It has been widely endorsed that a multifactorial etiology, including interaction between genetic and environmental factors, can contribute to Crohn’s Disease (CD) pathogenesis. More specifically, diet has proven to be able to shape gut microbiota composition and thus is suspected to play a significant role in inflammatory bowel disease (IBD) pathogenesis. Moreover, poor nutritional status and growth retardation, arising from several factors such as reduced dietary intake or nutrient leakage from the gastrointestinal tract, represent the hallmarks of pediatric CD. For these reasons, multiple research lines have recently focused on the utilization of dietary therapies for the management of CD, aiming to target concurrently mucosal inflammation, intestinal dysbiosis and optimization of nutritional status. The forerunner of such interventions is represented by exclusive enteral nutrition (EEN), a robustly supported nutritional therapy; however, it is burdened by monotony and low tolerance in the long term. Novel dietary interventions, such as Crohn’s Disease Exclusion Diet or Crohn’s Disease treatment with eating, have shown their efficacy in the induction of remission in pediatric patients with CD.
- Crohn’s disease
- children
- nutrition
- dietary management
1. Introduction
2. Mechanisms and Clinical Implications of Undernutrition in Children with CD
The inflammatory involvement in CD may extend throughout the length of the small bowel, thus impairing the absorption and processing of nutrients [36]. However, several other factors contribute to development of inadequate nutritional states in IBD [37]. One of the foremost determinants of malnutrition in IBD is reduced oral intake of food. Active disease often leads to reduced appetite due to abdominal symptom onset (abdominal pain, diarrhea, vomiting and nausea) [38]. Moreover, inflammation itself (i.e., via TNF-α and IL-6) can cause a reduction in appetite via catabolic effects and hypothalamic weight regulation [39]. In addition, some of the most commonly prescribed medications can induce nausea, vomiting and/or anorexia [40]. Lastly, some patients and/or their parents believe that certain foods may worsen, or even elicit, their symptoms. Therefore, they are inclined to modify their diet, excluding putative noxious triggers, in order to control their disease. According to a recent European survey, the most commonly charged foods are grains (29%), milk (28%), vegetables (18%) and fruits (11%) [41]. This behavior may have detrimental effects on nutritional status [42]. The intestinal epithelium can be easily disrupted during gut inflammation [43]. Impaired epithelial transport and loss of mucosal integrity are tightly associated with malabsorption. Indeed, deterioration of epithelial function leads to alterations of ionic transport, which consequently cause loss of fluids and electrolytes [38]. Furthermore, inflammation of the intestinal mucosa results in chronic leakage of blood and proteins [38]. Surgery is also associated with impairment of macro- and micronutrient absorption [38]. Bowel resections can cause accelerated intestinal transit and diarrhea, thus reducing the contact time of the luminal contents with the mucosal surface. Lastly, conflicting evidence exists regarding Resting Energy Expenditure (REE). REE represents the energy needs for an individual in resting condition [44]. Increased REE is thought to contribute to augmented caloric requirements in patients with active IBD. However, the latter issue remains controversial, as some studies have documented a positive correlation between REE and disease activity [45], whereas some others have not [46] (Figure 1).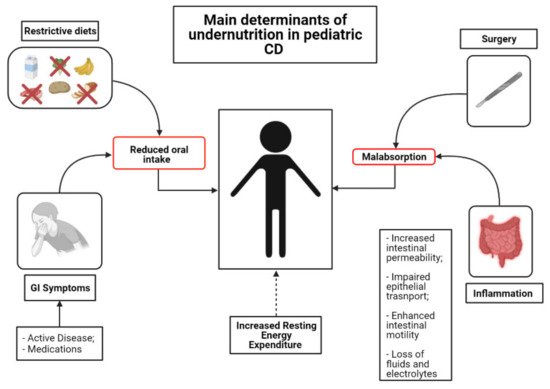
3. Nutritional Therapies
Nutritional status represents one of the foremost determinants of both clinical and surgical outcomes for patients affected by CD [118][64]. From this perspective, the identification, prevention and correction of nutritional deficiencies can be considered a therapeutic intervention as crucial as the choice of adequate pharmacological strategies. Indeed, malnutrition and impaired linear growth can be suggestive of active disease and their restoration should be considered as a treatment goal [44].3.1. EEN
3.1.1. Mechanism of Action
3.1.2. EEN for Induction of Remission in CD
| First Author | Study Design | Population | Intervention (Duration) |
Control Group | Key Findings |
|---|---|---|---|---|---|
| Morin [139][85] | R | 4 CD with growth failure | Elemental formula (6 weeks) |
N/A |
|
