Understanding the autistic brain and the involvement of genetic, non-genetic, and numerous signaling pathways in the etiology and pathophysiology of autism spectrum disorder (ASD) is complex, as is evident from various studies. Apart from multiple developmental disorders of the brain, autistic subjects show a few characteristics like impairment in social communications related to repetitive, restricted, or stereotypical behavior, which suggests alterations in neuronal circuits caused by defects in various signaling pathways during embryogenesis. Most of the research studies on ASD subjects and genetic models revealed the involvement of mutated genes with alterations of numerous signaling pathways like Wnt, hedgehog, and Retinoic Acid (RA).
1. Introduction
Autism Spectrum Disorder (ASD) is a chronic heterogeneous neurodevelopmental disorder characterized by impairment in social communications, related to restricted, repetitive, or stereotypical behavior
[1]. It is the principal cause of disability among children less than 5 years of age suffering from mental disorders. It is more than conduct disorder and ADHD (Attention Deficit Hyperactivity Disorder) together because it persists throughout the life span of children suffering from this disease
[2]. It is a great challenge for all developing countries, especially India, because of the severity of this disorder and its influence on the affected children and their families including the economic burden which imposes on the parents coupled with lack of knowledge about the disorder
[3]. A lack of knowledge and awareness, wrong diagnosis, and inclusion of autism disorder under the normal classification of intellectual disability, language, or speech disorders are most commonly observed
[4]. Some previous research studies suggested that early detection and intervention can improve language and speech abilities as well as reduce cognitive decline and behavior in affected children
[5]. They also supported the idea that ASD can be diagnosed in the first two years after birth
[6]; however, several studies report that a substantial proportion of affected children are not diagnosed until they are school-aged
[7].
Worldwide data collected by survey analysis and routine monitoring structures show that several countries since the 1990s have identified an estimated rise in the prevalence of ASD from 0.7 to 1.0%
[7]. The Centers for Disease Control and Prevention (CDC) in the United States conducted a study and reported that, from 11 sites, 1 in 54 children suffer from ASD, indicating an elevation in cases over the past two decades
[8]. ASD prevalence in Asian countries has also been estimated, and it was found that in India, 1.7 to 2 million individuals are affected with ASD
[9][10][9,10]. These rises in the number of ASD cases in the Indian population have increased the need to consider associated risk factors as well as to discover early detection techniques and therapeutic interventions.
Autism disorder was first identified in 1943 by Kanner in 11 children with similar symptoms like impaired speech and language, obsessive behavior, and deficits in social cognition. An epidemiological study on autism was conducted 23 years afterward and estimated a prevalence rate of 4.5 per 10,000 persons. This estimated ratio was drastically increased to 1 in 59 persons, with males being diagnosed three times more than females
[11]. This elevation in prevalence is the result of an increase in knowledge and awareness and advancement in the Diagnostic and Statistical Manual of Mental Disorder (DSM) standards, which covers a broad range of disorders
[12].
The etiology of ASD remains a topic of debate: Its origin, genetic and environmental factors, and their interplay form a range of cognitive, behavioral, and developmental features observed in affected individuals.
2. Types of Autism Spectrum Disorder
ASD involves Asperger’s syndrome, autistic disorders, pervasive developmental disorders not otherwise specified (PDD-NOS), and Rett syndrome
[13]. Asperger’s syndrome was identified in 1940 by pediatrician Hans Asperger. He observed symptoms of autism-like difficulty in communication and social interaction in boys with normal language development and intelligence. Many healthcare professionals have suggested that Asperger’s is a minor form of ASD and specified the term “high-functioning autism” to describe children affected with it
[14].
The Diagnostic and Statistical Manual of Mental Disorders (DSM) is the standard guideline used by psychiatrists and physicians in the United States to diagnose any type of mental illness. In DSM-V diagnostic criteria for ASD, patients have persistent deficits in three areas of social interaction and communication: social-emotional reciprocity; non-verbal communicative behaviors; and maintaining, understanding, developing relationships. It also includes four types of restricted and repetitive behaviors: stereotypical repetitive motor movements, insistence or sameness, highly restricted or fixated interest, and hyper- or hypo-reactivity to sensory signals
[15].
