Potentially toxic element (PTE) pollution is a major abiotic stress, which reduces plant growth and affects food quality by entering the food chain, and ultimately poses hazards to human health.
- slag
- immobilization
- plant
- potentially toxic elements
- tolerance
1. Introduction
With the rapid increase in the world population, similarly to other industries, steel industries are also more concerned about the safe and eco-friendly recycling of their by-products. In the past, steel industries were designed to produce iron and steel of a specific quality and quantity [1]. With the rapid growth of industrialization in recent decades, the increased volume of byproducts (slag) produced from iron/steel production has drawn attention to the need for its more effective recycling. [1]. Slags are widely used worldwide as a substitute for limestone and offer a cost-effective advantage to farmers. The main aim for researchers and environmentalists is to stop the entry of metals and metalloids into the food chain for better human health [2[2][3],3], and in this respect, the use of slags in various fields can help cope with this problem [2]. Potentially toxic element (PTE) contamination of soil refers to the excessive deposition of PTEs due to human activities [3,4,5,6][3][4][5][6]. In soil medium, the highest concentrations of PTE plant toxicity are represented by cadmium [7], lead [7], zinc [8], copper [9], nickel [10], vanadium [11], and arsenic [12], etc.
In the current scenario, the need for food is increased, and the use of fertilizers has been increased by humans, resulting in the deterioration of the environment [13,14,15][13][14][15]. Highly mobile PTEs can easily enrich the food chain and are highly hazardous to the environment. The remediation of soil becomes very difficult once it becomes polluted with PTEs. Soil cleanup is more complicated than air and water cleanup, according to previous reports [16], because PTEs in the soil form complexes and bonds with clay particles, and it becomes more challenging to break those bonds [17]. However, soil contamination in developed countries is considered a severe issue and more attention is paid to its remediation and public health. A wide range of compounds (TiO2, Fe3O3, FeO, Fe3O4, BaO, MgO, CaO, Al2O3, MnO and SiO2) and minerals are found on/in the layers of slags produced in various industrial operations [18,19][18][19]. In 2014, it was observed that each ton of steel produced by the steel industry generates 500 kg of steel slag [20]. China utilizes only 25% of steel slag out of 100 million tons produced, the production of steel slag accounting for 24% of the total solid waste produced in China [21,22][21][22]. Steel slag contains a variety of trace elements on its surface, which makes it an excellent fertilizer for better plant growth. Bearing in mind the importance of these benefits, Japan and Europe use steel slag as a soil amendment agent. Slag is used as a fertilizer and ground shifting agent in Japan and Europe because trace metal elements, such as Cr and V, are not readily released in slag. [23,24][23][24]. Slag fertilizers include slag–silicate fertilizers, slag–phosphate fertilizers, and unique iron matter fertilizers [4]. The presence of Ca2+ on the surface of steel slag produces stable PTE ions, with the aim to eliminate PTE ions from the contaminated medium.
Moreover, Wen et al. [25] concluded that applying steel slag to PTE acidic mining soils effectively raises the soil’s pH, increases soil microbial abundance, and immobilizes PTE ions, providing a desirable plant survival climate. Recently, a variety of studies have reported that there is a great commitment from slag-based fertilizer modification in agriculture to increase crop productivity [26[26][27],27], minimize soil acidification [28], and alleviate greenhouse gas (GHG). Slag fertilization’s beneficial results focus primarily on the shifts in microbial environments and microbial behaviors. In particular, soil microorganisms play a key role in almost all ecological processes at a system level and provide ecosystem services necessary for preserving soil quality and productivity [29]. Das et al. [1] reported that slag utilization in the PTE-contaminated soil improves crop production by affecting soil pH and greenhouse gas emissions. The fundamental processes of slag–microbial interactions and the importance of soil biota to ecosystem functionality are gradually deceptive.
