Avian pathogenic Escherichia coli (APEC), an extra-intestinal pathogenic E. coli (ExPEC), causes diverse local and systemic infections in poultry, including chickens, turkeys, ducks, and many other avian species. APEC possesses or utilizes different virulence and pathogenesis factors or mechanisms to cause colibacillosis in poultry.
- APEC
- virulence
- pathogenesis
- zoonosis
- antibiotic resistance
- vaccines
- virulence inhibitors
- infections
1. Introduction
Avian pathogenic Escherichia coli (APEC), an extra-intestinal pathogenic E. coli (ExPEC), causes diverse local and systemic infections in poultry, including chickens, turkeys, ducks, and many other avian species [1]. The most common infections caused by APEC in chickens are perihepatitis, airsacculitis, pericarditis, egg peritonitis, salphingitis, coligranuloma, omphalitis, cellulitis, and osteomyelitis/arthritis; these are commonly referred as avian colibacillosis [2]. APEC also causes swollen head syndrome in chickens and osteomyelitis complex in turkeys [2]. Colibacillosis is one of the leading causes of mortality (up to 20%) and morbidity in poultry and also results in decreased meat (2% decline in live weight, 2.7% deterioration in feed conversion ratio) and egg production (up to 20%), decreased hatching rates, and increased condemnation of carcasses (up to 43%) at slaughter [1][3][4]. Furthermore, APEC is responsible for high mortality (up to 53.5%) in young chickens [4]. Taken together, along with the treatment expenses, APEC costs the poultry industry hundreds of millions of dollars in economic losses worldwide [5]. In the United States (US), it has been estimated that economic losses to the broiler industry can be as high as $40 million annually only due to carcass condemnations [6].
APEC can affect all species of poultry in all types of production systems [3]. APEC is also prevalent (9.52% to 36.73%) in all age groups of chickens [7]. Broiler chickens between the ages of 4 and 6 weeks are more susceptible [1], whereas layer chickens can be affected by APEC throughout the grow and lay periods, particularly around the peak egg production and late lay period [1]. In the US, it is estimated that at least 30% of commercial flocks are affected by APEC at any point of time [8]. Multiple APEC serotypes have been associated with colibacillosis cases in the field outbreaks; however, three serotypes (O78, O2, and O1) account for the majority (more than 80%) of the cases [1][5]. APEC leads to systemic infections in chickens either as a primary pathogen or secondary to viral [infectious bronchitis (IBV), Newcastle disease (NDV), avian influenza (AIV)] and Mycoplasma (Mycoplasma gallisepticum (MG) infections, immunosuppressive disease [(infectious bursal disease (IBD)], or environmental stresses (overcrowding, high level of dust and ammonia) by entering through oral and respiratory routes [1][5]. Interestingly, studies have shown that APEC can colonize the gastrointestinal and respiratory tracts of chickens without causing disease and only translocate to extra-intestinal sites in the presence of stressors (production-related stress, immunosuppression, and concurrent infections) as an opportunistic pathogen [2][9]. APEC invades the gastrointestinal and respiratory tracts through abraded tracheal and intestinal epithelium in the presence of stressors and reaches bloodstream and internal organs [1][2][3]. Chickens get infected through contaminated feed and water and can spread to other birds through the feco-oral or aerosol route [1][2][3]. Furthermore, APEC can be vertically transmitted from infected breeders via contaminated eggs [1][2][3]. An overview of APEC infection in chickens is shown in Scheme 1.
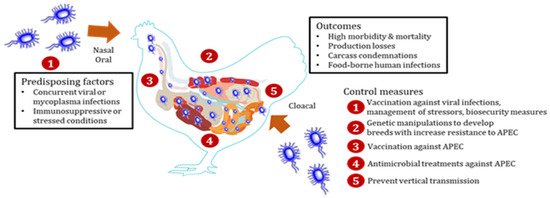
Scheme 1. Schematic diagram showing the overview of Avian pathogenic Escherichia coli (APEC) infection in chickens along with infection control checkpoints. After entry through oral, nasal, or cloacal routes, APEC colonizes the mucosal sites of gastrointestinal, respiratory, and reproductive tracts without causing disease in chickens. In the presence of concurrent viral or mycoplasma infections or under immunosuppressive or stressed conditions, APEC invades the mucosal layers and reach extra-intestinal organs (heart, liver, lung, spleen, kidney, reproductive organs, etc.) resulting in multi-systemic infections, which is commonly referred to as colibacillosis. Colibacillosis leads to high morbidity and mortality, production losses, and condemnation of carcasses as well as foodborne transmission risk to humans. Colibacillosis can be prevented by the management of stressors, biosecurity measures, and vaccination against APEC or associated viral infections. Breeds of chickens with high intrinsic resistance to APEC can be developed through genetic technologies. Vertical transmission of APEC from breeders through contaminated eggs should be monitored to prevent APEC entry into chicken flocks. Antibiotics are commonly used to treat chicken flocks affected with colibacillosis.