Without a diagnosis, PDD-NOS in children shows manifestations like restricted communications (verbal and non-verbal), social, and stereotypical behavior. Some epidemiological studies suggested that PDD-NOS are twice as common as autism
[16]. The diagnostic features associated with PDD-NOS are (i) onset of this disease after 3 years of age, (ii) atypical symptoms, and (iii) Fewer than six criteria and their subthreshold
[7][17][7,17]. A research study showed that children suffering from PDD-NOS were initially diagnosed with Attention Deficit Hyperactivity Disorder (ADHD) and that they did not differ from ADHD children with respect to all the symptoms of ASD, attention difficulties, or general psychopathology. No method has yet been established for differentiating ADHD and PDD-NOS
[18]. indicates the three severity levels of ASD.
Table 1. Three severity levels of ASD (modified from American Psychiatric Association, 2013)
[13].
|
| Levels |
|
| Clinical Symptoms |
|
| |
| Social Communication |
|
| Repetitive Behavior |
|
|
| Level 1 |
|
| Requires extensive medical support |
|
| Severe impairment in verbal and non-verbal communication; a deficit in social interactions; less response to social overtures (e.g., rarely starts an interaction if they have some words of intelligible speech and respond only to direct social overtures. |
|
| Rigid behavior; extreme problems coping with change; repetitive or restricted behavior marked by interferences in body functioning in all spheres; great difficulty changing action or focus. |
|
|
| Level 2 |
|
| Requires medical support |
|
| Marked impairment in verbal and non-verbal communication; a deficit in social interaction even with support; minimum responses to social overtures like simple spoken sentences; less interest in interaction, and odd behavior in non-verbal communication |
|
| Rigid behavior; difficulty coping with change, repetitive or restricted behavior affecting various functions in different contexts; trouble changing action or focus. |
|
|
| Level 3 |
|
| Requires support |
|
| Noticeable impairments in social interaction without support; problems initiating interactions with people and appears to have less interest in social communication (e.g., affected person speaks full sentences with others but to-and-fro conversation fails and attempts to make friends typically not successful and odd), |
|
| Rigid behavior causes difficulty with functioning in several contexts; problems switching from one activity to another; a deficit in behavior while organizing and planning inhibits independence. |
|
The predictive etiology of ASD characterized by deficits in social communication and interaction as well as repetitive behavior is very complex and has not yet been established. Several factors including environmental, genetic, and epigenetic are found to be associated with this disorder. Important information has been obtained from the characterization of candidate genes through association and case-controlled studies and whole-genome sequencing involving genomic hybridization. Mechanistic studies involving epimutations, genomic imprinting, and methylation have been identified
[19].
3. Etiology and Pathophysiology of ASD
Several factors are thought to be involved in the etiology of ASD. Non-specific signs like developmental delay, complications during pregnancy, dysmorphic features like an increase in head size indicate that ASD is a neuropsychiatric disease. Genetic and environmental factors play a key role in causing ASD. Some modeling studies reported that the interaction of several genes accounts for the underlying genetic complexity
[20]. Thalidomide-associated embryopathy and antiepileptic drugs taken during pregnancy are the primary evidence of environmental factors associated with the etiology of ASD
[21][22][21,22]. Some postmortem investigations using magnetic resonance imaging (MRI) identified an abundance of white matter and some structural impairment in cell alignment and density, especially in the limbic system
[23]. Atypical stimulation of the amygdala and associated structures are analyzed by functional imaging techniques in response to social stimuli in ASD-affected children
[24][25][24,25].
4. Impairment of Developmental Pathways
4.1. Wnt Protein and β-Catenin Signaling Pathways
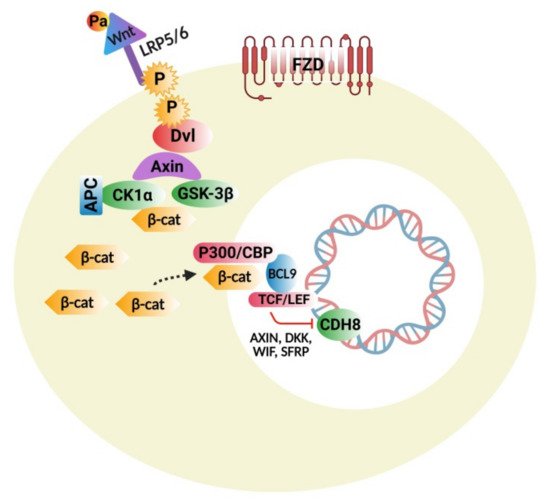
The Wnt protein family consists of several molecules that act as important regulators of stem-cell renewal, embryonic growth and development, cell proliferation, and tissue homeostasis [26]. This signaling cascade is divided mainly into two branches: β-catenin dependent canonical and non-canonical pathways, which are further classified into calcium dependent and a planar-cell polarity nerve tract. Figure 1 represents the Wnt/β-Catenin Signaling Pathway. Beta-catenin is an endogenous protein that is encoded by the gene CTNNB1, an adherent junction component bound to E-cadherin. The key components of the canonical pathway in humans consist of numerous Wnt ligands, low-density lipoprotein 5/6 co-receptors, frizzled receptors, and intracellular and extracellular modulators [27]. Wnt ligands are proteins rich in cysteine and have substantial post-translational modifications, including glycosylation and palmitoylation, which are important for biological actions [28].
