Mesenchymal stem cells (MSCs) have immunomodulatory and regenerative effects in many organs, including the kidney. Emerging evidence has shown that the trophic effects from MSCs are mainly mediated by the paracrine mechanism rather than the direct differentiation of MSCs into injured tissues. These secretomes from MSCs include cytokines, growth factors, chemokines and extracellular vesicles (EVs) containing microRNAs, mRNAs, and proteins. Many research studies have revealed that secretomes from MSCs have potential to ameliorate renal injury in renal disease models, including acute kidney injury and chronic kidney disease through a variety of mechanisms. These trophic mechanisms include immunomodulatory and regenerative effects. In addition, accumulating evidence has uncovered the specific factors and therapeutic mechanisms in MSC-derived EVs. We summarize the immunomodulatory and regenerative effects of EVs from MSCs.
- mesenchymal stem cell
- extracellular vesicles
- microRNA
In addition to the immunomodulatory effects, MSC-EVs have the potential to promote renal regeneration and protection in a variety of mechanisms (Figure 1).
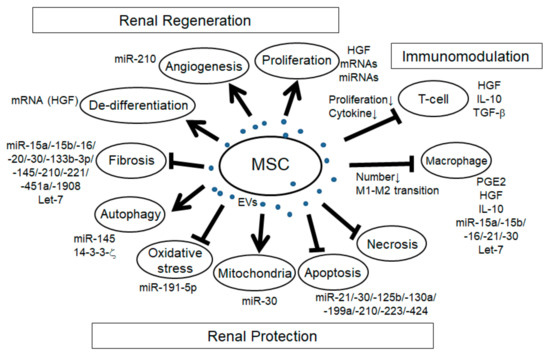
Figure 1. Schema of the effects of mesenchymal stem cell extracellular vesicles (MSC-EVs) in renal injury. Arrows: promotion of the processes; T-bars: inhibition of the processes.
Renal Regeneration
In many reports of AKI models induced by I/R and glycerol and cisplatin-induced rodent models, the increase of tubular proliferation was observed under the treatment of MSC-EVs [1][2][3][4][5][6][7][8][9], indicating the regenerative effect of MSC-EVs. Although it is not easy to identify the specific factors for cell proliferation, several factors might be involved. For example, HGF has been reported to enhance mitogenesis in I/R-AKI rat model [10]. mRNA and/or miRNA in MSC-EVs might be involved as well since the enhancement of tubular proliferation with MSC-EVs treatment in glycerol-induced AKI was abolished with RNase treatment. Angiogenesis is another mechanism of renal regeneration in rodent AKI models. MSC-EVs treatment in I/R-induced AKI rat model showed the increase of VEGF expression and capillary vessel density that was abolished with RNase treatment [5]. In addition, MSC-EVs treatment in I/R-induced AKI rat model showed the increase of VEGF and VEGFR2 expression as well as the increase of miR-210, and the overexpression of miR-210 in HUVEC-12 cells increased VEGF and VEGFR2 expression and promoted angiogenesis in vitro, indicating that miR-210 might be involved in MSC-EV-induced angiogenesis by targeting VEGF signaling [11]. The expression of angiogenesis indicators, CD31, von Willebrand factor (vWF), and angiopoietin was upregulated with MCS-EVs treatment in I/R-induced AKI rat model [12]. In addition, in the UUO-induced CKD mouse model, MSC-EVs treatment improved the rarefaction of peritubular capillaries detected by CD31 staining [13]. During renal regeneration after AKI, epithelial de-differentiation has been reported to be an important process that promotes cell survival, migration, and proliferation [14]. HGF, TGF-β1, IGF, and EGF have been reported to be involved in the de-differentiation [14][15]. Indeed, Ju et al. reported that MSC-EVs treatment in I/R-induced AKI rat model promoted tubular epithelial cell de-differentiation via HGF induction that was blocked with RNase treatment, suggesting that HGF induction might be mediated by mRNAs and/or miRNAs [16]. Furthermore, they revealed that the HGF mRNA from MSC-EVs entered the injured tubular cells and was translated into HGF protein, indicating foreign HGF synthesis. In summary, MSC-EVs have a variety of trophic mechanisms for renal regeneration through the regulation of cell proliferation, angiogenesis, and tubular cell de-differentiation.
