Rice bran arabinoxylan compound (RBAC) is derived from defatted rice bran hydrolyzed with Lentinus edodes mycelial enzyme. It has been marketed as a functional food and a nutraceutical with health-promoting properties. Some research has demonstrated this rice bran derivative to be a potent immunomodulator, which also possesses anti-inflammatory, antioxidant, and anti-angiogenic properties. To date, research on RBAC has predominantly focused on its immunomodulatory action and application as a complementary therapy for cancer. Nonetheless, the clinical applications of RBAC can extend beyond cancer therapy.
- complementary therapy
- BioBran
- MGN-3
- rice bran exo-biopolymer
- functional food
- nutraceuticals
1. Introduction
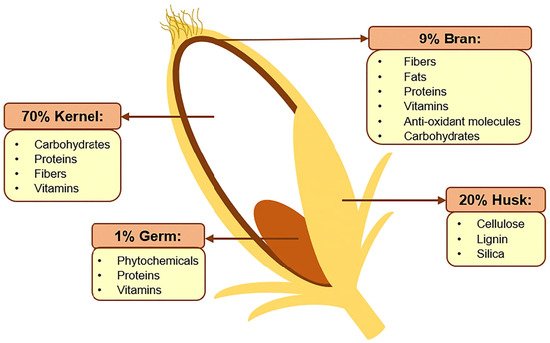
Rice bran is the rough brown layer sandwiched between the outer husk of paddy and its endosperm (kernel), as shown in Figure 1. The bran constitutes roughly 8% of a rice grain before milling and is a source of many nutrients, such as lipids (20–29%), carbohydrates (20–27%, including fibers), proteins (10–15%), B-complex vitamins, minerals, as well as a variety of phytochemicals and antioxidants [1,2][1][2]. Many of the bioactive compounds within rice bran possess cancer-preventing properties, including γ-oryzanol, ferulic acid, caffeic acid, tricin, coumaric acid, phytic acid, tocopherols, phytosterols, and carotenoids [3]. As such, this by-product of rice production is now gaining increasing attention for its nutritional, functional, and commercial potentials beyond its traditional use as animal feed [4]. For instance, there is a growing use of defatted rice bran as a functional ingredient in food production, such as baking, to increase the protein, dietary fiber, and antioxidant contents [5].
A primary component of the dietary fibers found in rice bran is arabinoxylan, a hemicellulose type belonging to the non-starch polysaccharide group. The structure of arabinoxylan consists of backbone chains of β-(1-4)-linked D-xylopyranosyl (xylan) with α-L-arabinofuranose units linked as side chains via the second and third carbon positions [6]. Along with arabinose, some galactose, xylose, and glucuronic acid residues can also exist as side branches. With a molecular weight ranging between 10 to 10,000 kDa, the cross-links of many side chains make arabinoxylan mostly resistant to extraction by water [7]. Additional treatments using enzymes, alkali solutions or mechanical methods are needed to remove arabinoxylans from the stable cross-link networks to produce soluble hemicellulose and arabinoxylan compounds with low molecular weights [7,8][7][8].
One approach to facilitate the extraction of arabinoxylan from rice bran is via the use of mycelium enzymes. In one study, rice bran was treated with enzymes cultured from nine different fungi types and tested for their macrophage stimulating activities [9]. Treatment with the enzyme from L. edodes (shiitake mushroom) exhibited the highest macrophage stimulating activity. Further research has shown that this rice bran arabinoxylan compound (RBAC) possesses immunomodulating, anti-inflammatory, antioxidant, and anti-angiogenesis properties. From this, RBAC has shown significant potential for clinical applications as a nutraceutical [8].
2. The Production and Composition of RBAC
RBAC is currently available as dietary supplements or nutraceuticals for immune system improvement and disease prevention. Most prominently, a functional food called Biobran MGN-3 is developed in Japan by Daiwa Pharmaceutical Co., Ltd. This product is marketed worldwide under many different brand names, such as Biobran, Ribraxx (in Australia), Lentin Plus (in Asia), or BRM4 (in the United States) [10]. Rice bran exo-biopolymer is another variant of RBAC independently developed in Korea by Erom Co., Ltd. [8,11,12][8][11][12]. It is the main ingredient of Erom’s immune-related products distributed not only in Korea but also in the United States and other countries. This review adopts RBAC as the generic name for any hydrolyzed extract of defatted rice bran modified with the L. edodes mycelial enzyme.