The Wnt protein family consists of several molecules that act as important regulators of stem-cell renewal, embryonic growth and development, cell proliferation, and tissue homeostasis [26]. This signaling cascade is divided mainly into two branches: β-catenin dependent canonical and non-canonical pathways, which are further classified into calcium-dependent and a planar-cell polarity nerve tract. represents the Wnt/β-Catenin Signaling Pathway. Beta-catenin is an endogenous protein that is encoded by the gene CTNNB1, an adherent junction component bound to E-cadherin. The key components of the canonical pathway in humans consist of numerous Wnt ligands, low-density lipoprotein 5/6 co-receptors, frizzled receptors, and intracellular and extracellular modulators [27]. Wnt ligands are proteins rich in cysteine and have substantial post-translational modifications, including glycosylation and palmitoylation, which are important for biological actions [28].
Figure 1. Wnt signaling pathway: Binding of Wnt to frizzled receptors (FZD) and LRP5/6; phosphorylation of LRP5/6 by GSK-3β; CK1α attracts the Dvl to the membrane and then inhibits the destruction complex; β-catenin in the cytoplasm is translocated to the nucleus, dislodging Groucho repressor and recruiting various BCL9 co-factors by binding to LEF/TCF. BCL9 and CBP/p300 permit the transcription of Wnt targeted genes, which are involved in cell differentiation, proliferation, and adhesion.
4.2. Hedgehog Signaling Pathway
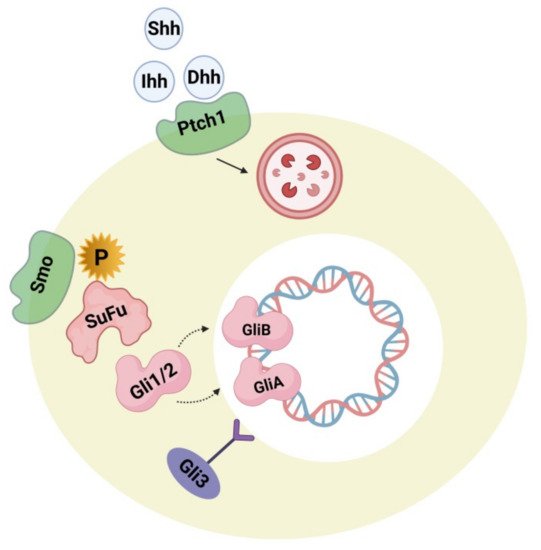
Hedgehog plays a major role during embryogenesis by acting directly on neural development and the dorsoventral pattern. During adulthood, it exerts a role in neuron generation, phenotype determination of neurons, cell cycle, stem-cell maintenance, and apoptosis. These processes include canonical and non-canonical mechanisms [29]. Figure 2 represents the hedgehog signaling pathway, the impairment of which is lethal during embryogenesis, causing serious birth defects like cyclopia and holoencephaly. In the adult human brain, the hedgehog-signaling pathway is executed in a paracrine manner through sonic hedgehog ligand-secreting neurons in the ventral forebrain, substantia nigra, and septum as well as by ligand-activated glial cells in the sub-ventricular, ventricular, sub-granular zone and cortex [30]. Some research studies supported evidence that the sonic hedgehog-signaling pathways perform an important role in cortical neuron fate modulation, circuit establishment, and astrocyte-arbitrated synaptic plasticity [31]. Changes in the cellular response of hedgehog production and pattern can be observed in brain-related disorders like neoplasia, brain-cell injury, and psychiatric disorders [32]. Some studies reported the correlation of components and hedgehog signaling-pathway activation (e.g., Patched activation, ligand concentration, cellular localization) leads smoothly to an etiology of ASD. Research in this field investigated the associated link between hedgehog components in the autistic brain and their synergism with several confounding factors, including congenital mutations in the gene component of the hedgehog pathway, impairment of the oxidative stress defense system, and inborn cholesterol metabolic errors. High serum levels of sonic hedgehog proteins were detected in autistic subjects when compared with an age-matched control group. The severity of the condition was positively correlated with the serum level of sonic hedgehog proteins. In addition to this, it was also observed that, in autistic subjects, blood levels of hydrogen peroxide (H Hedgehog plays a major role during embryogenesis by acting directly on neural development and the dorsoventral pattern. During adulthood, it exerts a role in neuron generation, phenotype determination of neurons, cell cycle, stem-cell maintenance, and apoptosis. These processes include canonical and non-canonical mechanisms [29]. represents the hedgehog signaling pathway, the impairment of which is lethal during embryogenesis, causing serious birth defects like cyclopia and holoencephaly. In the adult human brain, the hedgehog-signaling pathway is executed in a paracrine manner through sonic hedgehog ligand-secreting neurons in the ventral forebrain, substantia nigra, and septum as well as by ligand-activated glial cells in the sub-ventricular, ventricular, sub-granular zone and cortex [30]. Some research studies supported evidence that the sonic hedgehog-signaling pathways perform an important role in cortical neuron fate modulation, circuit establishment, and astrocyte-arbitrated synaptic plasticity [31]. Changes in the cellular response of hedgehog production and pattern can be observed in brain-related disorders like neoplasia, brain-cell injury, and psychiatric disorders [32]. Some studies reported the correlation of components and hedgehog signaling-pathway activation (e.g., Patched activation, ligand concentration, cellular localization) leads smoothly to an etiology of ASD. Research in this field investigated the associated link between hedgehog components in the autistic brain and their synergism with several confounding factors, including congenital mutations in the gene component of the hedgehog pathway, impairment of the oxidative stress defense system, and inborn cholesterol metabolic errors. High serum levels of sonic hedgehog proteins were detected in autistic subjects when compared with an age-matched control group. The severity of the condition was positively correlated with the serum level of sonic hedgehog proteins. In addition to this, it was also observed that, in autistic subjects, blood levels of hydrogen peroxide (H ), and hydroxyl radicals (OH
−) were significantly higher. Thus it was suggested that increased oxidative stress induces the activation of a sonic hedgehog-dependent neuroprotection mechanism [32][33]. Other reports also contributed to the hypothesis that additional anti-oxidative pathway components like BCL2 (B-cell CLL/lymphoma 2 apoptosis regulator) apoptosis regulator, glutathione peroxidase, superoxide dismutase, and the BDNF (brain-derived neurotrophic factor) might cause some changes to the sonic hedgehog protein concentration [34]. Other studies determined an interface between autism phenotypes and the Indian hedgehog and Desert hedgehog proteins. Investigations have shown that the serum concentration of desert hedgehog is lowered in autistic subjects with no exact correlation with disease severity [35]. Indian hedgehog proteins in serum were elevated in autistic subjects more significantly if there was a positive correlation with the severity of the disease [36]. Hence it is suggested that hedgehog proteins along with oxidative stress components may be significant biomarkers for ASD.
) were significantly higher. Thus it was suggested that increased oxidative stress induces the activation of a sonic hedgehog-dependent neuroprotection mechanism [32,33]. Other reports also contributed to the hypothesis that additional anti-oxidative pathway components like BCL2 (B-cell CLL/lymphoma 2 apoptosis regulator) apoptosis regulator, glutathione peroxidase, superoxide dismutase, and the BDNF (brain-derived neurotrophic factor) might cause some changes to the sonic hedgehog protein concentration [34]. Other studies determined an interface between autism phenotypes and the Indian hedgehog and Desert hedgehog proteins. Investigations have shown that the serum concentration of desert hedgehog is lowered in autistic subjects with no exact correlation with disease severity [35]. Indian hedgehog proteins in serum were elevated in autistic subjects more significantly if there was a positive correlation with the severity of the disease [36]. Hence it is suggested that hedgehog proteins along with oxidative stress components may be significant biomarkers for ASD.
