Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luis Cortés and Version 2 by Vivi Li.
Liver fibrosis is the consequence of different inflammatory processes occurring in any chronic liver disease. Its progression determines the development of cirrhosis and portal hypertension. The natural history of cirrhosis is characterized by a compensated phase, with or without portal hypertension, and a decompensated phase characterized by the appearance of major complications, such as ascites, portal hypertensive bleeding, encephalopathy, and jaundice. Malnutrition is frequent in patients with liver cirrhosis, which progresses in parallel with the worsening of the disease. Its etiology is multifactorial, given the great impact of liver disease on multiple processes related to nutrition.
- liver cirrhosis
- malnutrition
- malabsorption
- vitamins
- minerals
- sarcopenia
- liver transplant
- pancreatic exocrine insufficiency
- nutritional assessment
1. Consequences of Liver Disease on Nutritional Status
Liver fibrosis is the consequence of different inflammatory processes occurring in any chronic liver disease. Its progression determines the development of cirrhosis and portal hypertension. The natural history of cirrhosis is characterized by a compensated phase, with or without portal hypertension, and a decompensated phase characterized by the appearance of major complications, such as ascites, portal hypertensive bleeding, encephalopathy, and jaundice.
Malnutrition is frequent in patients with liver cirrhosis, which progresses in parallel with the worsening of the disease. Its etiology is multifactorial, given the great impact of liver disease on multiple processes related to nutrition [1][18]. In this section, we summarize the consequences of liver disease on nutritional status, focusing on the different components and functions that might be affected throughout the course of the disease (Table 1).
Table 1. Consequences of liver disease on nutritional status.
| Nutritional Consequence [Ref.] | Mechanisms in Chronic Liver Disease |
|---|---|
| 1. Impaired dietary intake [2][3][19,20] | Anorexia, dysgeusia, abdominal pain, bloating, early satiety secondary to ascites, prescription of restrictive diets, alcohol consumption |
| 2. Altered macro and micronutrient metabolism [4][5][6][7][8][9][10][11][13,14,15,16,21,22,23,24] | Lack of glycogen and vitamin storage, breakdown of fat and proteins as the principal energy source, decrease of vitamin and mineral levels |
| 3. Energy metabolism disturbances [12][25] | Hypermetabolic state, impaired glucose and lipid metabolism, sedentary lifestyle |
| 4. Increase in energy expenditure [13][14][26,27] | Increased catecholamines, malnutrition, immune compromise |
| 5. Nutrient malabsorption [15][16][28,29] | Decreased bile production, cholestasis, portosystemic shunting, portal hypertension gastropathy and enteropathy, small intestinal bacterial overgrowth, drug-related diarrhea |
| 6. Sarcopenia and muscle function [17][18][19][30,31,32] | Proteolysis as the energy source, inhibition of muscle growth, muscle autophagy, proinflammatory state |
| 7. Metabolic osteopathy [20][33] | Decrease in bone formation, increased bone resorption, dysbiosis, vitamin K and D deficiencies |
1.1. Impaired Dietary Intake
Anorexia is very common in patients with cirrhosis. It is caused by a mechanical effect, such as ascites, or by an imbalance between orexigenic and anorexigenic hormones (decrease of ghrelin and increase in leptin, respectively) [2][19]. Moreover, dietary restrictions imposed—sometimes quite rightly (sodium restriction for ascites) and sometimes based on false convictions (protein consumption restriction for encephalopathy)—may adversely limit dietary intake and cause taste alterations. The maintenance of an adequate nutritional status should prevail over dietary restrictions [3][20].
Regarding the etiology of liver disease, dietary intake is worse in alcoholic patients, given that alcohol represents their principal source of energy, rather than nutrient-rich foods. As a result, they develop nutrient deficiencies such as low serum levels of folate (B9), cobalamin (B12), and pyridoxine (B6), as well as macronutrient deficiencies.
1.2. Altered Macro and Micronutrients Metabolism
1.2.1. Plasma Proteins
Hepatocytes play a central role in the production of plasma proteins, and the liver is the site where 80% of blood proteins are formed, except for gamma-globulin, which is produced by the reticuloendothelial system in the Kupffer cells. Plasma proteins include albumin (55%), globulins (38%), and fibrinogen (7%). They also include clotting factors, carrier and transport proteins, hormones, apolipoproteins, and other proteins involved in homeostasis.
Albumin is the principal plasma protein and modulator of the fluid distribution in compartments of the body, accounting for about 70–75% of the total plasma oncotic pressure. It also has other important roles, such as antioxidation, and immune-modulatory function, and the maintenance of homeostasis. A reduced serum concentration of albumin is a common feature in patients with cirrhosis. It has an adverse prognosis and occurs in parallel with the severity of liver cirrhosis, reaching a 60–80% reduction in advanced cirrhosis. Hypoalbuminemia results from both the decreased synthesis and complications due to the disease progression, such as ascites, which dilutes extracellular fluid protein content. Moreover, the sustained systemic inflammatory and pro-oxidant state induces structural changes of albumin that compromise the non-oncotic properties of the molecule [8][21].
