Hydroxyurea (HU) is a non-alkylating agent administered for the management of different types of cancer or sickle cell disease.
HU has a cytostatic action, blocking cell cycle in S-phase and also inducing double-stranded breaks in DNA.
HU is generally well tolerated, however its widespread use has revealed the presence of adverse events related to tissues that have a high cellular turnover.
- Hydroxyurea
- hydroxycarbamide
- myeloproliferative neoplasms
- safety
Hydroxyurea (HU; also known as hydroxycarbamide) is a non-alkylating agent orally administered for the management of different types of cancer, such as of melanoma, resistant chronic myelocytic leukemia and recurrent, metastatic, or inoperable carcinoma of the ovary. HU is also widely employed for treatment of chronic myeloproliferative neoplasms (MPNs).
In addition to cancer management, HU is used to stimulate fetal hemoglobin production in sickle cell disease
[1]..
Mechanism of action
HU is a non-alkylating agent that is widely employed for treatment of chronic MPNs. HU exhibits non-competitive inhibition of ribonucleotide reductase, which leads to the depletion of deoxyribonucleotides. As a result, DNA synthesis is interrupted and the cell cycle blocked in S-phase (
Figure 1)
[2]. As ribonucleotide reductase is involved in DNA repair, HU also induces double-stranded breaks in DNA
[2] ..
Safety profile
Although HU is generally well tolerated, its widespread use, not only in MPNs, has revealed the presence of adverse events related to tissues that have a high cellular turnover due to the cytostatic action of HU. Multiple cutaneous alterations have been described in patients treated with HU. Among these, cutaneous ulcers and non-melanoma skin cancer lead to treatment discontinuation due to inacceptable toxicity with consequent modification of the treatment approach
[1]..
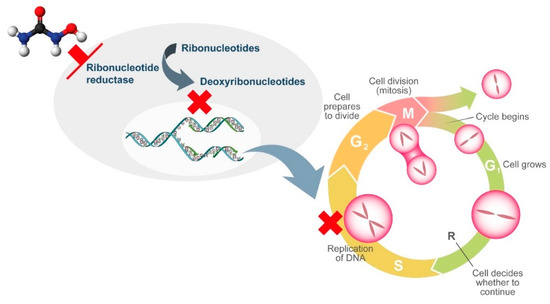
Mechanism of action of hydroxyurea.
Cutaneous Adverse Events Associated with Hydroxyurea in patients with MPNs
[1]
Cutaneous ulcers, typically in the perimalleolar area, are probably an underestimated side effect of HU. The pathogenesis of these lesions is likely multifactorial, and the cytotoxic effects of HU on basal cells of the epidermidis, keratinocytes, and endothelial cells likely plays a key role. HU-induced macrocytosis has also been recognized as a possible cause of microvascular disturbance due to deformability of red blood cells in capillaries and reduced oxygenation of the basal layer of the skin
[3]. On the other hand, MPNs induce alterations in both arterial and venous circulation, likely contributing to ischemia and delays in wound repair
[3]. However, cutaneous ulcers may often occur after long-term treatment with HU and when patients show hematological response.. However, cutaneous ulcers may often occur after long-term treatment with HU and when patients show hematological response.
Non-melanoma skin cancer (NMSC) and actinic keratosis (AK) have been reported to be induced by HU. Impaired DNA repair upon exposure to HU leads to somatic mutations and chromosomal damage, especially in sun-exposed areas, and UV-induced breaks in double-stranded DNA may also contribute to HU-mediated carcinogenesis.
Other HU-induced skin toxicities have only aesthetic implications, such as hyperpigmentation of skin and nails. Melanin deposition by melanocyte stimulation in the nail matrix by HU and photosensitization have been hypothesized as pathogenic mechanisms. Although alopecia represents a frequently described dermatological side effect with the use of more potent cytostatic drugs, it has also been reported in patients treated with HU. Its pathogenesis is related to the alterations in cell kinetics of the hair matrix HU (
Figure 2).).