APEC utilizes different virulence and pathogenesis factors to cause disease in chickens, primarily adhesins, invasins, protectins, iron acquisition systems, and toxins [2]. These factors facilitate adhesion, invasion, evasion from the host immune responses, colonization, proliferation, and systemic dissemination of APEC, thereby allowing the establishment of infection in chickens [2]. In addition to these factors, several other bacterial factors including but not limited to secretion systems (type III and VI), quorum sensing (QS) system, transcriptional regulators, two-component systems, and metabolism-associated genes also contribute to APEC pathogenesis in chickens [10][11][12][13][14][15][16][17][18]. An in-depth understanding of these factors and their roles in APEC pathogenesis will help to develop new effective preventative and therapeutic treatments.
Recent studies suggest APEC (particularly isolates belonging to sequence types ST95 and ST131 or O1, O2, and O18 serogroups) as a potential foodborne zoonotic pathogen as well as a source or reservoir of extra-intestinal infections in humans [4][19][20][21]. Particularly, APEC shares genetic similarity with human ExPECs, uropathogenic E. coli (UPEC), and neonatal meningitis E. coli (NMEC), and possesses UPEC-and NMEC-defining virulence genes with the ability to cause urinary tract infections (UTI) and meningitis in mice and rat models [4][22]. Furthermore, the detection of APEC-specific ColV (colicin V) plasmids in human ExPEC isolates suggests a possible zoonotic transmission of APEC from poultry to humans [21]. Therefore, APEC is a pathogen of importance to the poultry industry and public health.
Antibiotics (tetracyclines, sulfonamides, and aminoglycosides) are frequently used to control colibacillosis in chickens [23]. However, increasing resistance of APEC to different classes of antibiotics, including medically important antibiotics (β-lactams, colistin, and carbapenems), suggests challenges ahead in using antibiotics to control APEC infections in chickens [24]. Furthermore, there is no effective vaccine available to protect chickens against APEC infections, which is mainly due to the diversity of APEC serotypes associated with colibacillosis cases in the field outbreaks [5]. Currently, only two vaccines (live-attenuated APEC O78 ΔaroA Poulvac® E. coli vaccine and inactivated Nobilis® E. coli vaccine containing F11 fimbrial and FT flagellar antigens) are commercially available for use in chickens [5][25]. These scenarios necessitate the development of new and alternative therapies to control APEC infections in chickens. Probiotics, bacteriophages, and different new therapies (innate immune stimulants, growth and QS inhibitors, and antimicrobial peptides) have shown promising efficacy in reducing APEC infections in chickens [26][27][28][29][30]; however, none of these have advanced into field applications to date.
2. Virulence and Pathogenesis Factors
APEC possesses or utilizes different virulence and pathogenesis factors or mechanisms to cause colibacillosis in poultry [2][10][11][12][13][14][15][16][17][18][31]. These factors include but are not limited to adhesins, invasins, protectins, iron acquisition systems, toxins, two-component systems, a quorum-sensing (QS) system, transcriptional regulators, secretion systems, and genes associated with metabolism [2][10][11][12][13][14][15][16][17][18][31]. These factors play various roles in APEC infections, including attachment to host cells, invasion of the host cells, survival inside the phagocytic (macrophages) cells, colonization of tissues, persistence in the bloodstream, proliferation/replication inside the cells, cell lysis and damage, sequestering metals from body fluids for growth, resistance to the serum bactericidal activity and oxidative and environmental stresses, motility, and biofilm formation [2][10][11][12][13][14][15][16][17][18][31]. Table 1 provides the list of virulence and pathogenesis factors defined or characterized in APEC to date along with their roles in APEC pathogenesis/infection.