Figure 2. The hedgehog (hh) signaling pathway showing hh ligands (Shh, Dhh, Ihh) and their receptor Ptch 1. Upon binding with Ptch1, the pathway causes internalization, and Smo inhibition is released. After this, Smo is phosphorylated causing a cascade activation through downstream regulation, and Gli1/2 is processed into the activator forms GliA and GliB. After translocation of GliA into the nucleus, it stimulates target gene expression. The transcriptional repressor precursor Gli3 remains inactive.
During brain development, the hedgehog pathway is interrelated with several developmental and cell-survival mechanisms. Pathway activation can be implemented by both canonical (patched 1 mediated) and non-canonical processes. The non-canonical mechanism is mediated by several kinases like PKA (phosphokinase), GSK3-3β (Glycogen synthase kinase 3β), S6K (Ribosomal protein S6 kinase), DYRK1B (Dual-specificity tyrosine phosphorylation regulated kinase 1B) that can affect the condition of Gli1/2 phosphorylase, thus regulating its activity [37][38][39]. DYRK1B and S6K are also thought to be associated with the mTOR (mammalian target of rapamycin)/Akt (Protein kinase B) signaling processes, where DYRK1B regulates mTOR/Akt while mTORC1 phosphorylates S6K. Therefore it appears to be evident that there is a connection between Gli1/2 and Phosphoinositide 3-kinase (P13K) mTOR/Akt. Gli plays an important role in dual regulation. Hence, if there is an over-activation of mTOR signaling because of the influence of upstream regulation causing greater S6K activity, hedgehog signaling in the non-canonical pathway becomes upregulated. The biochemical and genetic biomarkers along with oxidative stress and the BDNF biomarker measured in serum, cerebrospinal fluid (CSF), and urine, have been suggested as potential biomarkers for ASD [40]. Some more research studies are required to check the accuracy and reliability of the hedgehog pathway-related tests in identifying ASD phenotypes. Some other studies investigated the role of hedgehog pathways in phenotypic acquisition and T-cell differentiation. Considering this as a reference, the T helper and CD4+ cells, along with high levels of Gli2A, were further differentiated into Th2 cells and secreted six-fold more interleukin 4 cells (IL-4) with normal levels of Gli2A after stimulation, suggesting that IL-4, like Gli, acts as a transcriptional target [41]. It has been reported that the blood serum profile of mothers of autistic offspring show elevated levels of interleukins (IL-4) and dysregulation of T-helper cells and the lymphocytes that regulate them [42] and that autistic patients [43] have increased levels of Sonic hedgehog (Shh) and Indian hedgehog (Ihh) ligands [36]. Conclusively, Gli factors play an important role in cell growth, differentiation, and survival in both the brain and immune system. Hence, more studies are required to prove the accuracy and reliability of the hedgehog-related pathway involvement in the detection of ASD phenotypes as well as modulators for designing novel therapeutic drug targets to treat ASD.
During brain development, the hedgehog pathway is interrelated with several developmental and cell-survival mechanisms. Pathway activation can be implemented by both canonical (patched 1 mediated) and non-canonical processes. The non-canonical mechanism is mediated by several kinases like PKA (phosphokinase), GSK3-3β (Glycogen synthase kinase 3β), S6K (Ribosomal protein S6 kinase), DYRK1B (Dual-specificity tyrosine phosphorylation regulated kinase 1B) that can affect the condition of Gli1/2 phosphorylase, thus regulating its activity [37-39]. DYRK1B and S6K are also thought to be associated with the mTOR (mammalian target of rapamycin)/Akt (Protein kinase B) signaling processes, where DYRK1B regulates mTOR/Akt while mTORC1 phosphorylates S6K. Therefore it appears to be evident that there is a connection between Gli1/2 and Phosphoinositide 3-kinase (P13K) mTOR/Akt. Gli plays an important role in dual regulation. Hence, if there is an over-activation of mTOR signaling because of the influence of upstream regulation causing greater S6K activity, hedgehog signaling in the non-canonical pathway becomes upregulated. The biochemical and genetic biomarkers along with oxidative stress and the BDNF biomarker measured in serum, cerebrospinal fluid (CSF), and urine, have been suggested as potential biomarkers for ASD [40]. Some more research studies are required to check the accuracy and reliability of the hedgehog pathway-related tests in identifying ASD phenotypes. Some other studies investigated the role of hedgehog pathways in the phenotypic acquisition and T-cell differentiation. Considering this as a reference, the T helper and CD4+ cells, along with high levels of Gli2A, were further differentiated into Th2 cells and secreted six-fold more interleukin 4 cells (IL-4) with normal levels of Gli2A after stimulation, suggesting that IL-4, like Gli, acts as a transcriptional target [41]. It has been reported that the blood serum profile of mothers of autistic offspring show elevated levels of interleukins (IL-4) and dysregulation of T-helper cells and the lymphocytes that regulate them [42] and that autistic patients [43] have increased levels of Sonic hedgehog (Shh) and Indian hedgehog (Ihh) ligands [36]. Conclusively, Gli factors play an important role in cell growth, differentiation, and survival in both the brain and immune system. Hence, more studies are required to prove the accuracy and reliability of the hedgehog-related pathway involvement in the detection of ASD phenotypes as well as modulators for designing novel therapeutic drug targets to treat ASD.