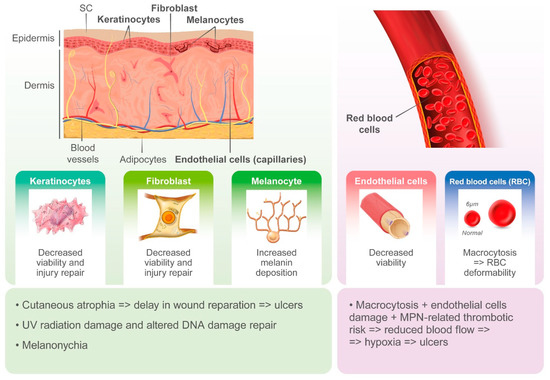
Pathogenesis of hydroxyurea-induced cutaneous toxicity.
Ulcers are the most frequent reported cutaneous adverse event in patients with MPNs undergoing treatment with HU (
Table 1).).
Reported cases of hydroxyurea-induced cutaneous toxicity.
| Study | Study Type | Reported Case | Sex | Age | Underlying Disease | Driver Mutation Gene | HU Dose (g/daily) | Duration of Treatment (months) | Toxicity Type | Site | Biopsy Performed | HU Discontinued | Intervention Type | Vascular Insufficiency | Sun Exposure |
|---|
| Antar, 2014[4] | Case report | 1 | F | 60 | ET | JAK2 | n/r | 60 | SSC | Leg | Yes | Yes | Surgical excision | n/r | n/r |
| Bader, 2000[5] | Case series | 3 | 1 F, 3 M | 84.6 | 2 PV, 1ET, | n/r | 0.66 (0.5–1) | 18–96 | Ulcers | Leg | Yes (2) | Yes (2) | Oral steroid and skin split graft (1) | 3 | n/r |
| Best, 1998[6] | Case series | 10 | 5 F, 4 M | 64.1 | 5 PV, 2ET, 2 MF, 1 u-MPN | n/r | 1.5 (1–2) | 84 (3–15) | Ulcers | Diffuse | Yes | n/r | n/r | n/r | n/r |
| Butler, 2014[7] | Case report | 1 | M | 64 | PV | JAK2 | 1.5 | 36 | Acral erythema | Hand/foot | No | n/r | n/r | n/r | n/r |
| Callot-Mellot, 1996[8] | Case series | 5 | 3 F, 2 M | 71 (64–76) | 2 PV, 3 ET | n/r | n/r | 78 (24–120) | 2 SCC, 3 BCC, actinic keratosis (5) | n/r | Yes | Yes | n/r | n/r | n/r |
| Cohen, 1999[9] | Case report | 1 | F | 70 | PV | n/r | 2–4 | 48 | Melanonychia | Fingernails and toenails | No | Yes | n/r | n/r | n/r |
| Daoud, 1997[10] | Case series | 3 | n/v | 56-69 | 1 PV, 2 ET | n/r | n/v | 61 (55–79) | 3 ulcers, 1 poikilodermatous eruption | Palms, toes, dorsal feet, ankles | Yes | Yes | n/r | n/r | n/r |
| De Benedettis, 2004[11] | Case report | 1 | M | 66 | PV | n/r | 1 | 204 | Ulcers, SCC | Leg, oral SCC | Yes | Yes | Surgical excision | n/r | n/r |
| Demicray, 2002[12] | Case series | 3 | 3 F | 61.6 (56–65) | 3 ET | n/r | 1 | 50 (6–84) | Ulcers | Leg | Yes (2) | Yes (1/3) | Oral steroids | 2/3 | n/r |
| Esteve, 2001[13] | Case report | 1 | F | 83 | PV | n/r | n/r | 156 | Actinic keratosis, SCC | Hands | Yes | Yes | Surgical excision | n/r | n/r |
| Hernandez-Martin, 1999[14] | Case report | 1 | M | 78 | ET | n/r | 1 | 5 | Melanonychia | Fingernails and toenails | No | No | None | n/r | n/r |
| Hirri, 2001[15] | Case Report | 1 | M | 66 | u-MPN | n/r | 1.5 | 8 | Ulcers | Leg | No | Yes | None | n/r | n/r |
| Hoff, 2009[16] | Case report | 1 | F | 68 | PV | n/r | n/r | 96 | Ulcers, actinic keratosis, SCC | Leg, head | Yes | Yes | Surgical excision, cryotherapy | No | n/r |