| Sanderson [140][86] | P/RCT | 8 pts with active CD | Elemental formula (6 weeks) |
8 CD patients treated with CS |
|
| Cohen-Dolev [24] | P/O | 60 patients with newly diagnosed CD | Any formula (6–8 weeks) |
87 matched patients treated with CS |
|
| Levine [142][88] | P/O | 43 patients with newly diagnosed CD | Any formula (6–8 weeks) |
114 patients treated with CS 29 with 5-ASA |
|
| Lee [143][89] | P/O | 22 patients with active CD | Any formula (6–8 weeks) |
52 patients treated with anti-TNF-α 16 pts with PEN + unrestricted diet |
|
| Grover [145][91] | P/O | 26 patients with active CD | Any formula (6 weeks) |
N/A |
|
| Borrelli [146][92] | P/RCT | 19 patients with active CD | Polymeric formula (10 weeks) |
18 patients treated with CS |
|
| Grover [147][93] | P/O | 54 patients with active disease | Any formula (6 weeks) |
N/A |
|
| Rubio [148][94] | R | 45 patients with CD who received oral EEN | Polymeric formula (8 weeks) |
61 patients treated with continuous EEN |
|
| Buchanan [149][95] | R | 110 patients with CD | Polymeric/ Elemental formula (8 weeks) |
N/A |
|
| Afzal [150][96] | R | 65 patients with active CD | Polymeric formula (8 weeks) |
N/A |
|
| Belli [152][98] | P | 8 patients with active CD and growth failure | Polymeric formula (intermittent administration over 1 year) |
4 matched CD patients not treated with EEN |
|
3.1.3. EEN for Preoperative Nutritional Optimization in Children with CD
3.1.4. EEN’s Effects on Body Mass Composition in Children with CD
3.2. Partial Enteral Nutrition (PEN) in CD
3.2.1. PEN for the Induction of Remission in CD
3.2.2. Maintenance of Enteral Nutrition (MEN) in CD
3.3. Food-Based Therapies in CD
| First Author | Study Design | Population | Intervention (Duration) |
Control Group | Key Findings |
|---|---|---|---|---|---|
| Sigall-Boneh [183][129] | R | 47 children and young adult pts with active CD | CDED + PEN (12 weeks, n = 40) CDED weeks, n = 7) |
N/A | Clinical remission achieved in 24/34 children and 9/13 adults at wk 6 and maintained in 27/33 patients at week 12; Significant fall in clinical disease activity and inflammatory markers. |
| Sigall-Boneh [187][133] | R | 21 children and young adult pts with treatment-refractory CD | CDED + PEN (12 weeks, n = 12) CDED (12 weeks, n = 4) Mod. EEN + CDED (2 + 12 weeks, n = 5) |
N/A | 13/21 pts refractory to biologic treatment achieved clinical remission; 9/17 of patients failing double biologic therapy achieved clinical remission; Significant decrease in serum markers of inflammation. |
| Levine [32] | P/RCT | 40 pts with mild-to-moderate CD | CDED + PEN (12 weeks) |
34 pts with mild-to-moderate CD treated with EEN | CDED+PEN was equally as effective as EEN in inducing remission at week 6; CDED+PEN was superior to EEN in maintaining remission at week 12; CDED+PEN was able to induce rapid remission (3 weeks); |
| Svolos [33] | OL | 5 pts with active CD (PCDAI ≥ 12.5) |
CD-TREAT (8 weeks) |
N/A | CD-TREAT was able to induce clinical response in 80% and remission in 60% of patients; 80% of pts showed decrease in fecal calprotectin |
3.3.1. Crohn’s Disease Exclusion Diet (CDED)
3.3.2. CD Treatment-with-Eating (CD-TREAT)
References
- Fumery, M.; Pariente, B.; Sarter, H.; Savoye, G.; Spyckerelle, C.; Djeddi, D.; Mouterde, O.; Bouguen, G.; Ley, D.; Peneau, A.; et al. Long-term outcome of pediatric-onset Crohn’s disease: A population-based cohort study. Dig. Liver Dis. 2019, 51, 496–502.
- Duricova, D.; Fumery, M.; Annese, V.; Lakatos, P.L.; Peyrin-Biroulet, L.; Gower-Rousseau, C. The natural history of Crohn’s disease in children: A review of population-based studies. Eur. J. Gastroenterol. Hepatol. 2017, 29, 10.
- Ghione, S.; Sarter, H.; Fumery, M.; Armengol-Debeir, L.; Savoye, G.; Ley, D.; Spyckerelle, C.; Pariente, B.; Peyrin-Biroulet, L.; Turck, D.; et al. Dramatic Increase in Incidence of Ulcerative Colitis and Crohn’s Disease (1988–2011): A Population-Based Study of French Adolescents. Am. J. Gastroenterol. 2018, 113, 265–272.
- E Roberts, S.; Thorne, K.; Thapar, N.; Broekaert, I.; A Benninga, M.; Dolinsek, J.; Mas, E.; Miele, E.; Orel, R.; Pienar, C.; et al. A Systematic Review and Meta-analysis of Paediatric Inflammatory Bowel Disease Incidence and Prevalence Across Europe. J. Crohn’s Colitis 2020, 14, 1119–1148.
- Van Limbergen, J.; Russell, R.K.; Drummond, H.E.; Aldhous, M.C.; Round, N.K.; Nimmo, E.R.; Smith, L.; Gillett, P.M.; McGrogan, P.; Weaver, L.T.; et al. Definition of Phenotypic Characteristics of Childhood-Onset Inflammatory Bowel Disease. Gastroenterology 2008, 135, 1114–1122.