4.3. Retinoic Acid (RA) Signaling Pathway
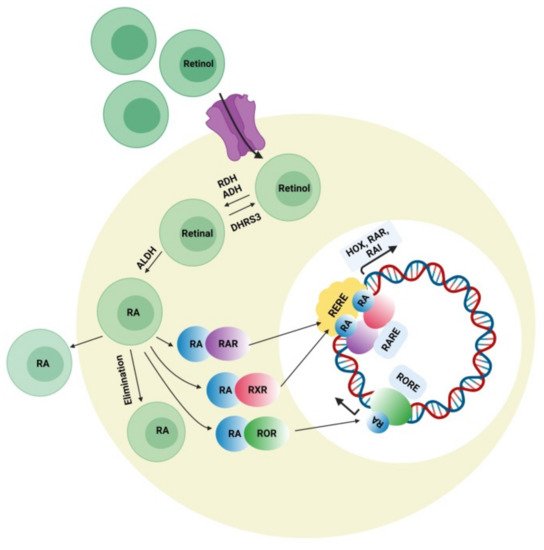
Retinoic acid can affect various developmental genes that contain retinoic acid response elements (RARE) together with their regulatory spaces. This role of RA suggests interconnections among neurodevelopmental disorders and RA signaling pathways. During embryonic development, retinoic acid helps regulate a set of HOX (homeobox) genes that, during embryogenesis, shapes the upper-body pattern both anteriorly and posteriorly and is involved in brain patterning. RA is engaged in neural-cell differentiation involving dopaminergic and GABAergic neurons. It is also a key induction component (together with sonic hedgehog) for the differentiation of motor neurons from pluripotent stem cells [44]. Moreover retinoic acid is also important for the normal functioning of motor neurons. It was also reported that RA is important for neural migration and neurogenesis in the granular zone of the hippocampus, sub-ventricular zone, and olfactory bulb [45]. Retinol concentration in adequate amount is required for normal functioning of RA signaling pathway presuming that all the enzymes related to RA pathway and nuclear factors work accordingly. Figure 3 represents the synthesis of retinoic acid and the retinoic acid signaling pathway. The deficiency of retinol is one of the major causes of decreased intracellular RA signaling. Some studies demonstrated that the deficiency of retinol in rats during pregnancy decreases RA receptor expression (RAR, beta isoform) in the hypothalamus, causing autistic-like symptoms in the neonates [46]. Interestingly, a lower level of retinol was detected in some autistic subjects when compared with the normal control group in China, which was possibly a synergistic factor in ASD symptom development [47]. Retinol supplements can activate RAR expression and lessen ASD symptoms. The important metabolic step in the RA pathway is the conversion of retinal to retinoic acid with the help of the enzyme (ALDH) retinaldehyde dehydrogenase, which ensures the concentration of RA in the cell. An increase in the degradation rate of this enzyme’s isoform ALDH 1A2 because of over-ubiquitinoylation by the enzyme UBE3A (ubiquitin ligase E3) was established in vitro, and autistic features were observed in mice with an overexpression of UBE3A [48]. The loss of function of UBE3A is related to a neurodevelopmental disorder (Angelman syndrome) showing its importance in brain development [49]. The nuclear receptors for RA have also been involved in ASD pathology. RORs (retinoic acid related orphan receptors) activate transcription of several genes by acting as transcriptional regulators upon retinoic acid-binding. A reduction in ROR gene expression due to hypermethylation was found in autistic subjects. In addition, an immunohistochemical analysis of the postmortem brains of autistic subjects confirmed a lower level of ROR alpha protein [50]. Disruption of the retinoic acid enzymatic production pathway was found to be associated with ASD phenotypes and retinoic acid nuclear receptors, which have also been involved in the pathophysiology of ASD; hence, additional studies have to be performed to establish the correlation between ASD pathogenesis and the involvement of RAR and ROR agonists for autism treatment. Quantitative EEG analysis is another signal-detection tool for diagnosing ASD. The details of the individual characterization of EEG fluctuations in ASD subjects could help examine issues of the brain, which would be useful for observing automatic groupings and random draws of the patient population when analyzing the sensory-processing issues of the brain and the peripheral system [51].