The production of RBAC is depicted in the following Figure 2.
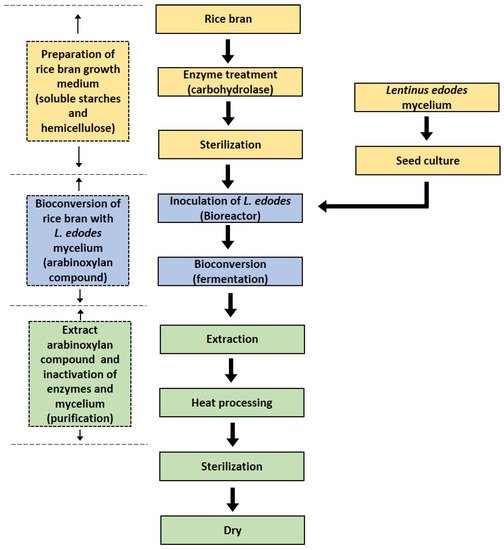
Biobran Research Foundation [10] described the steps to produce RBAC as follows:
Preparation: Defatted rice bran is thoroughly mixed with hot water at the ratio of 1:5. The insoluble polysaccharide is removed from the mixture leaving only soluble starch. Glucoamylase is then added to hydrolyze the starch by cleaving the 1,4-α-glycosidic bonds of the nonreducing end of the glycosidic chains resulting in the release of d-glucose. This process increases the content of fermentable carbohydrates [13]. L. edodes is cultured in a liquid medium. The mycelia of L. edodes and any insoluble residues are then removed to obtain the carbohydrase complexes, including xylanase, glucosidase, monosidase, and hemicellulose [10].
Bioconversion: This step involves mixing and heating the hemicellulose extract (from rice bran) with the carbohydrase complexes (from L. edodes), resulting in the bioconversion or fermentation of the raw materials.
Extraction: The bioconversion produces partially hydrolyzed rice bran hemicellulose, which has a high arabinoxylan content. The extract is then heated, sterilized, condensed, and added with excipient resulting in the final product as a powder [10].
This powdered product is 98.4% soluble in purified water consisting of heteropolysaccharide structures with molecular weights between 30–100 kDa [14,15][14][15]. The active ingredient responsible for its immunomodulatory property is likely to be a modified arabinoxylan with a xylose in its main chain and an arabinose polymer side chain [14], as shown in Figure 3.
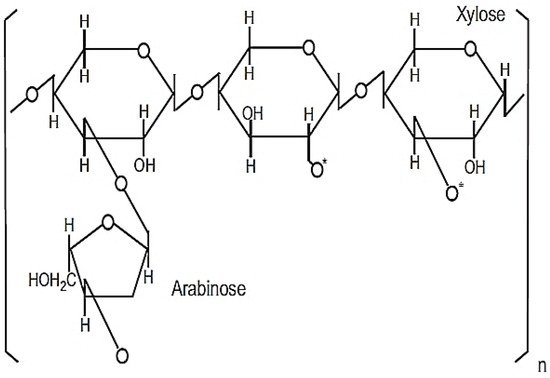
Figure 3. Chemical structure of MGN-3/Biobran. Reprinted from Wheat and Rice in Disease Prevention and Health, 1st ed., M. H. Ghoneum, Chapter 31—Apoptosis and arabinoxylan rice bran, pp. 401–408, Copyright 2014, with permission from Elsevier.
3. Health-Promoting Properties
3.1. Immunomodulatory Action
3.2. Anti-Inflammatory Effect
3.3. Antioxidant Action
3.4. Angiogenesis Inhibition
RBAC can affect new blood vessel growth through the vascular endothelial growth factor (VEGF) pathway. An in vitro study by Zhu et al. [49] demonstrated that RBAC significantly inhibited VEGF-induced tube formation in human umbilical vein endothelial cells co-cultured with human dermal fibroblasts. Furthermore, RBAC also suppressed the VEGF-induced proliferation and migration of human umbilical vein endothelial cells in a dose-dependent manner [49]. The anti-angiogenic mechanism of RBAC operates through the reduction of not only the VEGF-induced activation of VEGF receptor 2, but also the downstream proteins of protein kinase B, extracellular signal-regulated protein kinase 1/2, and p38 mitogen-activated protein kinase [49]. Therefore, RBAC can potentially mediate angiogenesis, implicated in the pathological progression of conditions such as cancer, cardiac and limb ischemia, diabetic retinopathy, rheumatoid arthritis, and neoplasms [50]. Figure 4 summarizes the medicinal actions and effects of rice bran arabinoxylan compound discussed in this section.