1.2.2. Vitamins and Minerals
The liver is a key storage site for several macro and micronutrients, including vitamin A, copper, manganese, iron, fatty acids, and glycogen, among others. It is also an important organ in terms of the production of binding, transport, and regulatory proteins required for micronutrient homeostasis and bile acids required for intestinal absorption. As a consequence, patients with chronic liver disease are at risk of the depletion of fat and water-soluble vitamins and minerals to a greater or lesser extent, depending on the etiology. For example, deficiencies of vitamin B12, folate, and zinc are the most well recognized symptoms of patients with alcoholic liver disease, whereas patients with cholestatic liver disease, deficiencies of fat-soluble vitamins prevail [5][6][7][14,15,16]. In end-stage liver disease, a lack of vitamins and minerals is nearly universal. Interestingly, vitamin D deficiency is present in 64% to 92%, regardless of the etiology of liver disease, and is associated with liver fibrosis and related to nonresponse to antiviral therapy for chronic hepatitis C [9][22]. In Table 2, the function of vitamins and minerals, their relationship with the liver, and the consequences of their deficit are summarized [10][11][23,24].
Table 2. Role of vitamins and minerals in the liver (RBP4: Retinol Binding Protein 4. HSc: Hepatic Stellate cells. MAFLD: Metabolic Associated Fatty Liver Disease).
| Vitamin [Ref.] | Liver Role | Deficiency and Liver Disease | |
|---|---|---|---|
| Fat-soluble vitamins | |||
| A (retinol) [5][6][7][14,15,16] | Production of RBP4 (transporter) Main storage in HSc (80%) |
Lost in vitamin A storage through the transformation of HSc into myofibroblasts. Deficiency is associated with nyctalopia (night blindness) and with hepatic encephalopathy | |
| D [5][6][7][9][14,15,16,22] | 25-hydroxylation site Production of binding proteins | Deficiency is associated with fibrosis, liver dysfunction, and mortality | |
| K [5][6][7][14,15,16] | Absorption of vitamin K trough bile acids | Deficiency is associated with coagulopathy and bone disease through an inadequate carboxylation of bone matrix proteins | |
| E [5][6][7][14,15,16] | Absorption of vitamin E trough bile acids | Deficiency is associated with hemolytic anemia, creatinuria, and neuronal degeneration | |
| Water-soluble vitamins | |||
| B [5][6][14[7],15,16] | B1 (thiamine) |
Normal thiamine function | Lost in activation and transport. Deficiency is associated with neurologic dysfunction (Wernicke encephalopathy) and high-output heart failure (wet beriberi) |
| B2 (riboflavin) |
Storage of riboflavin | Inadequate intake, increased utilization, and deficient storage. Deficiency is associated with inflammation of the gums and sores | |
| B6 (pyridoxine) |
Storage of pyridoxine | Deficiency is associated with anemia and neutropenia | |
| B9 (folate) |
Storage of folate | Deficiency is associated with anemia and macrocytosis | |
| B12 (cobalamin) |
Storage of cobalamin | Deficiency is associated with anemia and neutropenia | |
| C [5][6][7][14,15,16] | Storage of vitamin C | Deficiency is common in MAFLD. Deficiency is associated with bleeding, joint pain, and an increase of free radicals | |
| Minerals | |||
| Zinc (Zn) [5][6][7][14,15,16] | Absorption of Zn | Inadequate dietary intake, impaired absorption, and an increase in urinary loss. Deficiency is associated with hepatic encephalopathy and alterations in taste and smell | |
| Magnesium (Mg) [5][6][7][14,15,16] | Transport of Mg | Impaired transport and decrease intake. Deficiency is associated with dysgeusia, decreased appetite, muscle cramps, and weakness | |
| Manganese (Mn) [10][23] | Absorption trough bile acid production | Elevated if there is a decrease in biliary excretion Deficiency is associated with brain accumulation and parkinsonism |
|
| Carnitine [11][24] | Metabolism of carnitine | Poor intake. Deficiency is associated with muscle cramps | |
| Selenium (Se) [5][6][7][14,15,16] | Metabolism of Se | Deficiency related to severity liver disease Deficiency is associated with insulin resistance |
|
| Iron (Fe) [5][6][7][14,15,16] | Metabolism of Fe | Overload in alcoholic liver disease. Deficiency is associated with hepatic overload, fibrosis, and dysfunction | |
1.3. Energy Metabolism Disturbances
The human liver plays a central role in regulating fuel metabolism. The maintenance of glucose homeostasis, disposal of nitrogen by the urea cycle, and ketogenesis from fatty acids are some of the most important functions driven by the liver for maintaining internal homeostasis, and they are intimately linked. Liver disease leads to numerous metabolic disturbances. Firstly, due to the impairment of the ability to synthesize, store, and break down glycogen by hepatocytes, there is a decreased level of glycogenolysis and increased level of gluconeogenesis from muscle proteolysis, leading to a catabolism condition and sarcopenia. This not only leads to a decline in muscle mass, but also to a remarkable insulin resistance, which leads to glucose intolerance in up to 60% to 80% of patients, with cirrhosis and diabetes mellitus in 20% of cases. Moreover, it has been shown that the majority of energy is derived from fat oxidation. Conversely, in healthy subjects, fat oxidation accounts for only 40% of the total energy expenditure, after an overnight fast.