- Duricova, D.; Burisch, J.; Jess, T.; Gower-Rousseau, C.; Lakatos, P.L. Age-related differences in presentation and course of inflammatory bowel disease: An update on the population-based literature. J. Crohn’s Colitis 2014, 8, 1351–1361.
- Jakobsen, C.; Bartek, J., Jr.; Wewer, V.; Vind, I.; Munkholm, P.; Groen, R.; Paerregaard, A. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease—A population-based study: Differences between paediatric and adult IBD. Aliment. Pharmacol. Ther. 2011, 34, 1217–1224.
- Kelsen, J.; Baldassano, R.N. Inflammatory bowel disease: The difference between children and adults. Inflamm. Bowel Dis. 2008, 14, S9–S11.
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755.
- Huang, H.; International Inflammatory Bowel Disease Genetics Consortium; Fang, M.; Jostins, L.; Mirkov, M.U.; Boucher, G.; Anderson, C.A.; Andersen, V.; Cleynen, I.; Cortes, A.; et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nat. Cell Biol. 2017, 547, 173–178.
- Park, J.-H.; Wacholder, S.; Gail, M.H.; Peters, U.; Jacobs, K.B.; Chanock, S.J.; Chatterjee, N. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat. Genet. 2010, 42, 570–575.
- A Peters, L.; Perrigoue, J.; Mortha, A.; Iuga, A.; Song, W.-M.; Neiman, E.M.; Llewellyn, S.R.; Di Narzo, A.; A Kidd, B.; E Telesco, S.; et al. A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat. Genet. 2017, 49, 1437–1449.
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738.
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66.
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696.
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979.
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21.
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124.
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838.
- Dong, J.; Chen, Y.; Tang, Y.; Xu, F.; Yu, C.; Li, Y.; Pankaj, P.; Dai, N. Body Mass Index Is Associated with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0144872.
- Yerushalmy-Feler, A.; Galai, T.; Moran-Lev, H.; Ben-Tov, A.; Dali-Levy, M.; Weintraub, Y.; Amir, A.; Cohen, S. BMI in the lower and upper quartiles at diagnosis and at 1-year follow-up is significantly associated with higher risk of disease exacerbation in pediatric inflammatory bowel disease. Eur. J. Nucl. Med. Mol. Imaging 2021, 180, 21–29.
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111.
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2021, 15, 171–194.
- Kugathasan, S.; A Denson, L.; Walters, T.D.; Kim, M.-O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 2017, 389, 1710–1718.
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic Infections With Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2013, 108, 1268–1276.
- Magro, F.; Peyrin-Biroulet, L.; Sokol, H.; Aldeger, X.; Costa, A.; Higgins, P.D.; Joyce, J.C.; Katsanos, K.H.; Lopez, A.; De Xaxars, T.M.; et al. Extra-intestinal malignancies in inflammatory bowel disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J. Crohn’s Colitis 2014, 8, 31–44.
- Cohen-Dolev, N.; Sladek, M.; Hussey, S.; Turner, D.; Veres, G.; Koletzko, S.; Levine, A. Differences in Outcomes Over Time with Exclusive Enteral Nutrition Compared With Steroids in Children With Mild to Moderate Crohn’s Disease: Results From the GROWTH CD Study. J. Crohn’s Colitis 2018, 12, 306–312.
- Grover, Z.; Lewindon, P. Two-Year Outcomes After Exclusive Enteral Nutrition Induction Are Superior to Corticosteroids in Pediatric Crohn’s Disease Treated Early with Thiopurines. Dig. Dis. Sci. 2015, 60, 3069–3074.
- Scarallo, L. Mucosal and Histologic Healing in children with Inflammatory Bowel Disease treated with anti-Tumor Necrosis Factor-alpha. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 728–735.
- Qiu, Y.; Chen, B.-L.; Mao, R.; Zhang, S.-H.; He, Y.; Zeng, Z.-R.; Ben-Horin, S.; Chen, M.-H. Systematic review with meta-analysis: Loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J. Gastroenterol. 2017, 52, 535–554.
- Rubio, A. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding: Oral vs. continuous enteral nutrition for CD in children. Aliment. Pharmacol. Ther. 2011, 33, 1332–1339.
- Gerasimidis, K. Impact of exclusive enteral nutrition on body composition and circulating micronutrients in plasma and erythrocytes of children with active Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 1672–1681.
- Stewart, M.; Day, A.S.; Otley, A. Physician Attitudes and Practices of Enteral Nutrition as Primary Treatment of Paediatric Crohn Disease in North America. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 38–42.
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8.
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6.
- Hartman, C.; Eliakim, R.; Shamir, R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 2570–2578.
- Goh, J.; O’Morain, C.A. Nutrition and adult inflammatory bowel disease. Aliment. Pharm. Ther. 2003, 14, 307–320.
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372.
- Sanderson, I.R. Growth problems in children with IBD. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 601–610.