Renal Protection
The mechanism regulating cell apoptosis is one of the most important aspects of renal protection. Indeed, the anti-apoptotic pathway has been reported in many studies with MSC-EVs treatment in a variety of rodent renal injury models, including AKI induced by I/R [17][18][19][12][20][5][6][7][9][16][21], cisplatin [22][8][23], gentamicin [3], and glycerol [1], hypoxia-induced renal injury [24], aldosterone-induced renal injury [25], and UUO-induced CKD model [13]. Recent advances have gradually uncovered the specific miRNAs, their targets and signaling pathways involved in the effect of anti-apoptosis (Table 2), including PTEN, AKT, mTOR, dynamin-related protein 1 (DRP1), Sema3A, and ERK signaling pathway. Anti-necrosis with MSC-EVs treatment has also been reported in drug-induced AKI, such as with glycerol, cisplatin, and gentamicin [3][4][8]. Autophagy regulation is another aspect of trophic mechanism with MSC-EVs treatment for renal protection. In cisplatin-induced AKI model, the improvement of autophagy was observed with MSC-EVs treatment [26]. In the study, there was an increase of autophagy marker and LC3II expression in vivo and in vitro with MSC-EVs treatment, and it was associated with 14-3-3ζ expression, which regulates ATG-16L. Another group also indicated that MSC-EVs treatment increased the LC3B expression as well as the ATG-5 and ATG-7 gene expressions in normal rat kidney-52E (NRK-52E) cells through the inhibition of mTOR signaling [27]. In addition, Xiang et al. indicated that MSC-EVs enhanced the expression of LC3II and beclin 1 in HK-2 cells through miR-145 targeting PI3K/AKT/mTOR-signaling pathway [28]. Under pathological conditions, autophagy is induced as the adaptive and protective mechanism for cell survival, thus the regulation of autophagy is an important mechanism for renal protection. Preservation of mitochondria is also an important mechanism for renal protection from AKI. DRP1 is known as the key regulator for mitochondrial fission [29] and is rapidly activated after AKI [30]. Inhibition of DRP1 has been reported to protect the kidney from AKI injury caused by I/R and cisplatin [30][31]. Indeed, MSC-EVs treatment preserved mitochondrial function via transferring miR-30 that inhibited the expression of DRP1 [17]. Improvement of oxidative stress with MSC-EVs treatment is also an important mechanism for renal protection against cisplatin and I/R-induced AKI [12][6][8][21]. Oxidative stress via reactive ROS production induces apoptosis, necrosis, and inflammation in AKI. Zhang et al. reported that MSC-EVs treatment alleviated oxidative stress detected by the reduction of malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OhdG) as well as the enhancement of Nrf2 and HO-1 in I/R-induced AKI rat model [21]. NOX-2, known as the inducer of ROS production, declined with MSC-EVs treatment in I/R-induced AKI rat model [6]. In addition, Zhou et al. reported that MSC-EVs treatment ameliorated oxidative stress in cisplatin-induced AKI detected by the reduction of 8-OhdG-positive cells, which reduced the cell apoptosis [8]. HGF has also been reported to improve oxidative stress through the suppression of GLUT1 [32]. Taken together, several factors in MSC-EVs might be involved in the regulation of oxidative stress. Renal fibrosis is the pathological process of CKD, which is strongly associated with the progression of renal dysfunction. The improvement of renal fibrosis with MSC-EVs treatment was reported in a variety of renal injury models, including I/R-induced AKI rodent model [2][12][5][6][16], renovascular stenosis-induced pig CKD model [33], UUO-induced renal fibrosis model [34][35][13], diabetes nephropathy mouse model [36][37], and 5/6 subtotal nephrectomy mouse model [38]. In vitro experiment using HK-2 cells, MSC-EVs ameliorated TGF-β1-induced EMT, which was mediated by miR-133b-3p and miR-294 [39]. As described ahead, let-7c from MSC-EVs ameliorated renal fibrosis in UUO model with the downregulation of Col4a1, MMP-9, TGF-β1, and TGFBR1 [34]. In addition, miR-451 in MSC-EVs ameliorated renal fibrosis by targeting P15 and P19, thus inhibiting EMT in diabetic model [30]. MiR-29b in MSC-EVs might target snail, thereby regulating EMT [40]. Taken together, several MSC-EVs-derived miRNAs have the potential to ameliorate renal fibrosis, mainly through the regulation of TGF-β1-EMT axis. In summary, EV-MSCs have a variety of factors involved in many biological processes for renal generation and protection as well as immunomodulation.