Secondly, there is a hypermetabolic state due to the increased production of cytokines that activates gluconeogenesis from muscle proteins and leads to a breakdown of muscle cells via autophagy and sarcopenia. Due to this, both patients with cirrhosis and acute liver diseases, such as acute hepatitis, have a high incidence of wasting and malnutrition. This hypermetabolic state is also responsible for hyperdynamic circulation and its consequences, such as bacterial translocation from the gut and the chronic inflammation state.
Finally, sedentariness also contributes to energy metabolism disturbances. Physical activity is an important determinant of muscle anabolism and the correct use of energy. In cirrhotic patients, sedentariness is a frequent characteristic consequence of many factors, such as ascites or other concomitant diseases, which perpetuate sarcopenia and its metabolic disorders, such as hyperammonemia and fat oxidation. This energy metabolism disturbance leads to an increase in energy expenditure, which has been reported to be associated with metabolic risk factors, including insulin resistance and high blood pressure, turning cirrhosis into a risk state of developing metabolic syndrome [12][25].
1.4. Increase in Energy Expenditure
In healthy people, the energy supply must balance the total energy expenditure (TEE) to maintain nutritional equilibrium, which includes a combination of resting energy expenditure (REE), physical activity expenditure, and food-related thermogenesis.
Specifically, TEE is composed of the energy costs of the processes essential for life (basal metabolic rate (BMR), 60–80% of TEE), of the energy expended in order to digest, absorb, and convert food (diet-induced thermogenesis, ~10%), and the energy expended during physical activities (activity energy expenditure, ~15–30%) [13][26].
REE represents the amount of energy expended by a person at rest. In practice, REE and BMR differ by less than 10%, so the terms can be used interchangeably. As mentioned above, it contributes to 60–80% of TEE, being the largest component of total daily energy expenditure both in healthy and pathological subjects [13][26].
An increased REE has been proposed to be of pathophysiological importance in liver disease, given that cirrhosis is a state of accelerated starvation. It has been described to be raised to 120% of the expected value in more than 15–30% of patients with liver cirrhosis, and the principal mechanisms responsible for this state include hypermetabolism, defined as measured REE > 20% above predicted RE, malnutrition, and immunosuppression [4][14][13,27].
1.5. Nutrient Malabsorption
Several factors can contribute to the malabsorption of nutrients in cirrhotic patients. One of them is portosystemic-shunting, which makes nutrients bypass the liver without metabolic processing. Another factor to consider is a drop in bile production due to impaired liver function. As a result, micelles formation is defective, and the absorption of long chain fatty acids through the usual lymphatic route is missing. This has pathophysiologic implications and can result in an excess hepatic storage of fat, which can reduce liver function and the systemic availability of fat for organic functions. Small intestinal bacterial overgrowth (SIBO) is very common in cirrhotic patients and may contribute to malabsorption. Colonic bacteria colonize the small bowel and impair microvilli function, digestive enzyme production, and intestinal barrier dysfunction, causing a disturbed absorption and metabolism of nutrients and affecting intestinal motility. SIBO may also be involved in bacterial translocation and infectious complications, such as spontaneous bacterial peritonitis [15][28]. Finally, drug-related malabsorption due to diarrhea (e.g., lactulose, antibiotics, or diuretics) or interference with fat absorption (e.g., cholestyramine) can also contribute to the malabsorption of nutrients. Moreover, decompensations and several complications of end stage liver disease, like over hepatic encephalopathy or spontaneous bacterial peritonitis (SBP), are directly or indirectly linked with the gut microbiota. Several studies have evaluated how microbiota changes in cirrhosis. Overall, widespread dysbiosis is observed in cirrhotic patients, with reduction in autochthonous taxa and increase in pathogenic ones. Particularly, reduced Bacteroidetes with increased Proteobacteria at the phylum level, increased Veillonella and Streptococcus spp., a significantly higher abundance of Enterobacteriaceae, but lower Lachonospiraceae, Ruminococcaceae, and Blautia (7a-dehydroxylating bacteria) in the cirrhosis group compared to controls [16][29].