- Vasudevan, A.; Parthasarathy, N.; Con, D.; Nicolaides, S.; Apostolov, R.; Chauhan, A.; Bishara, M.; Luber, R.P.; Joshi, N.; Wan, A.; et al. Thiopurines vs methotrexate: Comparing tolerability and discontinuation rates in the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 1174–1184.
- Jowett, S.L.; Seal, C.J.; Phillips, E.; Gregory, W.; Barton, J.; Welfare, M.R. Dietary beliefs of people with ulcerative colitis and their effect on relapse and nutrient intake. Clin. Nutr. 2004, 23, 161–170.
- Guerreiro, C.S.; Cravo, M.; Costa, A.R.; Miranda, A.; Tavares, L.; Moura-Santos, P.; Marquesvidal, P.; Leitão, C.N. A Comprehensive Approach to Evaluate Nutritional Status in Crohn’s Patients in the Era of Biologic Therapy: A Case-Control Study. Am. J. Gastroenterol. 2007, 102, 2551–2556.
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46.
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708.
- Varille, V.; Cézard, J.P.; De Lagausie, P.; Bellaiche, M.; Tounian, P.; Besnard, M.; Faure, C.; Aigrain, Y.; Girardet, J.P.; Navarro, J. Resting Energy Expenditure before and after Surgical Resection of Gut Lesions in Pediatric Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 13–19.
- Wiskin, A.E.; Wootton, S.A.; Culliford, D.J.; Afzal, N.A.; Jackson, A.A.; Beattie, R.M. Impact of disease activity on resting energy expenditure in children with inflammatory bowel disease. Clin. Nutr. 2009, 28, 652–656.
- A Sentongo, T.; Semeao, E.J.; A Piccoli, D.; A Stallings, V.; Zemel, B.S. Growth, Body Composition, and Nutritional Status in Children and Adolescents With Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 33–40.
- Kanof, M.E.; Lake, A.M.; Bayless, T.M. Decreased Height Velocity in Children and Adolescents Before the Diagnosis of Crohn’s Disease. Gastroenterology 1988, 95, 1523–1527.
- Vasseur, F.; Gower-Rousseau, C.; Vernier-Massouille, G.; Dupas, J.L.; Merle, V.; Merlin, B.; Lerebours, E.; Savoye, G.; Salomez, J.L.; Cortot, A.; et al. Nutritional Status and Growth in Pediatric Crohn’s Disease: A Population-Based Study. Am. J. Gastroenterol. 2010, 105, 1893–1900.
- Pfefferkorn, M.; Burke, G.; Griffiths, A.; Markowitz, J.; Rosh, J.; Mack, D.; Otley, A.; Kugathasan, S.; Evans, J.; Bousvaros, A.; et al. Growth Abnormalities Persist in Newly Diagnosed Children With Crohn Disease Despite Current Treatment Paradigms. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 168–174.
- Lee, J.; Escher, J.; Shuman, M.; Forbes, P.; Delemarre, L.; Harr, B.; Kruijer, M.; Moret, M.; Allende-Richter, S.; Grand, R. Final adult height of children with inflammatory bowel disease is predicted by parental height and patient minimum height Z-score. Inflamm. Bowel Dis. 2010, 16, 1669–1677.
- Yerushalmy-Feler, A.; Ben-Tov, A.; Weintraub, Y.; Amir, A.; Galai, T.; Moran-Lev, H.; Cohen, S. High and low body mass index may predict severe disease course in children with inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 708–713.
- Thangarajah, D.; Hyde, M.J.; Konteti, V.K.S.; Santhakumaran, S.; Frost, G.; Fell, J.M.E. Systematic review: Body composition in children with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 142–157.
- Houttu, N.; Kalliomäki, M.; Grönlund, M.-M.; Niinikoski, H.; Nermes, M.; Laitinen, K. Body composition in children with chronic inflammatory diseases: A systematic review. Clin. Nutr. 2020, 39, 2647–2662.
- Ward, L.M.; Ma, J.; Rauch, F.; Benchimol, E.I.; Hay, J.; Leonard, M.B.; Matzinger, M.A.; Shenouda, N.; Lentle, B.; Cosgrove, H.; et al. Musculoskeletal health in newly diagnosed children with Crohn’s disease. Osteoporos. Int. 2017, 28, 3169–3177.
- Sylvester, F.A.; Leopold, S.; Lincoln, M.; Hyams, J.S.; Griffiths, A.M.; Lerer, T. A Two-Year Longitudinal Study of Persistent Lean Tissue Deficits in Children With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 452–455.
- Werkstetter, K.J.; Ullrich, J.; Schatz, S.B.; Prell, C.; Koletzko, B.; Koletzko, S. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J. Crohn’s Coliti 2012, 6, 665–673.
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482.
- Gerasimidis, K.; McGrogan, P.; Edwards, C.A. The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J. Hum. Nutr. Diet. 2011, 24, 313–326.