2. Mechanism of Slag Interaction with Potentially Toxic Elements in Soil
Bearing in mind the application benefits of slag-based fertilizers for better crop production in potentially toxic element (PTE)-contaminated soils, it is important to know the different mechanisms slag follows to bind toxic ions at the soil and plant levels (Figure 3). Many studies have mentioned the mechanisms of detoxification of PTEs in plants by slag. However, a better understanding of the mechanism of PTE detoxification in plants from contaminated soils is vital for the practical application of slag. At the soil level, the application of slag may cause the immobilization of PTE, changes in the soil pH, and changes in PTE fractions, and causes an improvement in the soil physicochemical and biological properties of soils. While at the plant level, the application of slag-based fertilizers causes an increase in the antioxidant defense system and causes a reduction in the translocation of PTEs to plant shoots.
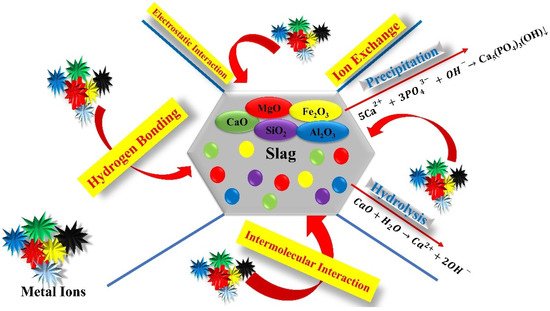
Figure 3. Proposed mechanism of slag-based fertilizers in the soil.
However, the mechanisms for PTE ion remediation by slag are similar to many other applied amendments in previous studies, such as lime [88][30], biochar [89[31][32][33][34],90,91,92], zeolite [93][35], chitosan [94][36], the indigenous strain Bacillus XZM [95][37], hydrogen sulfide [96][38], and sepiolite [97][39]. The basic PTE ion immobilization on the slag surface was attributed to the presence of silicate, ferrites, and calcium oxides on the surface [98][40]. Silicate ferrite present on the slag surface releases SiO44− and Fe2O3, and OH− interacts with PTE ions to make them immobile [98][40]. The presence of hydrogen oxides on the surface of slag has the ability to diffuse into the slag surface [98][40] due to small masses [99][41] and then can immobilize PTEs on the slag surface [100][42]. Moreover, PTEs exchange with the silicate and ferrite present on the slag surface and can be immobilized within the slag matrix [101][43]. The PTE ions present in the soil solution gradually enter the slag due to the absorption gradient.
Additionally, various metals form complexes with soil organic matter and become immobilized due to increased organic matter after slag addition to the soils. The increase in soil pH might result in the instability of PTE ion concentration in soil solutions. The unstable PTEs in the soil solutions exchange with Ca2+ in order to be immobilized within the silicate and ferrite fractions [100][42], while some PTE ions make bonds in soil organic matters. Moreover, various cations and anions are present on the slag surface, which increases the ionic strength and may affect PTE species. However, there are still unresolved areas for further studies to analyze the effects of ionic strength on the immobilization mechanism of PTEs under the slag-based fertilization of acidic polluting soils.
(References would be added automatically after the entry is online)
References
- Das, S.; Kim, G.W.; Hwang, H.Y.; Verma, P.P.; Kim, P.J. Cropping With Slag to Address Soil, Environment, and Food Security. Front. Microbiol. 2019, 10, 1320.
- Kimio, I. Steelmaking Slag for Fertilizer Usage. Nippon. STEEL SUMITOMO Met. Tech. Rep. 2015, 109, 130–136.
- Brevik, E.C.; Burgess, L.C. Soils and Human Health. In Soils and Human Health; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–403. ISBN 9781439844557.
- Chowdhury, S.; Mazumder, M.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488.
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212.
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181.
- Mehmood, S.; Rizwan, M.; Bashir, S.; Ditta, A.; Aziz, O.; Yong, L.Z.; Dai, Z.; Akmal, M.; Ahmed, W.; Adeel, M.; et al. Comparative Effects of Biochar, Slag and Ferrous–Mn Ore on Lead and Cadmium Immobilization in Soil. Bull. Environ. Contam. Toxicol. 2017, 100, 286–292.