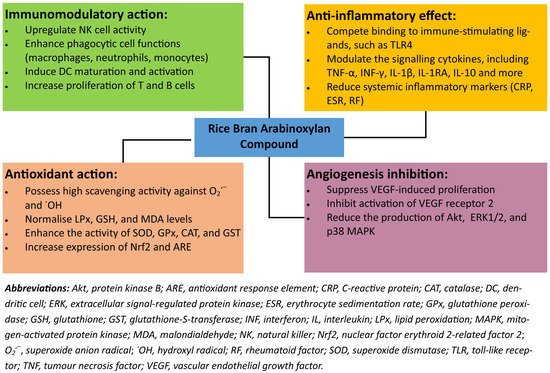
Figure 4. A summary of the key health-promoting attributes of rice bran arabinoxylan compound.
References
- Park, H.Y.; Lee, K.W.; Choi, H.D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943.
- Fraterrigo Garofalo, S.; Tommasi, T.; Fino, D. A short review of green extraction technologies for rice bran oil. Biomass Convers. Biorefin. 2021, 11, 569–587.
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653.
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816.
- Sairam, S.; Gopala Krishna, A.G.; Urooj, A. Physico-chemical characteristics of defatted rice bran and its utilization in a bakery product. J. Food Sci. Technol. 2011, 48, 478–483.
- Fadel, A.; Plunkett, A.; Nyaranga, R.R.; Ashworth, J. Arabinoxylans from rice bran and wheat immunomodulatory potentials: A review article. Nutr. Food Sci. 2018, 48, 97–110.
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxid. Med. Cell. Longev. 2018, 2018, 22.
- Hong, S. Development of immunostimulation materials from rice bran. Food Ind. Nutr. 2005, 10, 42–47.
- Yu, K.W.; Shin, K.S.; Choi, Y.M.; Suh, H.J. Macrophage stimulating activity of exo-biopolymer from submerged culture of Lentinus edodes with rice bran. J. Microbiol. Biotechnol. 2004, 14, 658–664.
- BioBran Research Foundation. The summary of Biobran/MGM-3. In BioBran/MGN-3 (Rice Bran Arabinoxylan Compound): Basic and Clinical Application to Integrative Medicine, 2nd ed.; BioBran Research Foundation: Tokyo, Japan, 2013; Chapter I-1; pp. 3–8.
- Kim, H.Y.; Kim, J.H.; Yang, S.B.; Hong, S.G.; Lee, S.A.; Hwang, S.J.; Shin, K.S.; Suh, H.J.; Park, M.H. A Polysaccharide Extracted from Rice Bran Fermented with Lentinus edodes Enhances Natural Killer Cell Activity and Exhibits Anticancer Effects. J. Med. Food 2007, 10, 25–31.
- Kim, H.Y.; Han, J.T.; Hong, S.G.; Yang, S.B.; Hwang, S.J.; Shin, K.S.; Suh, H.J.; Park, M.H. Enhancement of immunological activity in exo-biopolymer from submerged culture of Lentinus edodes with rice bran. Nat. Prod. Sci. 2005, 11, 183–187.
- Trono, D. Recombinant enzymes in the food and pharmaceutical industries. In Biomass, Biofuels, Biochem; Singh, R.S., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 13; pp. 349–387.
- Ghoneum, M. Apoptosis and arabinoxylan rice bran. In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Elsevier: San Diego, CA, USA, 2014; Chapter 31; pp. 399–404.
- Miura, T.; Chiba, M.; Miyazaki, Y.; Kato, Y.; Maeda, H. Chemical structure of the component involved in immunoregulation. In BioBran/MGN-3 (Rice Bran Arabinoxylan Coumpound): Basic and Clinical Application to Integrative Medicine, 2nd ed.; BioBran Research Foundation: Tokyo, Japan, 2013; Chapter I-3; pp. 14–22.