References
- Stefania Bruno; Cristina Grange; Maria Chiara Deregibus; Raffaele Calogero; Silvia Saviozzi; Federica Collino; Laura Morando; Alessandro Busca; Michele Falda; Benedetta Bussolati; et al.Ciro TettaGiovanni Camussi Mesenchymal stem cell-derived microvesicles protect against acute tubular injury.. Journal of the American Society of Nephrology 2009, 20, 1053-1067, 10.1681/ASN.2008070798.
- Xiangyu Zou; Guangyuan Zhang; Zhongliang Cheng; Deming Yin; Tao Du; Guanqun Ju; Shuai Miao; Guo-Hua Liu; Mujun Lu; Yingjian Zhu; et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Research & Therapy 2014, 5, 40, 10.1186/scrt428.
- Luciana A. Reis; Fernanda Teixeira Borges; Manuel De Jesus Simões; Andrea A. Borges; Rita Sinigaglia-Coimbra; N. Schor; Bone Marrow-Derived Mesenchymal Stem Cells Repaired but Did Not Prevent Gentamicin-Induced Acute Kidney Injury through Paracrine Effects in Rats. PLOS ONE 2012, 7, e44092, 10.1371/journal.pone.0044092.
- Stefania Bruno; Marta Tapparo; Federica Collino; Giulia Chiabotto; Maria Chiara Deregibus; Rafael Soares Lindoso; Francesco Neri; Sharad Kholia; Sara Giunti; Sicheng Wen; Peter Quesenberry; Giovanni Camussi; Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells.. Tissue Engineering Part A 2017, 23, 1262-1273, 10.1089/ten.TEA.2017.0069.
- Xiangyu Zou; Di Gu; Xiaoyu Xing; Zhongliang Cheng; Dongliang Gong; Guangyuan Zhang; Yingjian Zhu; Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. American journal of translational research 2016, 8, 4289-4299, null.
- Guangyuan Zhang; Xiangyu Zou; Shuai Miao; Jinjun Chen; Tao Du; Liang Zhong; Guanqun Ju; Guo-Hua Liu; Yingjian Zhu; The Anti-Oxidative Role of Micro-Vesicles Derived from Human Wharton-Jelly Mesenchymal Stromal Cells through NOX2/gp91(phox) Suppression in Alleviating Renal Ischemia-Reperfusion Injury in Rats. PLOS ONE 2014, 9, e92129, 10.1371/journal.pone.0092129.
- Hoon Young Choi; Sung Jin Moon; Brian B. Ratliff; Sun Hee Ahn; Ara Jung; Mirae Lee; Seol Lee; Beom Jin Lim; Beom Seok Kim; Matthew D. Plotkin; Sung Kyu Ha; Hyeong Cheon Park; Microparticles from Kidney-Derived Mesenchymal Stem Cells Act as Carriers of Proangiogenic Signals and Contribute to Recovery from Acute Kidney Injury. PLOS ONE 2014, 9, e87853, 10.1371/journal.pone.0087853.
- Ying Zhou; Huitao Xu; Wenrong Xu; Bingying Wang; Huiyi Wu; Yang Tao; Bin Zhang; Mei Wang; Fei Mao; Yongmin Yan; Shuo Gao; Hongbing Gu; Wei Zhu; Hui Qian; Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Research & Therapy 2013, 4, 34-34, 10.1186/scrt194.
- Stefano Gatti; Stefania Bruno; Maria Chiara Deregibus; Andrea Sordi; V. Cantaluppi; Ciro Tetta; Giovanni Camussi; Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrology Dialysis Transplantation 2011, 26, 1474-1483, 10.1093/ndt/gfr015.
- S B Miller; D R Martin; J Kissane; M R Hammerman; Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats.. American Journal of Physiology-Legacy Content 1994, 266, F129–F134.
- Zhang, C.; Ma, P.; Zhao, Z.; Jiang, N.; Lian, D.; Huo, P.; Yang, H.; miRNAmRNA regulatory network analysis of mesenchymal stem cell treatment in cisplatininduced acute kidney injury identifies roles for miR210/Serpine1 and miR378/Fos in regulating inflammation. Mol. Med. Rep. 2019, , 20, , 1509–1522..
- Kun-Chen Lin; Hon-Kan Yip; Pei-Lin Shao; Shun-Cheng Wu; Kuan-Hung Chen; Yen-Ta Chen; Chih-Chao Yang; Cheuk-Kwan Sun; Gour-Shenq Kao; Sheng-Yi Chen; et al.Han-Tan ChaiChia-Lo ChangChih-Hung ChenMel S. Lee Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia–reperfusion injury. International Journal of Cardiology 2016, 216, 173-185, 10.1016/j.ijcard.2016.04.061.