- Holt, D.Q.; Varma, P.; Strauss, B.J.G.; Rajadurai, A.S.; Moore, G.T. Low muscle mass at initiation of anti-TNF therapy for inflammatory bowel disease is associated with early treatment failure: A retrospective analysis. Eur. J. Clin. Nutr. 2017, 71, 773–777.
- Murthy, S.K.; Begum, J.; I Benchimol, E.; Bernstein, C.N.; Kaplan, G.G.; McCurdy, J.D.; Singh, H.; Targownik, L.; Taljaard, M. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut 2020, 69, 274–282.
- Alves, A.; Panis, Y.; Bouhnik, Y.; Pocard, M.; Vicaut, E.; Valleur, P. Risk Factors for Intra-Abdominal Septic Complications After a First Ileocecal Resection for Crohn’s Disease: A Multivariate Analysis in 161 Consecutive Patients. Dis. Colon Rectum 2007, 50, 331–336.
- Ryan, E. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73.
- Adamina, M.; Gerasimidis, K.; Sigall-Boneh, R.; Zmora, O.; Overstraeten, A.D.B.V.; Campmans-Kuijpers, M.; Ellul, P.; Katsanos, K.; Kotze, P.; Noor, N.; et al. DOP05 Perioperative Dietary Therapy in inflammatory bowel disease. J. Crohn’s Colitis 2020, 14, S044.
- Lochs, H.; Allison, S.; Meier, R.; Pirlich, M.; Kondrup, J.; Schneider, S.; Berghe, G.V.D.; Pichard, C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, Definitions and General Topics. Clin. Nutr. 2006, 25, 180–186.
- Narula, N.; Dhillon, A.; Zhang, D.; E Sherlock, M.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542.
- Knight, C.; El-Matary, W.; Spray, C.; Sandhu, B.K. Long-term outcome of nutritional therapy in paediatric Crohn’s disease. Clin. Nutr. 2005, 24, 775–779.
- Akobeng, A.K.; Thomas, A.G. Refeeding Syndrome Following Exclusive Enteral Nutritional Treatment in Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 364–366.
- Da Silva, J.S.V. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195.
- McCole, D.F. IBD Candidate Genes and Intestinal Barrier Regulation. Inflamm. Bowel Dis. 2014, 20, 1829–1849.
- Kleessen, B.; Kroesen, A.J.; Buhr, H.J.; Blaut, M. Mucosal and Invading Bacteria in Patients with Inflammatory Bowel Disease Compared with Controls. Scand. J. Gastroenterol. 2002, 37, 1034–1041.
- Zaidi, D.; Bording-Jorgensen, M.; Huynh, H.Q.; Carroll, M.W.; Turcotte, J.-F.; Sergi, C.; Liu, J.; Wine, E. Increased Epithelial Gap Density in the Noninflamed Duodenum of Children With Inflammatory Bowel Diseases. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 644–650.
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; Macdonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2000, 14, 281–289.
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: Cytokine production and endoscopic and histological findings. Inflamm. Bowel Dis. 2005, 11, 580–588.
- De Jong, N.S.H.; Leach, S.T.; Day, A.S. Polymeric Formula Has Direct Anti-Inflammatory Effects on Enterocytes in an in VitroModel of Intestinal Inflammation. Dig. Dis. Sci. 2007, 52, 2029–2036.
- Nahidi, L.; Corley, S.M.; Wilkins, M.R.; Wei, J.; Alhagamhmad, M.; Day, A.S.; Lemberg, D.A.; Leach, S.T. The major pathway by which polymeric formula reduces inflammation in intestinal epithelial cells: A microarray-based analysis. Genes Nutr. 2015, 10, 29.
- Alhagamhmad, M.H. Enteral Nutrition in the Management of Crohn’s Disease: Reviewing Mechanisms of Actions and Highlighting Potential Venues for Enhancing the Efficacy. Nutr. Clin. Pr. 2018, 33, 483–492.
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive enteral nutrition in active pediatric Crohn disease: Effects on intestinal microbiota and immune regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596.
- Wedrychowicz, A.; Kowalska-Duplaga, K.; Jedynak-Wasowicz, U.; Pieczarkowski, S.; Sladek, M.; Tomasik, P.; Fyderek, K. Serum Concentrations of VEGF and TGF-β1 During Exclusive Enteral Nutrition in IBD. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 150–155.
- Hahm, K.-B.; Im, Y.-H.; Parks, T.W.; Park, S.H.; Markowitz, S.D.; Jung, H.-Y.; Green, J.; Kim, S.J. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut 2001, 49, 190–198.
- Triantafillidis, J.K.; Tzouvala, M.; Triantafyllidi, E. Enteral Nutrition Supplemented with Transforming Growth Factor-β, Colostrum, Probiotics, and Other Nutritional Compounds in the Treatment of Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1048.
- I Keenan, J.; Hooper, E.M.; Tyrer, P.C.; Day, A.S. Influences of enteral nutrition upon CEACAM6 expression by intestinal epithelial cells. Innate Immun. 2013, 20, 848–856.