- Ghoneum, M. From bench to bedside: The growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer immunotherapy. Austin Immunol. 2016, 1, 1006.
- Ghoneum, M.; Brown, J. NK Immunorestoration and cancer patients by BioBran/MGN-3, a modified aracbynoxylan rice bran (Study of 32 patients followed for up to 4 years). In Anti-Aging Medical Therapeutics Vol III; Klatz, R.M., Goldman, R., Eds.; Health Quest Publications: Marina del Rey, CA, USA, 1999; Chapter 30; pp. 217–226.
- Ghoneum, M.; Abedi, S. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J. Pharm. Pharmacol. 2004, 56, 1581–1588.
- Pérez-Martínez, A.; Valentín, J.; Fernández, L.; Hernández-Jiménez, E.; López-Collazo, E.; Zerbes, P.; Schwörer, E.; Nuñéz, F.; Martín, I.G.; Sallis, H.; et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2015, 17, 601–612.
- Badr El-Din, N.K.; Noaman, E.; Ghoneum, M. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on ehrlich carcinoma-bearing mice. Nutr. Cancer 2008, 60, 235–244.
- Badr El-Din, N.K.; Abdel Fattah, S.M.; Pan, D.; Tolentino, L.; Ghoneum, M. Chemopreventive activity of MGN-3/Biobran against chemical induction of glandular stomach carcinogenesis in rats and its apoptotic effect in gastric cancer cells. Integr. Cancer Ther. 2016.
- Giese, S.; Sabell, G.R.; Coussons-Read, M. Impact of ingestion of rice bran and shitake mushroom extract on lymphocyte function and cytokine production in healthy rats. J. Diet. Suppl. 2008, 5, 47–61.
- Ghoneum, M. Enhancement of human natural killer cell activity by modified arabinoxylane from rice bran (MGN-3). Int. J. Immunother. 1998, 14, 89–99.
- Ghoneum, M.; Jewett, A. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect. Prev. 2000, 24, 314–324.
- Ali, K.H.; Melillo, A.B.; Leonard, S.M.; Asthana, D.; Woolger, J.M.; Wolfson, A.H.; Mcdaniel, H.R.; Lewis, J.E. An open-label, randomized clinical trial to assess the immunomodulatory activity of a novel oligosaccharide compound in healthy adults. Funct. Foods Health Dis. 2012, 2, 265–279.
- Elsaid, A.F.; Shaheen, M.; Ghoneum, M. Biobran/MGN-3, an arabinoxylan rice bran, enhances NK cell activity in geriatric subjects: A randomized, double-blind, placebo-controlled clinical trial. Exp. Ther. Med. 2018, 15, 2313–2320.
- Tsunekawa, H. Effect of long-term administration of immunomodulatory food on cancer patients completing conventional treatments. Clin. Pharmacol. Ther. 2004, 14, 295–302.
- Cholujova, D.; Jakubikova, J.; Sulikova, M.; Chovancova, J.; Czako, B.; Martisova, M.; Mistrik, M.; Pastorek, M.; Gronesova, P.; Hunakova, L.; et al. The effect of MGN-3 arabinoxylan on natural killer and dendritic cells in multiple myeloma patients. Haematologica 2011, 96, S117–S118.
- Cholujova, D.; Jakubikova, J.; Czako, B.; Martisova, M.; Hunakova, L.; Duraj, J.; Mistrik, M.; Sedlak, J. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol. Immunother. 2013, 62, 437–445.
- Ghoneum, M.; Matsuura, M. Augmentation of macrophage phagocytosis by modified arabinoxylan rice bran (MGN-3/Biobran). Int. J. Immunopathol. Pharmacol. 2004, 17, 283–292.
- Chae, S.; Shin, S.; Bae, M.; Park, M.; Song, M.; Hwang, S.; Yee, S. Effect of arabinoxylane and PSP on activation of immune cells. J. Korean Soc. Food Sci. Nutr. 2004, 33, 278–286.
- Ghoneum, M.; Matsuura, M.; Gollapudi, S. Modified arabinoxylan rice bran (Mgn-3/Biobran) enhances intracellular killing of microbes by human phagocytic cells in vitro. Int. J. Immunopathol. Pharmacol. 2008, 21, 87–95.