Table 1. APEC virulence and pathogenesis factors and their role in systemic infections.
| Virulence Factors | Genes/Proteins Involved | Role in Pathogenesis/Infection | Reference |
|---|---|---|---|
| Adhesins | fimH, fimC, papA, papC, papEF, papG I, papG II, papGIII, felA, sfa/sfaS, afaIBC, focGE, lpfA, lpf0141, lpf0154, flgE, crl, csg, bmaE, tsh, mat/ecpA, hra/hrlA/hek, iha, yqiG, kii | Adhesion, colonization, biofilm formation, motility, intracellular survival | [32][33][34][35][36][37][38][39][40] |
| yfc O | Adhesion, colonization, resistance to environmental stresses | [41] | |
| yad C | Adhesion, intracellular survival, motility | [42] | |
| aat A, aatB, upaB | Adhesion, colonization, biofilm formation | [43][44] | |
| fdtA, rluD, yjhB, ecpR, fdeC | Adhesion | [45] | |
| Invasins | ibeA, ibeB, tia, gimB | Invasion, resistance to oxidative stress, colonization, proliferation, biofilm formation | [35][46][47] |
| IbeR | Invasion, resistance to serum and environmental stresses, expression of virulence genes | [48] | |
| ych O | Motility, adhesion, invasion, biofilm formation, expression of membrane proteins and metabolism genes | [49] | |
| Iron acquisition systems | iutA, iucC, iucD, aerJ, iucA, iucB, iroBCDEN, fyuA, sitABCD, mntH, feoB, irp2, ireA, eitABCD, fepC, chuA, bfr | Iron and manganese uptake from the host, adhesion, invasion, colonization, persistence, expression of virulence genes, resistance to environmental stresses | [36][37][50][51][52][53][54][55][56] |
| entE, entS, tolC | Invasion, colonization, persistence | [57] | |
| Protectins | iss, traT, ompT, kpsMT(K1), kpsMT(II), kpsMT(III), neuC, neuS, neuD, kfiC-K5, betA | Protect from serum bactericidal activity and phagocytosis, adhesion, invasion, intracellular survival, colonization, proliferation | [36][40][50][52][58] |
| YbjX, PagP | Resistance to serum and environmental stresses, invasion, intracellular survival | [59][60] | |
| OmpA | Intracellular survival | [61] | |
| wzy | Adhesion, invasion, intracellular survival, colonization | [62] | |
| waa L | Motility, resistance to phagocytosis and environmental stresses, adhesion, invasion, biofilm formation | [62] | |
| sod A | Protect against ROS-mediated host defenses, biofilm formation | [63] | |
| lpx M | Invasion, intracellular survival, colonization, regulation of expression of cytokine genes and nitric oxide production | [64] | |
| Toxins | hlyF, hlyA, hlyE, cdtB, cdtS, vat, sat, stx2f, astA, pic, EAST-1, espC, ace4/35 | Cell lysis and damage, induce host cell vacuolization, colonization, motility, biofilm formation, agglutination, formation of outer membrane vesicles | [35][38][39][40][50][52][65][66][67][68][69] |
| Other virulence and pathogenesis factors | |||
| Quorum-sensing system (AI-2) | LuxS, LsrABCDFGK, ptsI, Pfs | Motility, biofilm formation, adherence, invasion, colonization, intracellular survival, persistence, expression of virulence genes, cell damage | [10][70][71][72][73] |
| Secretion systems | DotU, CpxRA, IcmF, Hcp, ClpV, VrgG (Type VI) | Interbacterial competition, adhesion, invasion, intracellular survival, colonization, motility, biofilm formation, production of type 1 fimbriae, resistance to serum bactericidal activity, modulation of intracellular host responses (IL-18, IL-1β) | [11][74][75][76][77][78][79] |
| EtrA, YqeI, EivC (Type III) | Motility, intracellular survival, resistance to phagocytosis and serum bactericidal activity, proliferation, expression of fimbriae genes, downregulation of pro-inflammatory cytokines | [12][80][81] | |
| Two-component systems | PhoPQ, tolC | Biofilm formation, motility, adhesion, invasion, intracellular survival, systemic infection, expression of virulence genes and genes associated with flagellar assembly, ABC transporters, quorum sensing, and bacterial chemotaxis | [13][82][83] |
| BasSR | Biofilm formation, APEC virulence and colonization in vivo | [84] | |
| KdpDE | Expression of flagella-related genes, flagellum formation, motility and resistance to serum bactericidal activity | [85] | |
| RstAB, hdeD | Iron acquisition, acid resistance, intracellular survival, colonization | [86][87] | |