- Budd, G.R.; Aitchison, A.; Day, A.S.; I Keenan, J. The effect of polymeric formula on enterocyte differentiation. Innate Immun. 2017, 23, 240–248.
- Nahidi, L.; Day, A.S.; Lemberg, D.A.; Leach, S.T. Differential effects of nutritional and non-nutritional therapies on intestinal barrier function in an in vitro model. J. Gastroenterol. 2011, 47, 107–117.
- Hansen, R.; Russell, R.K.; Reiff, C.; Louis, P.; McIntosh, F.; Berry, S.H.; Mukhopadhya, I.; Bisset, M.W.; Barclay, A.R.; Bishop, J.; et al. Microbiota of De-Novo Pediatric IBD: Increased Faecalibacterium Prausnitzii and Reduced Bacterial Diversity in Crohn’s But Not in Ulcerative Colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922.
- Hedin, C.R.; E McCarthy, N.; Louis, P.; Farquharson, F.M.; McCartney, S.; Taylor, K.; Prescott, N.J.; Murrells, T.; Stagg, A.J.; Whelan, K.; et al. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn’s disease and their unaffected siblings. Gut 2014, 63, 1578–1586.
- Treem, W.R.; Ahsan, N.; Shoup, M.; Hyams, J.S. Fecal Short-Chain Fatty Acids in Children with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 159–164.
- Bjerrum, J.T.; Wang, Y.; Hao, F.; Coskun, M.; Ludwig, C.; Günther, U.; Nielsen, O.H. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics 2015, 11, 122–133.
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539.
- Diederen, K.; Li, J.V.; Donachie, G.E.; De Meij, T.G.; De Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; Velde, A.A.T.; et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci. Rep. 2020, 10, 1–17.
- Lionetti, P.; Callegari, M.L.; Ferrari, S.; Cavicchi, M.C.; Pozzi, E.; De Martino, M.; Morelli, L. Enteral Nutrition and Microflora in Pediatric Crohn’s Disease. J. Parenter. Enter. Nutr. 2005, 29, S173–S178.
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in Presumptively Protective Gut Bacterial Species and Metabolites Are Paradoxically Associated with Disease Improvement in Pediatric Crohn’s Disease During Enteral Nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871.
- Logan, M.; Gkikas, K.; Svolos, V.; Nichols, B.; Milling, S.; Gaya, D.R.; Seenan, J.P.; Macdonald, J.; Hansen, R.; Ijaz, U.Z.; et al. Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn’s disease-new insights into dietary disease triggers. Aliment. Pharmacol. Ther. 2020, 51, 935–947.
- Quince, C.; Ijaz, U.Z.; Loman, N.; A Eren, M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children With Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729.
- Voitk, A.J. Experience With Elemental Diet in the Treatment of Inflammatory Bowel Disease: Is This Primary Therapy? Arch. Surg. 1973, 107, 329.
- Morin, C.L.; Roulet, M.; Roy, C.C.; Weber, A. Continuous elemental enteral alimentation in children with Crohn’s disease and growth failure. Gastroenterology 1980, 79, 1205–1210.
- Sanderson, I.R.; Udeen, S.; Davies, P.S.; O Savage, M.; A Walker-Smith, J. Remission induced by an elemental diet in small bowel Crohn’s disease. Arch. Dis. Child. 1987, 62, 123–127.
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.; Falzon, L.; Ferry, S.L. Systematic review with meta-analysis: Enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 46, 645–656.
- Levine, A. Comparison of Outcomes Parameters for Induction of Remission in New Onset Pediatric Crohn’s Disease: Evaluation of the Porto IBD Group “Growth Relapse and Outcomes with Therapy” (GROWTH CD) Study. Inflamm. Bowel Dis. 2014, 20, 278–285.
- Lee, D.; Baldassano, R.N.; Otley, A.R.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Lindsey, A.; Gilroy, E.; Nessel, L.; et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1786–1793.
- Shah, S.C.; Colombel, J.-F.; E Sands, B.; Narula, N. Systematic review with meta-analysis: Mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 317–333.
- Grover, Z.; Muir, R.; Lewindon, P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J. Gastroenterol. 2014, 49, 638–645.
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753.
- Grover, Z.; Burgess, C.; Muir, R.; Reilly, C.; Lewindon, P.J. Early Mucosal Healing with Exclusive Enteral Nutrition is Associated with Improved Outcomes in Newly Diagnosed Children with Luminal Crohn’s disease. J. Crohn’s Colitis 2016, 10, 1159–1164.
- Buchanan, E.; Gaunt, W.W.; Cardigan, T.; Garrick, V.; McGrogan, P.; Russell, R.K. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment. Pharmacol. Ther. 2009, 30, 501–507.
- Afzal, N.A.; Davies, S.; Paintin, M.; Arnaud-Battandier, F.; Walker-Smith, J.A.; Murch, S.; Heuschkel, R.; Fell, J. Colonic Crohn’s Disease in Children Does Not Respond Well to Treatment with Enteral Nutrition If the Ileum Is Not Involved. Dig. Dis. Sci. 2005, 50, 1471–1475.