- Hoon Young Choi; Hyun Gyu Lee; Beom Seok Kim; Sun Hee Ahn; Ara Jung; Mirae Lee; Jung Eun Lee; Hyung Jong Kim; Sung Kyu Ha; Hyeong Cheon Park; et al. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction.. Stem Cell Research & Therapy 2015, 6, 18, 10.1186/s13287-015-0012-6.
- Shuta Ishibe; Lloyd G Cantley; Epithelial-mesenchymal-epithelial cycling in kidney repair. Current Opinion in Nephrology and Hypertension 2008, 17, 379-385, 10.1097/mnh.0b013e3283046507.
- Glenda C. Gobe; David W. Johnson; Distal tubular epithelial cells of the kidney: Potential support for proximal tubular cell survival after renal injury. The International Journal of Biochemistry & Cell Biology 2007, 39, 1551-1561, 10.1016/j.biocel.2007.04.025.
- Guan-Qun Ju; Jun Cheng; Liang Zhong; Shuai Wu; Xiang-Yu Zou; Guang-Yuan Zhang; Di Gu; Shuai Miao; Ying-Jian Zhu; Jie Sun; et al.Tao Du Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Tubular Epithelial Cell Dedifferentiation and Growth via Hepatocyte Growth Factor Induction. PLOS ONE 2015, 10, e0121534, 10.1371/journal.pone.0121534.
- Di Gu; Xiangyu Zou; Guanqun Ju; Guangyuan Zhang; Erdun Bao; Yingjian Zhu; Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells International 2016, 2016, 2093940, 10.1155/2016/2093940.
- Xiaopeng Yuan; Xiaoping Wang; Chuanbao Chen; Jian Zhou; Ming Han; Bone mesenchymal stem cells ameliorate ischemia/reperfusion-induced damage in renal epithelial cells via microRNA-223.. Stem Cell Research & Therapy 2017, 8, 146, 10.1186/s13287-017-0599-x.
- Nana Song; Ting Zhang; Xialian Xu; Zhihui Lu; Xiaofang Yu; Yi Fang; Jiachang Hu; Ping Jia; Jie Teng; Xiaoqiang Ding; et al. miR-21 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Preventing Epithelial Cell Apoptosis and Inhibiting Dendritic Cell Maturation. Frontiers in Physiology 2018, 9, 790, 10.3389/fphys.2018.00790.
- Rulin Wang; Miao Lin; Liping Li; Long Li; Guisheng Qi; R. Rong; Ming Xu; Tongyu Zhu; [Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats].. Zhonghua yi xue za zhi 2014, 94, 3298–3303.
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y.; Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Press. Res. 2016,, 41,, 119–128..
- Chengjun Zhang; Shengqiang Yu; Binyan Zheng; Dongfu Liu; Fengchun Wan; Yue Ma; Jiantao Wang; Zhenli Gao; Zhengfei Shan; miR-30c-5p Reduces Renal Ischemia-Reperfusion Involving Macrophage.. Medical Science Monitor 2019, 25, 4362-4369, 10.12659/MSM.914579.
- Stefania Bruno; Cristina Grange; Federica Collino; Maria Chiara Deregibus; V. Cantaluppi; Luigi Biancone; Ciro Tetta; Giovanni Camussi; Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLOS ONE 2012, 7, e33115, 10.1371/journal.pone.0033115.
- Fen Liu; Yuan-Lei Lou; Jue Wu; Qiong-Fang Ruan; An Xie; Fei Guo; Su-Ping Cui; Zhi-Feng Deng; Yang Wang; Upregulation of MicroRNA-210 Regulates Renal Angiogenesis Mediated by Activation of VEGF Signaling Pathway under Ischemia/Perfusion Injury in vivo and in vitro. Kidney and Blood Pressure Research 2012, 35, 182-191, 10.1159/000331054.
- Rui Peng; Li Zhou; Yuru Zhou; Ya Zhao; Qianyin Li; Dongsheng Ni; Yanxia Hu; Yaoshui Long; Jianing Liu; Zhongshi Lyu; et al.Zhaomin MaoYue YuanLiyuan HuangHui ZhaoGe LiQin Zhou MiR-30a Inhibits the Epithelial—Mesenchymal Transition of Podocytes through Downregulation of NFATc3. International Journal of Molecular Sciences 2015, 16, 24032-24047, 10.3390/ijms161024032.