1.6. Sarcopenia and Muscle Function
Sarcopenia is defined by a loss of muscle mass and decreased functional capacity. This complication may be present in the early stages of liver disease, but it is more frequent and severe in the end-stage disease, with a prevalence of nearly 60%. It is associated with a higher mortality, increased hospital admissions, worse post-liver transplant outcomes, decreased quality of life, and increased risk of other complications associated with cirrhosis [17][30].
Again, multiple factors are thought to be involved in the development of sarcopenia, some of which are common to other components of malnutrition previously mentioned, such as an altered carbohydrate and lipid metabolism, malabsorption, hypermetabolism, and anorexia. However, the most well-documented factor that contributes to sarcopenia is hyperammonemia. Hyperammonemia is a metabolic condition characterized by raised levels of ammonia, a nitrogen-containing compound, that is a potent neurotoxin. Ammonia levels rise if the liver is unable to metabolize this toxic compound as a result of an enzymatic defect or hepatocellular damage. Normal levels of ammonia vary according to age. In healthy adults the normal level is less than 30 micromol/L [18][31]. The decrease in the hepatic clearance of ammonia is compensated by the muscle clearance, whereby energy and proteins are expended, and hence, muscle breakdown is increased. It also induces the up-regulation of myostatin, the main muscle growth inhibitory factor, which, together with the descent of IGF-1 and testosterone, the main muscle growth-promoting factors in liver cirrhosis, leads to sarcopenia [19][32].
Apart from the loss of muscle mass, patients with liver cirrhosis have a decreased muscle function due to a mitochondrial dysfunction and direct modifications of contractile proteins. Additionally, the increased muscle breakdown and reduced muscle quality, as determined by the fat infiltration, observed through imaging, contributes to the poor physical condition.
1.7. Metabolic Osteopathy
Osteoporosis and osteopenia are common complications in patients with cirrhosis, with a prevalence of approximately 12–55% higher than in healthy people [20][33]. First is a systemic bone disease, which is characterized by a low bone mineral density (BMD), micro architectural malformation, and susceptibility to fracture. Osteopenia is a low-grade osteoporosis.
In chronic liver diseases, bone loss refers to a decrease in bone formation and increase in bone resorption. The main mechanisms underlying osteoporosis in patients with chronic liver disease are vitamin K deficiency, vitamin D and calcium metabolism alterations, hormonal dysregulation, and proinflammatory cytokines related to “leaky gut syndrome” and IGF-1 deficiency [21][34]. These abnormalities differ depending on the etiology: in cholestatic liver disease, deficiencies of vitamin K and D represent the main cause of metabolic osteopathy; in hemochromatosis, there is an associated hypogonadism that can explain this condition; in Metabolic Associated Fatty Liver Disease (MAFLD), viral hepatitis and alcoholic liver disease, the increase in proinflammatory cytokine production represents the pathophysiological mechanism.
1.8. Interplay between MAFLD and Diet
The prevalence of MAFLD is increasing as the rate of obesity rises as well as sedentary lifestyles and other components of metabolic syndrome. Fortunately, only a small percentage of patients develop inflammation and subsequently fibrosis and chronic liver disease. Obesity does not rule out malnutrition. In fact, several studies have described a significant association between sarcopenia and MAFLD, independent of obesity and insulin resistance [22][35]. Moreover, MAFLD is also present in 7% of normal-weight (lean) persons.
The underlying pathophysiological mechanism of MAFLD remains unclear. The “multiple hit” hypothesis considers multiple insults acting together on genetically predisposed subjects, such as insulin resistance, hormones secreted from the adipose tissue, nutritional factors, gut microbiota and genetic and epigenetic factors [23][36]. Recently, a complex interplay between the gut microbiota, intestinal barrier and nutrition has been described. The dietary factors may alter the gut microbiota and intestinal barrier function, directly affecting hepatic organelles and cell-to-cell communications, favoring the occurrence of metabolic endotoxemia and low-grade inflammation and generating an adverse microenvironment which could in which several hepatocytes select anti-apoptotic programs and mutations that may allow survival and proliferation [24][25][37,38]. These facts may facilitate MAFLD progression from simple steatosis to nonalcoholic steato-hepatitis (NASH) and cirrhosis and development of hepatocellular carcinoma (HCC) [24][25][37,38].
2. Nutritional Assessment of Patients with Chronic Liver Disease
2.1. Nutritional Screening and Risk of Malnutrition
Nutritional screening should be performed in all patients with liver cirrhosis, especially if they have portal hypertension or liver failure.