| Hwang, 2009[17] | Case report | 1 | M | 75 | ET | n/r | 2 | 48 | Ulcers, melanonychia | Leg, fingernails and toenails | Yes | Yes | None | n/r | n/r |
| Kelly, 1994[18] | Case report | 1 | M | 61 | PV | n/r | 1.5–2 | 72 | Actinic keratosis, BCC | Diffuse | Yes | No | Surgical excision, topical steroids | n/r | Yes |
| Kluger, 2011[19] | Case report | 1 | F | 74 | ET | n/r | 0.6 | 36 | Melanonychia | Toenails | No | No | None | n/r | n/r |
| Kwong, 1996[20] | Case report | 1 | F | 69 | ET | n/r | 2–3 | 6 | Melanonychia | Fingernails and Toenails | No | n/r | None | n/r | n/r |
| Simeonovski, 2018[21] | Case report | 1 | M | 52 | ET | n/r | 1.5 | >120 | Perimalleolar and nummular lesions, actinic keratosis, BCC | Less, arms, nose | Yes | Yes | Surgical excision, cryotherapy | n/r | n/r |
| Accurso, 2019[22] | Case report | 1 | F | 72 | MPN | JAK2 | n/r | ≈84 | Desquamative dermatitis | Diffuse facial | No | Yes | Topical and systemic steroids | n/r | n/r |
ET: Essential Thrombocythemia; MPN: Myeloproliferative neoplasms; PV: Polycythemia Vera; SSC: Squamous Cell Carcinoma; BCC: Basal Cell carcinoma; n/r: not reported.
Altogether, 27 papers have been published, including a randomized controlled clinical trial, retrospective studies, case series, and case reports, accounting for a total of 249 cases
[5][6][10][11][12][16][23][24][25][26][27][28][29][30][31][32][33][34]. The demographic and clinical features of MPN have not always have been described, but when available the median age of patients with skin ulcers was 67 years (range 19–91) and there was a higher prevalence of HU-related ulcers in women (61.4%, 108/176) compared with men (38.6%, 68/176). Underlying MPN pathologies were distributed as follows: PV 32.4% (67/207), ET 54.6% (113/207), MF 12.1% (25/207), and u-MPN 1% (2/207). The median time from initiation of HU to the detection of a skin ulcer was 60 months (range 1–262) at a median daily dose of 1 g (range 0.25–2)
[5][6][10][11][12][16][17][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38]. The most frequent localization was the leg, at the perimalleolar side, and concomitant venous or arterial insufficiency was reported in 38 cases. To ensure healing of the lesion, discontinuation of therapy is mandatory and was described in virtually all cases in which intervention was specified (
Figure 3).).
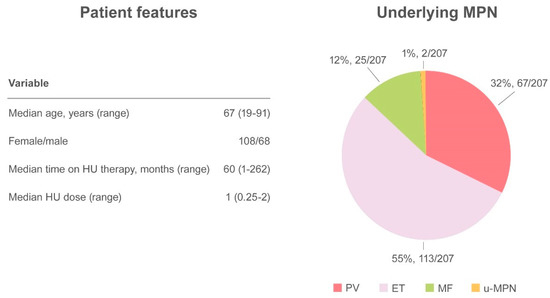
Summary of cutaneous ulcers as an adverse event.
AK and NMSC are notable adverse events in HU-treated patients (
Figure 4).).
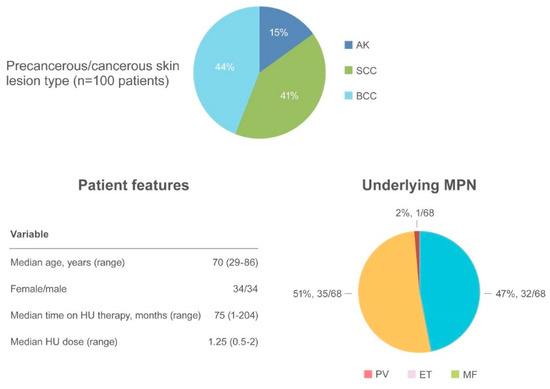
Summary of actinic keratosis and non-melanoma skin cancer as an adverse event.