| BarA-UvrY | Adhesion, invasion, persistence, intracellular survival, resistance to serum bactericidal activity and oxidative stress, regulation of exopolysaccharide production and type 1 and P fimbriae | [88] | |
| Transcriptional regulators | AutA/AutR | Expression of K1 capsule and acid resistance systems, adaptive lifestyle change | [14] |
| FNR | Adhesion, invasion, expression of type 1 fimbriae and type VII secretion system, resistance to oxidative stress | [15] | |
| YjjQ | Flagellar motility | [89] | |
| McbR | Biofilm formation, response to H2O2 | [90] | |
| tyrR | Invasion, motility, intracellular survival | [37] | |
| RfaH | Invasion, intracellular survival, resistance to serum bactericidal activity | [91] | |
| Metabolism-associated genes | acs -yjcH-actP | Intracellular survival, proliferation, colonization, production of pro-inflammatory cytokines and nitric oxide | [92] |
| PotE, PotF | Colonization, adhesion | [16] | |
| NirC | Adhesion, colonization | [17] | |
| ArcA | Chemotaxis, motility | [18] | |
| Miscellaneous | OmpF, OmpC | Adhesion, invasion, colonization, proliferation | [93] |
| Prophage phiv142-3 (orf20) and phiv205-1 | Resistance to serum and environmental stresses, adhesion, invasion, intracellular survival, colonization, biofilm formation, formation of flagella and I fimbriae | [94][95][96] | |
| YicS | Motility, biofilm formation, invasion | [97] | |
| cpd B | Colonization | [98] | |
| pst B | Resistance to serum bactericidal activity and oxidative stress, colonization | [99] | |
| tmRNA-SmpB | Colonization, persistence, replication, intracellular survival | [100] | |
| mli C | Resistance to serum bactericidal activity | [101] | |
| malX, frz, cvaABC, cvi, cba, cib/cibI, cbi, cma, eaeA, sopB, yfcV, gad, mchBCF, mcmA, bor, air, eilA, celB, pabB, capU, cif, tir, tccp, nleB, iaL, cjrC, mig-14p | Unknown/not clearly known functions | [34][39][102][40][52][53][56][103][104][105] | |
| Genes essential for systemic infections and adaptation | metH, lysA, pntA, purL, serS, ybjE, ycdK (rutC), wcaJ, gspL, sdsR, irp2, eitD, ylbE, yjiY, tkt1, pilN, pilQ, tsh, hpb, TcfD, Z5222, waaO, waaY, iutA, iucA, iucD, iroC, ColE2, traK, traG, traT, SopA, psiA, hkaG, hkbV, hkbQ, Z3370, Int, CC0532, TM0427, YPO3000, rhsH, RSp0733, bioABFCD, rnfA, rfnE, gene encoding endonuclease III, creABCD, yehD, potF, flgE, tyrR, bfr | Systemic APEC infections and adaptation | [37][106][107][108] |
2.1. Adhesins
Adhesins are appendages or cell-surface components of bacteria that facilitate adhesion or adherence to other cells or to surfaces, usually in the host they are living in or infecting [2][31]. Adherence is required for colonizing a new host and is an essential step in bacterial pathogenesis or infection [2][31]. Adherence in APEC is facilitated primarily by type 1 fimbriae, P fimbriae, and S fimbriae [2][31]. Several genes encoding these fimbriae and additional adhesins, fimH, fimC (type 1 fimbriae), papA, papC, papEF, papG I, papG II, papG III, felA (P fimbriae), sfa/sfaS (S fimbriae), focGE (F1C fimbriae), afaIBC (afimbriae), lpfA, lpf0141, lpf0154 (long polar fimbriae), mat/ecpA (fimbrillin), flgE (flagellar hook), crl, csg (curli), tsh (temperature-sensitive haemagglutinin), bmaE (M hemagglutinin), hra/hrlA/hek (heat-resistant agglutinin), iha (IrgA homologue adhesin), yqiG (putative outer membrane usher protein), and kii (K capsule encoding genes) have been reported in APEC [32][33][34][35][36][37][38][39][102][40]. These adhesins also mediate motility, biofilm formation, and APEC survival in macrophages [31]. Furthermore, the fimbriae-encoding gene, yfcO, facilitates adhesion, colonization, and resistance to environmental stresses [41], whereas yadC, promotes adhesion, intracellular survival, and motility [42]. Similarly, autotransporter adhesin genes (aatA, aatB and upaB) contribute to adhesion, colonization, and biofilm formation [43][44]. By screening a random transposon mutant library, multiple other genes (fdtA, rluD, yjhB, ecpR, and fdeC) were found to be responsible for adhesion to chicken and human cell lines [45].