The European Society for Clinical Nutrition and Metabolism (ESPEN) states that screening tools should be simple and quick, and untrained personnel should be able to administer them. A good screening tool has to be easy to apply and have a reasonable specificity, but above all, it should have an excellent sensitivity [26][39].
There are a large number of nutritional screening tools, each with its own strengths and weaknesses. Table 3 shows the most frequently used tools [27][28][29][30][31][32][33][40,41,42,43,44,45,46]. There are variables common to most of them, such as BMI and weight loss, and other ones which vary, such as muscle mass assessment, food intake, appetite, etc. In recent years, specific tools have been developed for patients with liver diseases, such as the Royal Free Hospital Nutritional Prioritizing Tool (RFH-NPT) and Liver Disease Undernutrition Screening Tool (LDUST). All general tools that take into account BMI or weight loss as a variable may be inaccurate due to the presence of oedema and/or ascites, which is very prevalent in the liver cirrhosis patient population. What LDUST and RFH-NPT have in common is that they seek to exclude or limit the impact of weight gain through fluid retention, as shown through anthropometric assessment. RFH-NPT has shown a higher diagnostic and complication predictive capacity. In addition, LDUST has a higher degree of subjectivity, since at least two of the questions depend on the patient’s assessment and may have a high degree of variability.
Table 3. Most frequently used screening tools for patients with liver cirrhosis.
| Screening Tool [Ref.] | Target Population | Variables | Strengths and Weaknesses | Usefulness in Patients with Liver Cirrhosis |
|---|---|---|---|---|
| MST [27][40] | Hospitalized patients | 1—Weight loss 2—Food intake 3—Appetite |
Quick and easy No calculations No training Self-administered |
May be inaccurate due to fluid overload. Low sensitivity in patients with liver cirrhosis. |
| MUST [28][41] | Hospitalized patients and outpatients | 1—BMI 2—Weight loss 3—Acute illness and impact on dietary |
Quick and easy Adds acute illness Offers advice |
May be inaccurate due to fluid overload. Low sensitivity in patients with liver cirrhosis. |
| MNA-SF [29][42] | Elderly patients | 1—Weight loss 2—Appetite 3—Mobility 4—Neuropsycho problems 5—BMI 6—Acute illness |
Full evaluation, not only nutritional aspects BMI can be replaced by calf diameter |
Good performance in liver cirrhosis. High sensitivity and good specificity. |
| NRS-2002 [30][43] | Hospitalized patients | 1—BMI 2—Weight loss 3—Food intake 4—Illness severity |
Adds illness severity and age | May be inaccurate due to fluid overload. Low sensitivity in liver cirrhosis. High specificity |
| CONUT [31][44] | Informatic tool Hospitalized patients and outpatients |
1—Albumin 2—Cholesterol 3—Lymphocytes 4—Age 5—Illness severity 6—Length of illness 7—Treatment |
Automated screening of large populations Blood test required Low specificity |
Predictor of survival and complications after liver resection. Predictor of survival in end-stage liver disease. |
| SNAQ [32][45] | Hospitalized patients and outpatients | 1—Weight loss 2—Appetite 3—Nutritional supplements 4—BMI 5—Albumin 6—Lymphocytes |
Simple and quick Provides a recommendation Blood test required |
Limited data on the population with liver cirrhosis, but correlation with the Child–Pugh stage. |
| RFH-NPT [33][46] | Patients with liver cirrhosis | 1—Transplant 2—Fluid overload 3—Weight loss 4—Food intake 5—BMI (in absence of fluid overload) 6—Acute illness |
Adds transplantation Reduces the impact of fluid retention Adds acute illness |
Superior results compared to other tests in liver cirrhosis. High sensitivity and specificity. |
| LDUST [32][45] | Patients with liver cirrhosis | 1—Food intake, 2—Weight loss 3—Body fat loss 4—Muscle mass loss 5—Fluid overload 6—Functional capability |
Reduces the impact of fluid retention Adds functional capacity Includes subjective variables |
Limited data in clinical practice. High sensitivity and specificity. |
Weaknesses appear in italics. Abbreviations: Malnutrition Screening Tool (MST); Malnutrition Universal Screening Tool (MUST); Nutrition Risk Screening (NRS-2002); Controlling Nutritional Status (CONUT); Short Nutritional Assessment Questionnaire (SNAQ); Royal Free Hospital-Nutrition Prioritizing Tool (RFH-NPT); Liver Disease Universal Screening Tool (LDUST).
As for the other tests, several studies have recently been published showing that the Mini Nutritional Assessment Short Form (MNA®-SF) may have an important role in patients with liver cirrhosis. The limited mobility of these patients and their neuropsychiatric problems may make this test a useful tool.
Finally, in patients on surgery waiting lists, the CONtrolling NUTritional status (CONUT) tool has an important capacity to predict complications and post-surgical mortality. In addition, it is quick and easy and may sometimes be automatically available for blood analyses.