AK has been reported in 15 patients
[29][35][39][40][41][42][43]often preceding the appearance of NMSC. SCC occurred in 41 patients and BCC in 44 patients
[6][16][24][29][31][34][35][41][42][44][45]. The median age of onset was 70.6 years (range 29–86), with a similar incidence of NMSC in women (34 patients) and men (34 patients). The most frequently involved regions were photo-exposed areas, such as the scalp (43 patients), ears/neck (5 patients), hands (5 patients), and diffuse pattern (5 patients). When reported, the underlying MPN disease were as follows: PV (32/68, 47%), ET (35/68, 51.5%) and MF (1/68, 1.5%). The median time to NMSC after initiating HU was 75 months (range 1–204) at a median HU daily dose of 1.25 g (range 0.5–2). Surgical excision of the suspected lesion and HU discontinuation were the most frequent types of intervention.. The median age of onset was 70.6 years (range 29–86), with a similar incidence of NMSC in women (34 patients) and men (34 patients). The most frequently involved regions were photo-exposed areas, such as the scalp (43 patients), ears/neck (5 patients), hands (5 patients), and diffuse pattern (5 patients). When reported, the underlying MPN disease were as follows: PV (32/68, 47%), ET (35/68, 51.5%) and MF (1/68, 1.5%). The median time to NMSC after initiating HU was 75 months (range 1–204) at a median HU daily dose of 1.25 g (range 0.5–2). Surgical excision of the suspected lesion and HU discontinuation were the most frequent types of intervention.
References
- Alessandra Malato; Elena Rossi; Giuseppe Alberto Palumbo; Paola Guglielmelli; Novella Pugliese; Drug-Related Cutaneous Adverse Events in Philadelphia Chromosome-Negative Myeloproliferative Neoplasms: A Literature Review. International Journal of Molecular Sciences 2020, 21, 3900, 10.3390/ijms21113900.
- AmanPreet Singh; Yong-Jie Xu; The Cell Killing Mechanisms of Hydroxyurea. Genes 2016, 7, 99, 10.3390/genes7110099.
- Filippo Quattrone; Valentina Dini; Sabrina Barbanera; Nicola Zerbinati; Marco Romanelli; Cutaneous ulcers associated with hydroxyurea therapy. Journal of Tissue Viability 2013, 22, 112-121, 10.1016/j.jtv.2013.08.002.
- Ahmad Antar; Rim S. Ishak; Zaher K. Otrock; Nadim El Majzoub; Samer Ghosn; Rami Mahfouz; Ali T. Taher; Successful treatment of hydroxyurea-associated chronic leg ulcers associated with squamous cell carcinoma. Hematology/Oncology and Stem Cell Therapy 2014, 7, 166-169, 10.1016/j.hemonc.2014.09.008.
- U. Bader; M. Bányai; R. Böni; G. Burg; J. Hafner; Leg Ulcers in Patients with Myeloproliferative Disorders: Disease- or Treatment-Related?. Dermatology 2000, 200, 45-48, 10.1159/000018315.
- Patricia J. Best; Mazen S. Daoud; Mark R. Pittelkow; Robert M. Petitt; Hydroxyurea-induced leg ulceration in 14 patients.. Annals of Internal Medicine 1998, 128, 29-32, 10.7326/0003-4819-128-1-199801010-00005.
- Daniel Butler; Vinod E. Nambudiri; Tina Nandi; Hydroxyurea-associated acral erythema in a patient with polycythemia vera. American Journal of Hematology 2014, 89, 931-932, 10.1002/ajh.23698.
- C. Callot-Mellot; Cutaneous carcinoma during long-term hydroxyurea therapy: a report of 5 cases. Archives of Dermatology 1996, 132, 1395-1397, 10.1001/archderm.132.11.1395.