- Ghoneum, M.; Agrawal, S. Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int. J. Immunopathol. Pharmacol. 2011, 24, 941–948.
- Cholujova, D.; Jakubikova, J.; Sedlak, J. BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma 2009, 56, 89–95.
- Ghoneum, M.; Agrawal, S. MGN-3/Biobran enhances generation of cytotoxic CD8+ T cells via upregulation of DEC-205 expression on dendritic cells. Int. J. Immunopathol. Pharmacol. 2014, 27, 523–530.
- Lissoni, P.; Messina, G.; Brivio, F.; Fumagalli, L.; Rovelli, F.; Maruelli, L.; Miceli, M.; Marchiori, P.; Porro, G.; Held, M.; et al. Modulation of the anticancer immunity by natural agents: Inhibition of T regulatory lymphocyte generation by arabinoxylan in patients with locally limited or metastatic solid tumors. Cancer Ther. 2008, 6, 1011–1016.
- Endo, Y.; Kanbayashi, H. Modified rice bran beneficial for weight loss of mice as a major and acute adverse effect of cisplatin. Pharmacol. Toxicol. 2003, 92, 300–303.
- Choi, J.Y.; Paik, D.J.; Kwon, D.Y.; Park, Y. Dietary supplementation with rice bran fermented with Lentinus edodes increases interferon-γ activity without causing adverse effects: A randomized, double-blind, placebo-controlled, parallel-group study. Nutr. J. 2014, 13, 35.
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316.
- Ichihashi, K. Experience with administration of BioBran in patients with chronic rheumatism. Clin. Pharmacol. Ther. 2004, 14, 459–463.
- Ghoneum, M.H.; El Sayed, N.S. Protective effect of Biobran/MGN-3 against sporadic Alzheimer’s disease mouse model: Possible role of oxidative stress and apoptotic pathways. Oxid. Med. Cell. Longev. 2021, 2021, 8845064.
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Addition of rice bran arabinoxylan to curcumin therapy may be of benefit to patients with early-stage B-cell lymphoid malignancies (monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, or stage 0/1 chronic lymphocytic leukemia). Integr. Cancer Ther. 2016, 15, 183–189.
- Kamiya, T.; Shikano, M.; Tanaka, M.; Ozeki, K.; Ebi, M.; Katano, T.; Hamano, S.; Nishiwaki, H.; Tsukamoto, H.; Mizoshita, T.; et al. Therapeutic effects of biobran, modified arabinoxylan rice bran, in improving symptoms of diarrhea predominant or mixed type irritable bowel syndrome: A pilot, randomized controlled study. Evid.-Based Complement. Altern. Med. 2014, 2014, 828137.
- Tazawa, K.; Namikawa, H.; Oida, N.; Itoh, K.; Yatsuzuka, M.; Koike, J.; Masada, M.; Maeda, H. Scavenging activity of MGN-3 (arabinoxylane from rice bran) with natural killer cell activity on free radicals. Biotherapy 2000, 14, 493–495.
- Noaman, E.; Badr El-Din, N.K.; Bibars, M.A.; Abou Mossallam, A.A.; Ghoneum, M. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008, 268, 348–359.
- Ghoneum, M.; Badr El-Din, N.K.; Fattah, S.M.A.; Tolentino, L. Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body γ-irradiation in mice via restoration of hematopoietic tissues. J. Radiat. Res. 2013, 54, 419.
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462.
- Tan, D.F.S.; Flores, J.A.S. The immunomodulating effects of arabinoxylan rice bran (Lentin) on hematologic profile, nutritional status and quality of life among head and neck carcinoma patients undergoing radiation therapy: A double blind randomized control trial. Radiol. J. Off. Publ. Philipp. Coll. Radiol. 2020, 12, 11–16.
- Zhu, X.; Okubo, A.; Igari, N.; Ninomiya, K.; Egashira, Y. Modified rice bran hemicellulose inhibits vascular endothelial growth factor-induced angiogenesis in vitro via VEGFR2 and its downstream signaling pathways. Biosci. Microbiota Food Health 2017, 36, 45–53.
- Pang, R.W.C.; Poon, R.T.P. Clinical implications of angiogenesis in cancers. Vasc. Health Risk Manag. 2006, 2, 97–108.