2.2. Diagnosis of Malnutrition
When nutritional screening with any of the tools is positive, a comprehensive nutritional assessment should be carried out, which, in addition to confirming the diagnosis of malnutrition, allows for an assessment of the cause, severity, repercussions, deficits, and potential interventions to be carried out (Figure 1).
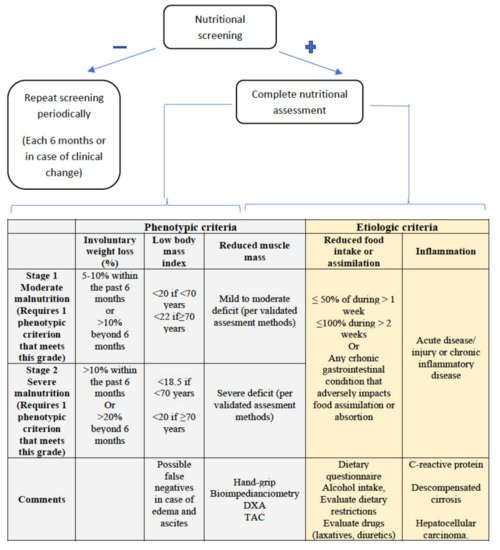

Figure 1. GLIM criteria for malnutrition diagnosis. At least one phenotypic criterion and one etiologic criterion are required.
New diagnostic criteria for malnutrition have recently been published by the Global Leadership Initiative on Malnutrition, called the GLIM criteria [34][47], with the participation of the main nutrition societies worldwide. As can be seen in Figure 1, they consist of two groups of criteria, phenotypic and etiological, with the presence of at least one criterion from each group being necessary to establish a diagnosis of malnutrition. Severity (moderate or severe) is established according to the phenotypic criteria. According to the GLIM criteria, malnutrition affected 38.1% of patients with cirrhosis, being severe in 22% of the patients in a recently published study [35][48]. Using this tool as a guide, we will now discuss some particularities of the assessment of these points in patients with cirrhosis of the liver.
2.2.1. Assessment of Reduced Intake
There are several ways to assess dietary intake, some more sensitive than others. The simplest way is to ask the patient directly about what the proportion of their usual intake they are currently eating. Previously, the existing criteria established different cut-off points and also took into account the time of evolution. The suggestion of the GLIM group is to set at least 50% of energy requirements for at least one week or any reduction for at least 2 weeks for any chronic GI condition that adversely impacts food assimilation or absorption.
However, it is recommended to keep at least a 24-h nutritional diary, which allows for a more accurate and reliable assessment, or to apply one of the validated questionnaires, such as the “Patient Generated Subjective Global Assessment” [36][49].
In patients with liver cirrhosis, it is particularly important to stress the importance of protein-rich food consumption and to assess the impact of alcohol consumption on the quantity and quality of a patient’s diet.
2.2.2. Weight Loss and Body Mass Index
In almost all screening tools and in virtually all comprehensive assessment methods or diagnostic criteria, weight loss and BMI play an important role. However, their assessment is particularly difficult in patients with liver cirrhosis, since, as mentioned above, they can be inaccurate due to the presence of fluid, either in the form of ascites or edema.
Some previous diagnostic criteria, such as the ESPEN criteria [37][50], attached great importance to BMI, making them insensitive in patients with a high baseline BMI or who might have a falsely elevated BMI, like patients with cirrhosis or heart failure. The GLIM criteria, on the other hand, do not require this item to be met, allowing a diagnosis of malnutrition to be made in this group of patients, even though they may have a normal BMI or no current weight loss.
2.2.3. Muscle Mass and Body Composition
Among the complications associated with malnutrition in patients with liver cirrhosis, sarcopenia is particularly relevant. However, the assessment of body composition in patients with fluid overload can be particularly complex. There are several described methods that can be used in cirrhotic patients, such as bioimpedance [38][51], or dual-energy X-ray absorptiometry (DXA) [39][52]. An indirect, but more widely available, method is the assessment of muscle strength by hand-grip, although its correlation with the stage of cirrhosis is unclear [40][53]. In terms of cut-off points, there are different recommendations according to the different societies, which should be adjusted for age and sex.
As a complement to these techniques, which can be influenced by water retention, computed axial tomography allows for a more objective assessment of muscle mass, although with the disadvantage of the associated irradiation [41][54]. Moreover, the cut-off points are not properly established.
2.2.4. Disease Burden/Inflammation
The presence of an inflammatory condition is the most difficult criterion to assess in patients with chronic disease. According to the GLIM criteria consensus document, the mere presence of a chronic disease, such as a neoplasm or liver cirrhosis, is not a sufficient condition to meet this criterion, and it is necessary to demonstrate the progression or decompensation of the disease, for which clinical, radiological, or analytical elements, such as C-reactive protein, can be used. The presence of an acute condition, such as an infection or another similar factor, would meet this criterion.