- A. D. Cohen; Dafnah Hallel-Halevy; Lev Hatskelzon; E Peretz; Sima Halevy; Longitudinal melanonychia associated with hydroxyurea therapy in a patient with essential thrombocytosis.. Journal of the European Academy of Dermatology and Venereology 1999, 13, 137-139, 10.1111/j.1468-3083.1999.tb00868.x.
- Mazen S. Daoud; Lawrence E. Gibson; Mark R. Pittelkow; Hydroxyurea dermopathy: A unique lichenoid eruption complicating long-term therapy with hydroxyurea. Journal of the American Academy of Dermatology 1997, 36, 178-182, 10.1016/s0190-9622(97)70276-7.
- M. De Benedittis; Massimo Petruzzi; C. Giardinà; Lorenzo Lo Muzio; Gianfranco Favia; Rosario Serpico; Oral squamous cell carcinoma during long-term treatment with hydroxyurea. Clinical and Experimental Dermatology 2004, 29, 605-607, 10.1111/j.1365-2230.2004.01586.x.
- Zeynep Demircay; Asuman Cömert; Cafer Adigüzel; Leg ulcers and hydroxyurea: report of three cases with essential thrombocythemia. International Journal of Dermatology 2002, 41, 872-874, 10.1046/j.1365-4362.2002.01623.x.
- Eric Esteve; V Georgescu; P Heitzmann; L Martin; [Multiple skin and mouth squamous cell carcinomas related to long-term treatment with hydroxyurea].. Annales de Dermatologie et de Vénéréologie 2001, 128, 919-921.
- Ángela Hernández‐Martín M.D.; Santiago Ros-Forteza; P. De Unamuno; Longitudinal, transverse, and diffuse nail hyperpigmentation induced by hydroxyurea.. Journal of the American Academy of Dermatology 1999, 41, 333-334, 10.1016/s0190-9622(99)70379-8.
- H. M. Hirri; P. J. Green; Skin lesion caused by hydroxyurea.. European Journal of Haematology 2002, 67, 328-329, 10.1034/j.1600-0609.2001.00567.x.
- N.-P. Hoff; S. Akanay-Diesel; U. Pippirs; K.-W. Schulte; S. Hanneken; Kutane Nebenwirkungen einer Hydroxyurea-Therapie bei Polycythaemia vera. Der Hautarzt 2009, 60, 783-787, 10.1007/s00105-009-1844-8.
- Seon-Wook Hwang; Soon-Kwon Hong; Sang-Hyun Kim; Jong-Keun Seo; Deborah Lee; Ho-Suk Sung; A Hydroxyurea-induced Leg Ulcer. Annals of Dermatology 2009, 21, 39-41, 10.5021/ad.2009.21.1.39.
- R I Kelly; R H Bull; A Marsden; Cutaneous manifestations of long-term hydroxyurea therapy.. Australasian Journal of Dermatology 1994, 35, 61-64.
- Nicolas Kluger; Magali Naud; Pierre Francès; Toenails melanonychia induced by hydroxyurea. La Presse Médicale 2012, 41, 444-445, 10.1016/j.lpm.2011.06.024.
- Y.L Kwong; Hydroxyurea-induced nail pigmentation. Journal of the American Academy of Dermatology 1996, 35, 275-276, 10.1016/s0190-9622(96)90353-9.
- Viktor Simeonovski; Hristina Breshkovska; Silvija Duma; Ivana Dohcheva-Karajovanov; Katerina Damevska; Suzana Nikolovska; Hydroxyurea Associated Cutaneous Lesions: A Case Report. Open Access Macedonian Journal of Medical Sciences 2018, 6, 1458-1461, 10.3889/oamjms.2018.320.
- M Santoro; Vincenzo Accurso; V Caputo; S Fiorella; P Casimiro; A case of severe dermatitis in a patient with Polycythemia Vera during cytoreductive therapy. Archives of Hematology Case Reports and Reviews 2019, 4, 020-021, 10.17352/ahcrr.000020.