- Day, A.S.; E Whitten, K.; A Lemberg, D.; Clarkson, C.; Vitug-Sales, M.; Jackson, R.; Bohane, T.D. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: A feasible and effective approach. J. Gastroenterol. Hepatol. 2006, 21, 1609–1614.
- Belli, D.; Seidman, E.; Bouthillier, L.; Weber, A.; Roy, C.; Pletincx, M.; Beaulieu, M.; Morin, C. Chronic intermittent elemental diet improves growth failure in children with Crohn’s disease. Gastroenterology 1988, 94, 603–610.
- Polk, D.B.; Hattner, J.A.T.; Kerner, J.A. Improved Growth and Disease Activity After Intermittent Administration of a Defined Formula Diet in Children With Crohn’s Disease. J. Parenter. Enter. Nutr. 1992, 16, 499–504.
- Patel, K.V.; Darakhshan, A.A.; Griffin, N.; Williams, A.B.; Sanderson, J.D.; Irving, P.M. Patient optimization for surgery relating to Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 707–719.
- Heerasing, N.; Thompson, B.; Hendy, P.; Heap, G.A.; Walker, G.; Bethune, R.; Mansfield, S.; Calvert, C.; Kennedy, N.A.; Ahmad, T.; et al. Exclusive enteral nutrition provides an effective bridge to safer interval elective surgery for adults with Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 45, 660–669.
- Ge, X.; Tang, S.; Yang, X.; Liu, W.; Ye, L.; Yu, W.; Xu, H.; Cao, Q.; Zhou, W.; Cai, X. The role of exclusive enteral nutrition in the preoperative optimization of laparoscopic surgery for patients with Crohn’s disease: A cohort study. Int. J. Surg. 2019, 65, 39–44.
- Grass, F.; Pache, B.; Martin, D.; Hahnloser, D.; Demartines, N.; Hübner, M. Preoperative Nutritional Conditioning of Crohn’s Patients-Systematic Review of Current Evidence and Practice. Nutrients 2017, 9, 562.
- Jacobson, S. Early postoperative complications in patients with Crohn’s disease given and not given preoperative total parenteral nutrition. Scand. J. Gastroenterol. 2012, 47, 170–177.
- Wang, H.; Zuo, L.; Zhao, J.; Dong, J.; Li, Y.; Gu, L.; Gong, J.; Liu, Q.; Zhu, W. Impact of Preoperative Exclusive Enteral Nutrition on Postoperative Complications and Recurrence After Bowel Resection in Patients with Active Crohn’s Disease. World J. Surg. 2016, 40, 1993–2000.
- Li, Y.; Zuo, L.; Zhu, W.; Gong, J.; Zhang, W.; Gu, L.; Guo, Z.; Cao, L.; Li, N.; Li, J. Role of Exclusive Enteral Nutrition in the Preoperative Optimization of Patients With Crohn’s Disease Following Immunosuppressive Therapy. Medicine 2015, 94, e478.
- Li, G.; Ren, J.; Wang, G.; Hu, D.; Gu, G.; Liu, S.; Ren, H.; Wu, X.; Li, J. Preoperative exclusive enteral nutrition reduces the postoperative septic complications of fistulizing Crohn’s disease. Eur. J. Clin. Nutr. 2014, 68, 441–446.
- Harris, R.E.; Duncan, H.; Buchanan, E.; Cardigan, T.; Garrick, V.; Curtis, L.; Gervais, L.; Barclay, A.; Haddock, G.; Hansen, R.; et al. Prehabilitation: The Impact of Preoperative Exclusive Enteral Nutrition on Paediatric Patients With Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 503–507.
- Whitten, K.E.; Leach, S.T.; Bohane, T.D.; Woodhead, H.J.; Day, A.S. Effect of exclusive enteral nutrition on bone turnover in children with Crohn’s disease. J. Gastroenterol. 2009, 45, 399–405.
- Strisciuglio, C.; Scarpato, E.; Cenni, S.; Serra, M.; Giugliano, F.; Mainolfi, C.; Dolce, P.; Martinelli, M.; Staiano, A.; Miele, E. Improvement of body composition and bone mineral density after enteral nutrition in pediatric Crohn disease. Dig. Liver Dis. 2020, 52, 630–636.
- Lev-Tzion, R.; Ben-Moshe, T.; Abitbol, G.; Ledder, O.; Peleg, S.; Millman, P.; Shaoul, R.; Kori, M.; Assa, A.; Cohen, S.; et al. The Effect of Nutritional Therapy on Bone Mineral Density and Bone Metabolism in Pediatric Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 2021.
- Johnson, T.; Macdonald, S.; Hill, S.M.; Thomas, A.; Murphy, M.S. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: A randomised controlled trial. Gut 2006, 55, 356–361.
- Gupta, K.; Noble, A.; Kachelries, K.E.; Albenberg, L.; Kelsen, J.R.; Grossman, A.B.; Baldassano, R.N. A Novel Enteral Nutrition Protocol for the Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 1374–1378.