Within the nutritional assessment of patients with cirrhosis of the liver, certain particularities must be taken into account with respect to the general population. While macronutrient deficiencies have to be assessed as in other patients, patients with cirrhosis are at increased risk of micronutrient deficiencies, such as zinc and magnesium due to diuretic use, as well as vitamin A or vitamin D. The particular characteristics of patients with alcohol consumption, which is very prevalent in patients with cirrhosis of the liver, must also be taken into account.
In summary, the screening and diagnosis of malnutrition in patients with liver disease is complex and influenced by factors intrinsic to the cirrhosis itself. Alterations in body composition mean that global assessment must be adapted in order to be reliable. Therefore, the development of specific tools may be an important advance in nutritional assessment. As for the new GLIM criteria, their validity and prognostic ability remains to be demonstrated, although their lower dependence on BMI make them potentially interesting.
3. Nutritional Intervention in Liver Disease
In patients with liver cirrhosis, nutritional intervention aims to supply at least 35 kcal/Kg/day (in non-obese patients) and a protein intake of 1.2–1.5 g/Kg/day, or even more than 1.5 g/Kg/day if sarcopenia is already present [42][43][1,55]. The main nutrition strategies to achieve these goals include nutritional counselling, frequent feeding, and nutritional supplementation.
In a retrospective study that included 232 patients with liver cirrhosis, patients that received nutritional counselling through teaching sessions given by a multidisciplinary team (including physicians, nurses, pharmacists, and dieticians) showed improved survival rates and an improved quality of life [44][56]. Therefore, nutritional counselling is strongly recommended for patients with chronic liver diseases [45][57]. On the other hand, in patients with liver cirrhosis, the daily intake should be split into six meals or snacks, and the late-evening snack is essential. The late-evening snack shortens nocturnal fasting and decreases skeletal muscle proteolysis, thus improving quality of life [46][58]. In a meta-analysis that included eight studies and 341 patients with cirrhosis, a late-evening snack improved liver biochemical parameters and liver dysfunction [47][59].
Nutritional supplementation includes oral nutritional supplements—mainly branched chain amino acid (BCAA) supplements, enteral nutrition (EN), and parenteral nutrition (PN). Three randomized clinical trials (RCTs) [48][49][50][60,61,62] with BCAA showed beneficial results in cirrhotic patients. In one RCT with 174 patients with advanced cirrhosis, one-year supplementation with BCAA prevented progressive hepatic failure and decreased the hospital admission rate [48][60]. In another RCT, the administration of 12 g/day of BCAA for two years improved the event-free survival, serum albumin concentration, and quality of life of patients with decompensated cirrhosis [49][61]. Finally, a third multicenter RCT, developed in Spain, showed that a supplement of 30 g of BCAA showed an improvement in neuropsychological tests and an increase of muscle mass in patients with cirrhosis and a previous episode of hepatic encephalopathy (HE) [50][62]. Consistent with these results, the ESPEN guidelines on clinical nutrition in liver diseases recommend long-term treatment with oral BCAA in patients with advanced cirrhosis in order to improve their clinical evolution [45][57].
EN has been proposed in malnourished cirrhotic patients admitted to hospital, but systematic meta-analyses have not shown relevant positive results in terms of survival [51][52][63,64], so the routine use of EN in hospitalized cirrhotic patients is not supported. However, in malnourished cirrhotic patients who are unable to obtain correct dietary intake (even with oral supplements), a short treatment with EN should be performed [42][43][45][1,55,57]. In these patients, a nasogastric tube can be placed, even in the presence of esophageal varices. PN is recommended in cirrhotic patients who cannot receive adequate oral and/or EN, for example, in patients with intestinal ileus [45][57]. The composition of a PN solution in cirrhotic patients can be the same as that of a standard solution, because specific solutions, like the BCAA-enriched solution, did not show better results in terms of survival or other outcomes, such as quality of life or nutritional parameters [53][65].
In addition to nutritional supplementation, exercise is an important factor in preventing sarcopenia in cirrhotic patients [42][1]. Despite the absence of large studies, some clinical trials published in recent years showed hopeful conclusions. In a small prospective study, eight weeks of supervised exercise improved the aerobic capacity and muscle mass and decreased fatigue of patients with Child–Pugh class A or B cirrhosis [54][66]. Besides, in another study, moderate exercise for 16 weeks reduced the body weight and the hepatic venous pressure gradient in overweight/obese patients with cirrhosis [55][67]. As a general recommendation, exercise should include aerobic and resistance actions and should have a mild duration, for example, about 30–60 min [43][56][55,68].