- Roberto Latagliata; Antonio Spadea; Michele Cedrone; Jonny Di Giandomenico; Marianna De Muro; Nicoletta Villivà; Massimo Breccia; Barbara Anaclerico; Raffaele Porrini; Francesca Spirito; et al.Angela RagoGiuseppe AvvisatiGiuliana AlimenaMarco MontanaroAlessandro AndrianiAnd Gruppo Laziale Smpc Ph1 Neg Symptomatic mucocutaneous toxicity of hydroxyurea in Philadelphia chromosome-negative myeloproliferative neoplasms. Cancer 2011, 118, 404-409, 10.1002/cncr.26194.
- Yves Najean; Jean-Didier Rain; Treatment of Polycythemia Vera: The Use of Hydroxyurea and Pipobroman in 292 Patients Under the Age of 65 Years. Blood 1997, 90, 3370-3377, 10.1182/blood.v90.9.3370.
- Maria L Randi; Elisabetta Ruzzon; Fabiana Tezza; Guido Luzzatto; Fabrizio Fabris; Toxicity and side effects of hydroxyurea used for primary thrombocythemia. Platelets 2005, 16, 181-184, 10.1080/09537100400020179.
- F. Ravandi-Kashani; J. Cortes; P. Cohen; M. Talpaz; S. O'brien; A. Markowitz; H. Kantarjian; Cutaneous Ulcers Associated with Hydroxyurea Therapy in Myeloproliferative Disorders. Leukemia & Lymphoma 1999, 35, 109-118, 10.3109/10428199909145710.
- Marco Romanelli; Valentina Dini; Paolo Romanelli; Hydroxyurea-Induced Leg Ulcers Treated With a Protease-Modulating Matrix. Archives of Dermatology 2007, 143, 1310-1313, 10.1001/archderm.143.10.1310.
- Guillermo José Ruiz-Argüelles; Guillermo J. Ruiz-Delgado; Guillermo Ruiz-Reyes; Selbert G. Chernoff; Anagrelide-Induced Relapse of a Hydroxyurea-Induced Leg Ulcer in a Patient With Primary Thrombocythemia. Mayo Clinic Proceedings 1998, 73, 1125, 10.4065/73.11.1125.
- V. Salmon-Ehr; G. Leborgne; J.P. Vilque; G. Potron; P. Bernard; Effets secondaires cutanés de l'hydroxyurée : étude prospective de 26 patients consultant dans un service de dermatologie. La Revue de Médecine Interne 2000, 21, 30-34, 10.1016/s0248-8663(00)87226-4.
- P. Senet; Selim Aractingi; M. Pornkuf; P. Perrin; M. Duterque; Hydroxyurea-induced dermatomyositis-like eruption. British Journal of Dermatology 1995, 133, 455-459, 10.1111/j.1365-2133.1995.tb02677.x.
- Tamar Stone; Alexandra Berger; Sheila Blumberg; Daniel O’Neill; Frank Ross; Alexander Mcmeeking; Weiliam Chen; Irena Pastar; A multidisciplinary team approach to hydroxyurea-associated chronic wound with squamous cell carcinoma. International Wound Journal 2011, 9, 324-329, 10.1111/j.1742-481x.2011.00887.x.
- Antonio Vélez; José‐María García‐Aranda; José-Carlos Moreno; Hydroxyurea-induced leg ulcers: is macroerythrocytosis a pathogenic factor?. Journal of the European Academy of Dermatology and Venereology 1999, 12, 243-244, 10.1111/j.1468-3083.1999.tb01037.x.
- G. Weinlich; Gerold Schuler; Richard Greil; Heinz Kofler; Peter Fritsch; Leg ulcers associated with long-term hydroxyurea therapy.. Journal of the American Academy of Dermatology 1998, 39, 372-374, 10.1016/s0190-9622(98)70394-9.
- Elisa Zaccaria; Emanuele Cozzani Md; Aurora Parodi; Secondary cutaneous effects of hydroxyurea: Possible pathogenetic mechanisms. Journal of Dermatological Treatment 2006, 17, 176-178, 10.1080/09546630600780494.