- Wilschanski, M.; Sherman, P.; Pencharz, P.; Davis, L.; Corey, M.; Griffiths, A. Supplementary enteral nutrition maintains remission in paediatric Crohn’s disease. Gut 1996, 38, 543–548.
- Duncan, H.; Buchanan, E.; Cardigan, T.; Garrick, V.; Curtis, L.; McGrogan, P.; Barclay, A.; Russell, R.K. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: Supplemental enteral nutrition is better than nothing! BMC Gastroenterol. 2014, 14, 50.
- Schulman, J.M.; Pritzker, L.; Shaoul, R. Maintenance of Remission with Partial Enteral Nutrition Therapy in Pediatric Crohn’s Disease: A Retrospective Study. Can. J. Gastroenterol. Hepatol. 2017, 1–7.
- Gavin, J.; Ashton, J.J.; Heather, N.; Marino, L.V.; Beattie, R.M. Nutritional support in paediatric Crohn’s disease: Outcome at 12 months. Acta Paediatr. 2017, 107, 156–162.
- Logan, M.; Clark, C.M.; Ijaz, U.Z.; Gervais, L.; Duncan, H.; Garrick, V.; Curtis, L.; Buchanan, E.; Cardigan, T.; Armstrong, L.; et al. The reduction of faecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment. Pharmacol. Ther. 2019, 50, 664–674.
- Takagi, S.; Utsunomiya, K.; Kuriyama, S.; Yokoyama, H.; Takahashi, S.; Iwabuchi, M.; Kinouchi, Y.; Hiwatashi, N.; Funayama, Y.; Sasaki, I.; et al. Effectiveness of an ’half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 1333–1340.
- Hanai, H.; Iida, T.; Takeuchi, K.; Arai, H.; Arai, O.; Abe, J.; Tanaka, T.; Maruyama, Y.; Ikeya, K.; Sugimoto, K.; et al. Nutritional therapy versus 6-mercaptopurine as maintenance therapy in patients with Crohn’s disease. Dig. Liver Dis. 2012, 44, 649–654.
- Hirai, F.; Takeda, T.; Takada, Y.; Kishi, M.; Beppu, T.; Takatsu, N.; Miyaoka, M.; Hisabe, T.; Yao, K.; Ueki, T. Efficacy of enteral nutrition in patients with Crohn’s disease on maintenance anti-TNF-alpha antibody therapy: A meta-analysis. J. Gastroenterol. 2020, 55, 133–141.
- Gkikas, K.; Gerasimidis, K.; Milling, S.; Ijaz, U.Z.; Hansen, R.; Russell, R.K. Dietary Strategies for Maintenance of Clinical Remission in Inflammatory Bowel Diseases: Are We There Yet? Nutrients 2020, 12, 2018.
- Jones, V.A. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn’s disease. Dig. Dis. Sci. 1987, 32, S100–S107.
- Woolner, J.T.; Parker, T.J.; Kirby, G.A.; Hunter, J.O. The development and evaluation of a diet for maintaining remission in Crohn’s disease. J. Hum. Nutr. Diet. 1998, 11, 1–11.
- Faiman, A.; Mutalib, M.; Moylan, A.; Morgan, N.; Crespi, D.; Furman, M.; Kader, A. Standard versus rapid food reintroduction after exclusive enteral nutritional therapy in paediatric Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2014, 26, 276–281.
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel. Dis. 2013, 19, 1322–1329.
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015, 6, 78–83.
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nat. Cell Biol. 2015, 519, 92–96.
- Nickerson, K.P.; Homer, C.R.; Kessler, S.P.; Dixon, L.J.; Kabi, A.; Gordon, I.O.; Johnson, E.E.; De La Motte, C.A.; McDonald, C. The Dietary Polysaccharide Maltodextrin Promotes Salmonella Survival and Mucosal Colonization in Mice. PLoS ONE 2014, 9, e101789.
- Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. EEN Yesterday and Today CDED Today and Tomorrow. Nutrients 2020, 12, 3793.
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360.
- Boneh, R.S.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759.
- Debruyn, J.C.; Jacobson, K.; El-Matary, W.; Carroll, M.; Wine, E.; Wrobel, I.; Van Woudenberg, M.; Huynh, H.Q. Long-term Outcomes of Infliximab Use for Pediatric Crohn Disease: A Canadian Multicenter Clinical Practice Experience. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 268–273.
- Faubion, W.A.; Dubinsky, M.; Ruemmele, F.M.; Escher, J.; Rosh, J.; Hyams, J.S.; Lazar, A. Long-term Efficacy and Safety of Adalimumab in Pediatric Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 453–460.
- Boneh, R.S.; Shabat, C.S.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy With the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohn’s Colitis 2017, 11, 1205–1212.
- Turner, D.; Griffiths, A.M.; Wilson, D.; Mould, D.R.; Baldassano, R.N.; Russell, R.K.; Dubinsky, M.; Heyman, M.B.; De Ridder, L.; Hyams, J.; et al. Designing clinical trials in paediatric inflammatory bowel diseases: A PIBDnet commentary. Gut 2020, 69, 32–41.