Other conditions requiring particular management include obesity in cirrhotic patients, HE, and acute alcoholic hepatitis. In obese patients with cirrhosis, a reduction >5–10% of body weight is associated with a decrease of the disease progression [57][69]. In these obese patients, a strategy of exercise and a hypocaloric diet (between 500 and 800 Kcal/day) is recommended to obtain this reduction of body weight. Compliance with a calorie-restricted diet over the long term is associated with the mobilization of liver fat and an improvement in cardiovascular risk. The specific macronutrient composition of the diet appears to be less relevant than the sustained weight loss. However, diet should incorporate an adequate amount of protein (>1.5 g/kg/day) to avoid a loss of muscle mass [42][1].
Regarding HE, in the past, some studies with methodological flaws suggested that decreasing protein intake in patients with HE showed better outcomes. Nevertheless, more recent and better studies have not confirmed these results, and protein restriction is now considered to be detrimental both in patients with acute HE and those with chronic HE [42][45][1,57]. In general, vegetable and dairy protein is better tolerated than meat protein and can develop a prebiotic and laxative action [42][1]. BCAA supplements have been documented to promote muscle protein synthesis and improve muscle mass loss. Both of these effects are involved in the pathophysiology of HE, thus establishing the rational basis for its use in HE. In addition, in patients with HE, BCAA supplements are recommended, because they have a beneficial effect on overt HE, according to a Cochrane meta-analysis including 16 RCTs. Unfortunately, BCAA supplements had no effect on mortality [53][65].
Moreover, recent data published by Ericksen R.E. et al. suggest a positive correlation between BCAA intake and cancer risk in humans [58][70]. In this study, transcriptomic and metabolomic analysis of primary HCCs and animal liver cancer models also identified an important role for BCAA catabolism in tumor development, progression, and growth [58][70]. Specifically, the loss of BCAA catabolism and accumulation during carcinogenesis lead to a stimulation of mTORC1 activity, which promotes HCC development and progression in mice models [58][70]. Furthermore, the authors observed that BCAA catabolic enzyme expression predicts tumor aggressiveness and patient survival and that dietary BCAAs correlate with tumor burden in mice and cancer mortality in humans [58][70]. Other results also suggest that BCAAs may mediate pathways related to cancer development and progression, possibly due to their involvement in insulin metabolism [59][71]. Nowadays, this safety concern could represent a limitation for BCAA supplementation therapy.
Severe acute alcoholic hepatitis is a life-threatening condition with a high mortality, and EN could play a relevant role. In an RCT, 71 patients with severe alcoholic hepatitis were randomized to receive prednisolone or enteral tube feeding for 28 days. The mortality during treatment was equal in both groups, but occurred earlier with EN. However, patients in the prednisolone group had a higher rate of infections, which was associated with a higher mortality during a one-year follow-up [60][72]. In another study, 136 patients with alcoholic hepatitis were randomized to receive EN plus methylprednisolone or conventional nutrition plus methylprednisolone. No significant difference between the treatments in terms of 6-month mortality was shown, although there may be confounding factors, like active alcohol intake. Nonetheless, patients in the study with a daily calorie intake of less than 21.5 kcal/kg/day had a lower survival [61][73].
Additionally, in patients with chronic liver disease, micronutrient and vitamin deficiencies are frequent and are associated with hepatic dysfunction. Therefore, confirmed or clinically suspected deficiencies of micronutrients or vitamins must be treated [42][45][1,57]. Specifically, a deficiency of vitamin D is very common in cirrhotic patients and should be evaluated in all these patients [62][74]. If vitamin D levels are below 20 ng/mL, this deficiency should be corrected to achieve vitamin D levels above 30 ng/mL [42][1]. Other vitamin deficiencies, such as vitamin K in cholestatic diseases or vitamin B in alcoholic cirrhosis, should also be considered and treated. In addition, zinc deficiency is associated with HE, changes in taste and smell, and hair loss [43][63][55,75]. Zinc supplementation could cause an improvement in the taste and palatability of food, but the data on mental activity are not conclusive [64][76].
Finally, other nutritional interventions for sarcopenia, frailty, and malnutrition are emerging [43][55]. A recent 1-year clinical controlled trial of intramuscular testosterone in male patients with cirrhosis and low serum testosterone demonstrated that testosterone safely increases muscle mass without a clear effect on muscle function [65][77]. Larger-scale investigations are warranted, before this is implemented into routine clinical practice.
Reducing portal pressure by transjugular intrahepatic portosystemic shunt (TIPS) placement may have beneficial effects on muscle mass and decrease mortality in patients, showing an improvement of sarcopenia after the TIPS placement [66][78]. Nevertheless, caution should be exercised when performing TIPS in malnourished patients with cirrhosis, as sarcopenia is a risk factor for the development of HE and acute-on-chronic liver failure after TIPS placement.
