor 2 (FGFR2) through suppression of the Src/Id1 signaling pathway [5][6][7][41,42,43]. The molecular effect and mechanism of action of S. baicalensis and its compounds at different concentrations showed strong inhibitory properties in NSCLC cells, as reported by in vitro studies. However, the potential molecular mechanisms by which S. baicalensis and its isolated compounds are thought to be useful in the treatment of asthma have not been clearly studied. Indeed, the mechanisms of the anti-lung cancer and asthma action of S. baicalensis containing flavones compounds in nicotine-induced NSCLC and asthma are unclear. The significant link between NSCLC and asthma could have health benefits for smokers in terms of using S. baicalensis and its compounds. Given that S. baicalensis is used in several therapeutic practices, targeting the flavones extracted from such a plant and understanding its molecular mechanisms of action might help in providing new therapeutic strategies for the treatment of NSCLC and asthma, particularly in smoker patients.
2. Therapeutic Role of Scutellaria baicalensis and Their Flavone Compounds in Nicotine-Induced NSCLC
S. baicalensis and its extracts possess anti-lung cancer activity demonstrated in NSCLC cells. Park et al. [8] showed that the aqueous extract of
S. baicalensis
S. baicalensis downregulates cyclinD1, cyclin-dependent kinase 4 (CDK4) and MMP2 production in A549 cells. Kim et al. [9] reported that the aqueous extract of
S. radix
S. radix also induces autophagy via increasing microtubule-associated protein 1A/1B-light chain 3 (LC3)-II/LC3-I expression. A study by Wang et al. [10] assessed the anti-lung tumor effect of
S. radix
Gao et al. [11] studied the anti-lung cancer effect of
S. baicalensis
S. baicalensis is reported as high against A549 and SK-LU-1 cells due to the stochiometric combination of extracts. The extracts reported cell cycle and apoptosis-inducing in NSCLC cells via upregulating expressions of p53 and Bax and downregulating expressions of cyclinA. The study concludes that the cytotoxicity of extracts may control growth of NSCLC cells via inducing cell cycle arrest and apoptosis, suggesting the significance of cell growth arrest and apoptosis as potential mechanisms in the cytotoxicity of flavones treatment. Gong et al. [12] studied the apoptotic and anti-inflammatory effects of baicalin, baicalein and wogonin extracted from
S. baicalensis on nicotine-induced A549 and H1299 cells. The extracts upregulate expression of Bax and downregulate expressions of MMP2, MMP9, caspase-3, bcl-2/bax ratio and bcl-2. The extracts showed anti-inflammatory activity by inhibiting expressions of NF-κB, TNF-α, IL-6 and I kappa B-alpha (IκB-α) in NSCLC cells. Zhao et al. [5] reported the effects of three major
S. baicalensis
2.1. Baicalein
3.1. Baicalein
Baicalein has been reported to promote apoptosis and/or suppress cellular growth and proliferation of NSCLC cells. A study has shown that baicalein promotes sensitivity to cisplatin drugs in A549 and H460 cells by inducing apoptosis and inhibiting cell proliferation through suppression of the PI3K/Akt signaling pathway and the expression of microRNA 424-3p (miR-424-3p) targeting phosphatase and tensin homolog (PTEN) gene expression [13]. In another study, baicalein enhances antitumor activity by reducing the induction of vasculogenic mimicry (VM), a pattern of tumor microcirculation, via inhibiting the expression of VM-associated factors (e.g., MMP9, VE-cadherin) and the RhoA/rho-associated coiled-coil kinase (RhoA/ROCK) signaling pathway, leading to the suppression of motility and viability of A549 cells [14]. A study by Li et al. [15] reported that baicalein suppresses MAP4K3 expression via reducing its stability. The results also indicated that inhibition of the mTOR signaling pathway in A549 and H1299 cells by RNA interference could be sufficient in mediating baicalein-induced autophagy.
In a study carried out by Zhang et al. [16], the A549 and H1299 cells were treated with different concentrations of baicalein to study the mechanisms by which baicalein suppresses cell metastasis. The results showed that baicalein inhibits cell metastasis by inhibiting ezrin tension transduction, leader cell production and inducible nitric oxide synthase (iNOS)-mediated ezrin S-nitrosylation (SNO) tension, which are responsible for A549 and H1299 cell aggression in the inflammatory milieu. A study by Cathcart et al. [6] reported that the angiogenesis of NSCLC cells (A549, H460 and SKMES1) is significantly inhibited via downregulating VEGF-A, FGFR-2 and RB-1 expression. Su et al. [17] reported baicalein increases the protein expression of E-cadherin and inhibits the EMT-induced N-cadherin and vimentin in A549 and H1299 cells by downregulating the Notch signaling pathway. Similarly, a study on the anti-tumor effect of baicalein on NSCLC cells showed that baicalein causes an inhibitory effect on A549 cells by suppressing the expressions of N-cadherin, vimentin, VEGF-1, and upregulating the expression of E-cadherin through Src-Id1 signaling pathway blocking [7]. Therefore, baicalein showed effective results in the inhibition of NSCLC cells through anti-proliferative/metastatic, anti-angiogenesis and apoptosis/autophagy induction. The mechanisms underlying the effect are linked to the inhibition of different cellular signaling pathways.