- Elisabetta Antonioli; Paola Guglielmelli; Lisa Pieri; Mariachiara Finazzi; Elisa Rumi; Vincenzo Martinelli; Nicola Vianelli; Maria Luigia Randi; Irene Bertozzi; Valerio De Stefano; et al.Tommaso ZaElena RossiMarco RuggeriElena ElliRossella CacciolaEmma CacciolaE.M. PoglianiFrancesco RodeghieroMichele BaccaraniFabrizio PassamontiGuido FinazziAlessandro RambaldiAlberto BosiMario CazzolaT BarbuiAlessandro Maria Vannucchion behalf of the AGIMM Investigators Hydroxyurea-related toxicity in 3,411 patients with Ph'-negative MPN. American Journal of Hematology 2012, 87, 552-554, 10.1002/ajh.23160.
- S Natarajan; D Williamson; J Grey; Keith Harding; Ra Cooper; Healing of an MRSA-colonized, hydroxyurea-induced leg ulcer with honey. Journal of Dermatological Treatment 2001, 12, 33-36, 10.1080/095466301750163563.
- Elisabetta Ruzzon; Maria L Randi; Fabiana Tezza; Guido Luzzatto; Raffaella Scandellari; Fabrizio Fabris; Leg ulcers in elderly on hydroxyurea: a single center experience in Ph− myeloproliferative disorders and review of literature. Aging Clinical and Experimental Research 2006, 18, 187-190, 10.1007/bf03324647.
- M E Sirieix; C Debure; N Baudot; L. Dubertret; M E Roux; P Morel; C Frances; S Loubeyres; C Beylot; D Lambert; et al.P HumbertO GauthierM DandurandBernard GuillotL. VaillantGérard LoretteJ M BonnetblancC LokJ P Denoeux Leg ulcers and hydroxyurea: forty-one cases.. Archives of Dermatology 1999, 135, 818-820.
- Jerald P. Radich; When to Consider Allogeneic Transplantation in CML. Clinical Lymphoma Myeloma and Leukemia 2016, 16, S93-S95, 10.1016/j.clml.2016.02.008.
- Maria Luigia Randi; Elisabetta Ruzzon; Guido Luzzatto; Fabiana Tezza; A. Girolami; Fabrizio Fabris; Safety profile of hydroxyurea in the treatment of patients with Philadelphia-negative chronic myeloproliferative disorders.. Haematologica 2005, 90, 261-262.
- Tatjana Roš; Branislava Gajic; Zorica Gajinov; Milana Ivkov-Simic; Slobodan Stojanovic; Zoran Golušin; Hydroxyurea and nonmelanoma skin cancers: Report of three cases and review of the literature. Vojnosanitetski pregled 2017, 74, 1089-1093, 10.2298/vsp160218298r.
- Carla Sanchez-Palacios; Joan Guitart; Hydroxyurea-associated squamous dysplasia. Journal of the American Academy of Dermatology 2004, 51, 293-300, 10.1016/j.jaad.2003.11.059.
- Rosita Saraceno; Miriam Teoli; Sergio Chimenti; Hydroxyurea associated with concomitant occurrence of diffuse longitudinal melanonychia and multiple squamous cell carcinomas in an elderly subject.. Clinical Therapeutics 2008, 30, 1324-1329, 10.1016/s0149-2918(08)80057-4.
- Montse Gómez; Vicent Guillem; Arturo Pereira; Francisca Ferrer-Marín; Alberto Alvarez-Larrán; Ana Kerguelen; Natàlia Estrada; Joaquín Martínez-López; Anna Angona; Paula Amat; et al.Blanca NavarroCarles BessesJuan Carlos Hernández-Boluda Risk factors for non-melanoma skin cancer in patients with essential thrombocythemia and polycythemia vera. European Journal of Haematology 2015, 96, 285-290, 10.1111/ejh.12588.
- Jennifer M. Levine; Michael Weiner; Kara M. Kelly; Routine Use of PET Scans After Completion of Therapy in Pediatric Hodgkin Disease Results in a High False Positive Rate. Journal of Pediatric Hematology/Oncology 2007, 29, 667, 10.1097/mph.0b013e3181462320.