Retinoic acid can affect various developmental genes that contain retinoic acid response elements (RARE) together with their regulatory spaces. This role of RA suggests interconnections among neurodevelopmental disorders and RA signaling pathways. During embryonic development, retinoic acid helps regulate a set of HOX (homeobox) genes that, during embryogenesis, shapes the upper-body pattern both anteriorly and posteriorly and is involved in brain patterning. RA is engaged in neural-cell differentiation involving dopaminergic and GABAergic neurons. It is also a key induction component (together with sonic hedgehog) for the differentiation of motor neurons from pluripotent stem cells [44]. Moreover retinoic acid is also important for the normal functioning of motor neurons. It was also reported that RA is important for neural migration and neurogenesis in the granular zone of the hippocampus, sub-ventricular zone, and olfactory bulb [45]. Retinol concentration in adequate amount is required for normal functioning of RA signaling pathway presuming that all the enzymes related to RA pathway and nuclear factors work accordingly. represents the synthesis of retinoic acid and the retinoic acid signaling pathway. The deficiency of retinol is one of the major causes of decreased intracellular RA signaling. Some studies demonstrated that the deficiency of retinol in rats during pregnancy decreases RA receptor expression (RAR, beta isoform) in the hypothalamus, causing autistic-like symptoms in the neonates [46]. Interestingly, a lower level of retinol was detected in some autistic subjects when compared with the normal control group in China, which was possibly a synergistic factor in ASD symptom development [47]. Retinol supplements can activate RAR expression and lessen ASD symptoms. The important metabolic step in the RA pathway is the conversion of retinal to retinoic acid with the help of the enzyme (ALDH) retinaldehyde dehydrogenase, which ensures the concentration of RA in the cell. An increase in the degradation rate of this enzyme’s isoform ALDH 1A2 because of over-ubiquitinoylation by the enzyme UBE3A (ubiquitin ligase E3) was established in vitro, and autistic features were observed in mice with overexpression of UBE3A [48]. The loss of function of UBE3A is related to a neurodevelopmental disorder (Angelman syndrome) showing its importance in brain development [49]. The nuclear receptors for RA have also been involved in ASD pathology. RORs (retinoic acid-related orphan receptors) activate transcription of several genes by acting as transcriptional regulators upon retinoic acid-binding. A reduction in ROR gene expression due to hypermethylation was found in autistic subjects. In addition, immunohistochemical analysis of the postmortem brains of autistic subjects confirmed a lower level of ROR alpha protein [50]. Disruption of the retinoic acid enzymatic production pathway was found to be associated with ASD phenotypes and retinoic acid nuclear receptors, which have also been involved in the pathophysiology of ASD; hence, additional studies have to be performed to establish the correlation between ASD pathogenesis and the involvement of RAR and ROR agonists for autism treatment. Quantitative EEG analysis is another signal-detection tool for diagnosing ASD. The details of the individual characterization of EEG fluctuations in ASD subjects could help examine issues of the brain, which would be useful for observing automatic groupings and random draws of the patient population when analyzing the sensory-processing issues of the brain and the peripheral system [51].
Figure 3. Retinoic acid (RA) signaling pathway. Retinoic acid is synthesized intracellularly from retinol, which first is converted into retinaldehyde by the enzyme alcohol dehydrogenase or retinol dehydrogenase. The reversible conversion of retinal to retinol is mediated by the enzyme retinal reductase (DHRS3). Retinal is irreversibly oxidized to become retinoic acid (RA) by the enzyme retinaldehyde dehydrogenase (ALDHs). Retinoic acid inside the cell binds to receptors present on the surface of the nucleus (RAR, ROR, and RXR) and recognizes consistent response elements (RARE, RORE, and RXRE) along with the DNA, which activates the expression of different target genes.