- Jia, H.; Liu, W.; Zhang, B.; Wang, J.; Wu, P.; Tandra, N.; Liang, Z.; Ji, C.; Yin, L.; Hu, X.; et al.et al. HucMSC exosomes-delivered 14-3-3zeta enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am. J. Transl. Res. 2018, , 10, , 101–113..
- Bingying Wang; Haoyuan Jia; Bin Zhang; Juanjuan Wang; Cheng Ji; Xueming Zhu; Yongmin Yan; Lei Yin; Jing Yu; Hui Qian; et al.Wenrong Xu Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy.. Stem Cell Research & Therapy 2017, 8, 75, 10.1186/s13287-016-0463-4.
- Jin Xiang; Tingting Jiang; Wenying Zhang; Wei Xie; Xun Tang; Jun Zhang; Human umbilical cord-derived mesenchymal stem cells enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the PI3K/AKT/mTOR signaling pathway.. Experimental Cell Research 2019, 378, 198-205, 10.1016/j.yexcr.2019.03.019.
- Marc Liesa; Manuel Palacín; Antonio Zorzano; Mitochondrial Dynamics in Mammalian Health and Disease. Physiological Reviews 2009, 89, 799-845, 10.1152/physrev.00030.2008.
- Ming Zhan; Craig Brooks; Fuyou Liu; Lin Sun; Zheng Dong; Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology.. Kidney International 2013, 83, 568-581, 10.1038/ki.2012.441.
- Craig Brooks; Qingqing Wei; Sung-Gyu Cho; Z Dong; Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models.. Journal of Clinical Investigation 2009, 119, 1275-1285, 10.1172/JCI37829.
- Shasha Lv; Jing Cheng; Aili Sun; Junhua Li; Weiwei Wang; Guangju Guan; Gang Liu; Moran Su; Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Research and Clinical Practice 2014, 104, 143-154, 10.1016/j.diabres.2014.01.011.
- Alfonso Eirin; Xiang-Yang Zhu; Amrutesh S. Puranik; Hui Tang; Kelly A. McGurren; Andre J. Van Wijnen; Amir Lerman; Lilach O. Lerman; Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation.. Kidney International 2017, 92, 114-124, 10.1016/j.kint.2016.12.023.
- Bo Wang; Kevin Yao; Brooke M Huuskes; Hsin-Hui Shen; Junli Zhuang; Catherine Godson; Eoin P Brennan; Jennifer L. Wilkinson-Berka; Andrea F Wise; Sharon D. Ricardo; et al. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Molecular Therapy 2016, 24, 1290-1301, 10.1038/mt.2016.90.
- Juan He; Yan Wang; Xingyan Lu; Bei Zhu; Xiaohua Pei; Jianqing Wu; Weihong Zhao; Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591-600, 10.1111/nep.12490.
- Cristina Grange; Stefania Tritta; Marta Tapparo; Massimo Cedrino; Ciro Tetta; Giovanni Camussi; Maria Felice Brizzi; Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy.. Scientific Reports 2019, 9, 4468, 10.1038/s41598-019-41100-9.
- Ling Zhong; Guangneng Liao; Xiaojiao Wang; Lan Li; Jie Zhang; Younan Chen; Jingping Liu; Shuyun Liu; Lingling Wei; Wengeng Zhang; Yanrong Lu; Mesenchymal stem cells–microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Experimental Biology and Medicine 2018, 243, 1233-1242, 10.1177/1535370218819726.
- Juan He; Yan Wang; Shu Sun; Meining Yu; Cuiyu Wang; Xiaohua Pei; Bei Zhu; Jianqing Wu; Weihong Zhao; Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493-500, 10.1111/j.1440-1797.2012.01589.x.
- Yan Wang; Bo Fu; Xuefeng Sun; Diangeng Li; Qi Huang; Weihong Zhao; Xiangmei Chen; Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-β1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Research & Therapy 2015, 6, 185, 10.1186/s13287-015-0179-x.
- Liu, H.; He, X.J.; Li, G.J.; Ding, Q.X.; Liang, W.X.; Fan, J.; Effects of microRNA-145 on epithelial-mesenchymal transition of TGF-beta1-induced human renal proximal tubular epithelial cells. Zhongguo Dang Dai Er Ke Za Zhi 2017,, 19,, 712–718..