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of Zinc in Plant Nutrition—A Review. Am. J. Exp. Agric. 2013, 3, 374–391.
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883.
- Tariq, W.; Saifullah, M.; Anjum, T.; Javed, M.; Tayyab, N.; Shoukat, I. Removal of Heavy Metals from Chemical Industrial Wastewater Using Agro Based Bio-Sorbents. Acta Chem. Malays. 2018, 2, 9–14.
- Imtiaz, M.; Ashraf, M.; Rizwan, M.S.; Nawaz, M.A.; Mehmood, S.; Yousaf, B.; Yuan, Y.; Ditta, A.; Mumtaz, M.A.; Ali, M.; et al. Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: Effects on cell death, ROS and antioxidative systems. Ecotoxicol. Environ. Saf. 2018, 158, 139–144.
- Hettick, B.E.; Cañas-Carrell, J.E.; French, A.D.; Klein, D.M. Arsenic: A Review of the Element’s Toxicity, Plant Interactions, and Potential Methods of Remediation. J. Agric. Food Chem. 2015, 63, 7097–7107.
- Isherwood, K.F. Mineral Fertilizer Use; IFA: Paris, France, 2000; ISBN 2950629938.
- Smith, L.; Siciliano, G. A comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agric. Ecosyst. Environ. 2015, 209, 15–25.
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060.
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Front. Plant Sci. 2016, 7, 303.
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266.
- Lim, J.; Chew, L.; Choong, T.S.; Tezara, C.; Yazdi, M. Overview of Steel Slag Application and Utilization. MATEC Web Conf. 2016, 74, 26.
- Liu, C.; Shih, K.; Sun, C.; Wang, F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci. Total Environ. 2012, 416, 507–512.
- Hocheng, H.; Su, C.; Jadhav, U.U. Bioleaching of metals from steel slag by Acidithiobacillus thiooxidans culture supernatant. Chemosphere 2014, 117, 652–657.
- Pang, B.; Zhou, Z.; Xu, H. Utilization of carbonated and granulated steel slag aggregate in concrete. Constr. Build. Mater. 2015, 84, 454–467.
- Yüksel, I. A review of steel slag usage in construction industry for sustainable development. Environ. Dev. Sustain. 2016, 19, 369–384.
- Fujisawa, N.; Fukushima, M.; Yamamoto, M.; Iwai, H.; Komai, T.; Kawabe, Y.; Liu, D. Structural alterations of humic acid fractions in a steel slag-compost fertilizer during fertilization. Analysis by pyrolysis/methylation-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2012, 95, 126–133.
- Gómez-Nubla, L.; Aramendia, J.; De Vallejuelo, S.F.-O.; Carrero, J.A.; Madariaga, J.M. Focused ultrasound energy over steel slags as a fast tool to assess their environmental risk before and after their reuse in agriculture and civil constructions. Microchem. J. 2017, 132, 268–273.
- Wen, T.; Yang, L.; Dang, C.; Miki, T.; Bai, H.; Nagasaka, T. Effect of basic oxygen furnace slag on succession of the bacterial community and immobilization of various metal ions in acidic contaminated mine soil. J. Hazard. Mater. 2020, 388, 121784.
- Gwon, H.S.; Khan, M.I.; Alam, M.A.; Das, S.; Kim, P.J. Environmental risk assessment of steel-making slags and the potential use of LD slag in mitigating methane emissions and the grain arsenic level in rice (Oryza sativa L.). J. Hazard. Mater. 2018, 353, 236–243.
- White, B.; Tubana, B.S.; Babu, T.; Mascagni, H.; Agostinho, F.; Datnoff, L.E.; Harrison, S. Effect of Silicate Slag Application on Wheat Grown Under Two Nitrogen Rates. Plants 2017, 6, 47.