2.2. Baicalin
A few in vitro studies have shown potent anti-lung cancer activity of baicalin in NSCLC cell lines. Zhang et al. [18] reported the anti-lung cancer activities of baicalin in A549 and H2009 cells at different concentrations, in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a potential anticancer agent which modulates signaling pathways in NSCLC cells. Baicalin sensitizes lung cancer cells to TRAIL-induced apoptosis via the activation of MAPK and reactive oxygen species (ROS) production. Another study reported a high anti-lung cancer effect of baicalin in combination with DDP (cisplatin) on proliferation and invasion of A549 cells. Baicalin suppresses protein expression of microtubule affinity-regulating kinase 2 (MARK2) and Akt in A549/DDP cells, which are involved in cell proliferation and the pathogenesis of lung cancer [19]. Diao et al. [20] used baicalin to examine its inhibitory effect on NSCLC cells. The inhibitory effect of baicalin is observed against H441, H1975 and H1299 cells with a high expression level of PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK), a novel mitotic kinase which promotes cell invasion. You et al. [21] reported that baicalin stimulates apoptosis and inhibits the invasion abilities in A549 and H1299 cells. This is observed through activation of the sirtuin 1 gene/AMPK (SIRT1/AMPK) signaling pathway, known as tumor suppressor genes, and inhibition of the expression levels of MMP2 and MMP9 in NSCLC cells. From these studies, it can be suggested that baicalin has significant anti-proliferative/invasive and apoptotic effects, and the mechanisms of its therapeutic effect in NSCLC cells are related to the modulation of cellular signaling pathways.
23.3. Wogonin
Wogonin possesses antitumor properties against NSCLC cells, as demonstrated by a few in vitro studies. The study conducted by He et al. [22] found high anti-tumor activity against A549 cells of wogonin in combination with cisplatin. The anti-tumor effect of wogonin is reported as enhancing cisplatin-induced cell death through activating apoptosis, cleavage of the caspase-3 substrate PARP, caspase-3 and ROS production. Zhao et al. [23] assessed the inhibitory effect of wogonin in A549 cells by targeting the inflammatory microenvironment. The results demonstrated inhibition in human monocytic leukemia cell line (THP-1 CM)-induced migration of A549 cells, IL-6-induced EMT, vimentin, N-cadherin, Twist and Snail, and activation of E-cadherin. The inactivation of signal transducer and activation of the transcription 3 (STAT3) signaling pathway was found to be the mechanisms for this effect.
Wang et al. [24] performed a proliferation inhibition assay on A549 cells. The study reported a significant cell proliferation inhibition rate of 54% in A549 when treated with wogonin. Wogonin also showed an effect on energy metabolism by reducing lactate dehydrogenase (LDH) activity in A549 cells. In a study performed to assess the effect of wogonin on NSCLC cells and to explore their mechanism of action as an anti-tumor inhibitor, wogonin showed a significant cell viability inhibition rate of 31% and 34% in A549 and A427 cells, respectively. The mechanism of NSCLC cell inhibition is linked to the activation of apoptosis-inducing enzymes, autophagy and formation of ROS [25]. In the study by Chen et al. [26], the anticancer effects of wogonin in different concentrations on A549 cells are examined through regulating cellular pathways to assess cell viability. Results showed that wogonin inhibits cell viability and induces apoptosis in A549 cells through down-regulating HDAC1 and HDAC2 at mRNA and protein levels, and c-Myc oncogene, S-phase kinase-associated protein 2 (Skp2), F-box and WD repeat domain-containing 7 (Fbw7) and Glycogen synthase kinase 3b (GSK3b) at the protein level. A study of the inhibitory effect of wogonin on cell viability of NSCLC cells, Shi et al. [27] reported cell cycle arrest, senescence and apoptosis-induction in A549 cells by the downregulation of protein serum levels and glucocorticoid-inducible kinase 1 (SGK1). Furthermore, wogonin upregulates cellular levels of apoptotic protease activating factor-1 (APAF1), Bax, p21, promyelocytic leukemia protein (PML), growth arrest and DNA damage-inducible 45A(GADD45A) and yippee-like 3 (YPEL3) in A549 cells, which are the key factors involved in apoptosis, senescence and cell cycle arrest. Therefore, it can be suggested that wogonin has a strong anti-proliferative/viability, anti-inflammatory and apoptotic activities against NSCLC cells through regulation of several cellular signaling pathways as the mechanisms for these activities.
23.4. Wogonoside
Studies that have examined the in vitro activities of wogonoside on NSCLC cells are limited. A study by Luo et al. [28] reported the inhibition of A549 cells via inducing apoptosis and cell cycle arrest when treated with wogonoside. Wogonoside treatment upregulates cleaved caspase-3/9 and Bax expression, downregulates Bcl-2 expression, and promotes the levels of mitochondrial cytochrome
c in the cytosol of A549 cells. Furthermore, wogonoside induces apoptosis and cell cycle arrest by reducing phospho-mTOR and its downstream target p70S6K and increasing phospho-AMPK in A549 cells, suggesting that the AMPK/mTOR signaling pathway may be involved in the apoptotic activity of wogonoside. A study by Wang et al. [29] did not show any anti-proliferative effects of wogonoside on six cancer cell lines, including NSCLC (DMEM). However, the study observed the anti-proliferative activity on DMEM treated with wogonin (the deglycosylation of wogonoside).
23.5. Oroxylin A
A few in vitro studies have revealed that oroxylin A (OA) possesses strong anti-lung cancer activity in NSCLC cell lines. Shen et al. [30] studied the anti-inflammatory effect of OA on T
regs
regs production in H460 cells. Treatment with OA causes an inhibitory effect on the H460 cells cocultured with Jurkat cells by suppressing TGFβ-activated Smad3, ERK1/2, c-JUN NH2-terminal kinase (JNK) and p38 signaling pathways. OA also significantly inhibits the DNA binding activity of NF-κB/p65 in H460 cells by reducing phospho-Kappa B kinase α, β (IKKα, IKKβ). A study by Wei et al. [31] has been performed to assess the anti-invasive effect of OA on NSCLC cells and the molecular mechanisms involved. The study reported inhibition in A549 and 95-D cell migration/invasion with a 70% and 73% inhibition rate OA, respectively. The mechanism of inhibition is attributed to the suppression of Snail/TGFβ-induced EMT through ERK/Glycogen synthase kinase-3β (GSK-3β) signaling pathway blocking. As a result, OA downregulates expression of CD44v6, MMP-9, and vimentin and upregulates the expression of E-cadherin, and this could lead to the suppression of invasion and migration in Snail-expressing NSCLC cells. Another study by Wei et al. [32] reported the effect of OA on anoikis (cell death)-sensitization and glycolysis-inhibition in NSCLC cells. Results showed that OA suppresses the growth of detached A549 cells and induces anoikis through Src/PI3K/AKT signal pathway inhibition at the Tyr418 and ser316 sites. The study also showed that OA induces anoikis in A549 cells by lowering ATP synthesis, the level of glycolysis, lactic acid production and hexokinase II (HK II) glycolytic enzyme activity in mitochondria. A study by Liu et al. [33] was conducted to evaluate the effect of OA with cisplatin on suppression of NSCLC cells under hypoxic conditions. Flow cytometric and CCK8 assays reported that OA promotes cisplatin-induced cell death in H460 cells under hypoxia through inhibiting xeroderma pigmentosum group C (XPC), a DNA damage recognition protein upregulated by HIF-1α, which is implicated in the activation of nucleotide excision repair (NER). It is, therefore, suggested that OA has significant anti-invasive and anti-inflammatory activities in nicotine-induced NSCLC cells, due to their ability to inhibit cellular signaling pathways involved in NSCLC development. Taken together,
S. baicalensis
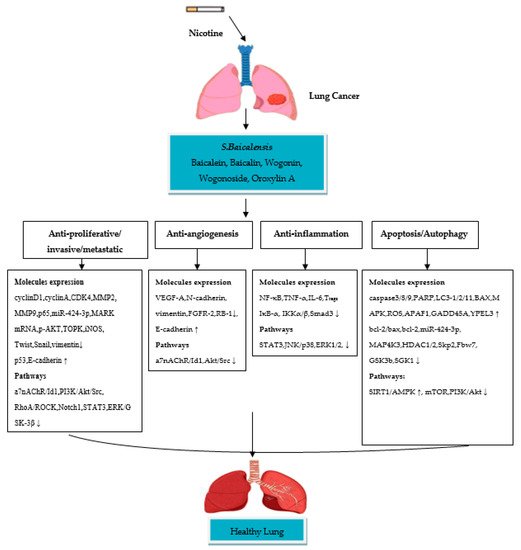
Figure 1.
S. baicalensis