- Ning, D.; Liang, Y.; Liu, Z.; Xiao, J.; Duan, A. Impacts of Steel-Slag-Based Silicate Fertilizer on Soil Acidity and Silicon Availability and Metals-Immobilization in a Paddy Soil. PLoS ONE 2016, 11, e0168163.
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted Cattle Manure Increases Microbial Activity and Soil Fertility More Than Composted Swine Manure in a Submerged Rice Paddy. Front. Microbiol. 2017, 8, 1702.
- Singh, J.; Kalamdhad, A.S. Effect of carbide sludge (lime) on bioavailability and leachability of heavy metals during rotary drum composting of water hyacinth. Chem. Speciat. Bioavailab. 2014, 26, 76–84.
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751.
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167.
- Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I. Effect of Biochar Application on Heavy Metal Mobility in Soils Impacted by Copper Smelting Processes. Pol. J. Environ. Stud. 2020, 29, 1749–1757.
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576.
- Bashir, S.; Salam, A.; Rehman, M.; Khan, S.; Gulshan, A.B.; Iqbal, J.; Shaaban, M.; Mehmood, S.; Zahra, A.; Hu, H. Effective Role of Biochar, Zeolite and Steel Slag on Leaching Behavior of Cd and Its Fractionations in Soil Column Study. Bull. Environ. Contam. Toxicol. 2019, 102, 567–572.
- Yuvaraja, G.; Pang, Y.; Chen, D.-Y.; Kong, L.-J.; Mehmood, S.; Subbaiah, M.V.; Rao, D.S.; Pavuluri, C.M.; Wen, J.-C.; Reddy, G.M. Modification of chitosan macromolecule and its mechanism for the removal of Pb(II) ions from aqueous environment. Int. J. Biol. Macromol. 2019, 136, 177–188.
- Irshad, S.; Xie, Z.; Wang, J.; Nawaz, A.; Luo, Y.; Wang, Y.; Mehmood, S. Faheem Indigenous strain Bacillus XZM assisted phytoremediation and detoxification of arsenic in Vallisneria denseserrulata. J. Hazard. Mater. 2020, 381, 120903.
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Zhou, Y.; Adeel, M.; Mehmood, S.; Javed, R.; Imtiaz, M.; Aziz, O.; Ikram, M.; et al. Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol. Biochem. 2019, 138, 100–111.
- Bashir, S.; Ali, U.; Shaaban, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Husain, A.; Ahmed, N.; Mehmood, S.; Kamran, M.; et al. Role of sepiolite for cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J. Environ. Manag. 2020, 258, 110020.
- Yang, L.; Wei, T.; Li, S.; Lv, Y.; Miki, T.; Yang, L.; Nagasaka, T. Immobilization persistence of Cu, Cr, Pb, Zn ions by the addition of steel slag in acidic contaminated mine soil. J. Hazard. Mater. 2021, 412, 125176.
- Lü, R.; Xi, Q.; Li, T.; Li, R.; Zhang, X.; Liu, J.; Fan, C.; Feng, J.; Zhang, L.; Wang, Z.; et al. Adsorption equilibrium, kinetics, and dynamic separation of Ca2+ and Mg2+ ions from phosphoric acid–nitric acid aqueous solution by strong acid cation resin. Chin. J. Chem. Eng. 2019, 27, 2930–2936.
- Yang, L.; Wen, T.; Wang, L.; Miki, T.; Bai, H.; Lu, X.; Yu, H.; Nagasaka, T. The stability of the compounds formed in the process of removal Pb(II), Cu(II) and Cd(II) by steelmaking slag in an acidic aqueous solution. J. Environ. Manag. 2019, 231, 41–48.
- Chen, G.; Yang, L.; Chen, J.; Miki, T.; Li, S.; Bai, H.; Nagasaka, T. Competitive mechanism and influencing factors for the simultaneous removal of Cr(III) and Zn(II) in acidic aqueous solutions using steel slag: Batch and column experiments. J. Clean. Prod. 2019, 230, 69–79.