2.2. Invasins
Invasins are a class of proteins associated with the entry of pathogens into the host cells [2][31]. Invasins play a role in promoting entry during the initial stage of the infection [2][31]. Multiple genes encoding invasins, ibeA (also called ibe10), ibeB (invasion protein), tia (toxigenic invasion locus), and gimB (genetic island associated with neonatal meningitis) have been reported in APEC [35][46][47]. In addition, invasins also contribute to APEC resistance to oxidative stress induced by macrophages, biofilm formation, colonization, and proliferation in the host [46][47]. IbeR, a regulator of ibeRAT operon, contributes to invasion, resistance to serum and environmental stresses, and the expression of virulence genes [48]. Similarly, ychO, a putative invasin gene, plays a role in motility, adhesion, invasion, biofilm formation, and the expression of membrane proteins and metabolism genes [49].
2.3. Iron Acquisition Systems
Iron is an essential micronutrient required for bacterial growth and proliferation inside the host, once bacteria successfully colonize and/or invade the host [2][31]. APEC possesses different iron acquisition systems consisting of multiple siderophores (aerobactin, salmochelin, yersiniabactin) and transporters to sequester iron from the body fluids [2][31]. Several genes encoding the iron uptake and transport systems, iucCD, iutA, aerJ (aerobactin), iroBCDEN (salmochelin), fyuA (yersiniabactin), sitABCD, mntH (iron and manganese transporter), irp2 (iron repressible protein), feoB (ferrous ion transporter), fepC (ferric enterobactin transporter), ireA (iron-regulated virulence gene), eitABCD (putative iron transporter), chuA (outer membrane hemin receptor), and bfr (bacterioferritin) have been reported in APEC [36][37][102][50][51][52][53][54][55][56]. In addition, these siderophores and transporters also mediate APEC adhesion, invasion, resistance to environmental stresses, expression of other virulence genes, colonization, and persistence in the host [51][55][109][110]. Furthermore, enterobactin synthesis and transport genes (entE and entS) in coordination with gene encoding outer membrane efflux protein (tolC) also facilitate invasion, colonization, and persistence [57].
2.4. Protectins
Protectins protect bacteria from the host immune system as well as various unfavorable conditions [2][31]. In particular, protectins include bacterial capsules, outer membrane proteins, and lipopolysaccharide (LPS) components, and they provide protection against phagocytic engulfment by macrophages and complement-mediated bactericidal effect in the host serum [2][31]. Several genes encoding multiple protectins, iss (increased serum survival), traT (complement resistance protein), ompT (outer membrane protease), kpsMT(K1), kpsMT(II), kpsMT(III), neuC, neuS, neuD (capsule), kfiC-K5 (glycosyl transferase), and betA (choline dehydrogenase) have been reported in APEC [36][40][50][52][58]. These protectins also mediate APEC adhesion, invasion, intracellular survival, colonization, and proliferation in the host, in addition to protection from host defense [58]. The outer membrane proteins, YbjX and PagP, also play a role in resistance to serum and environmental stresses, invasion, and intracellular survival [59][60]. Similarly, OmpA, another outer membrane protein, promotes APEC survival in macrophages [61]. The genes involved in LPS biosynthesis, wzy (O-antigen polymerase) and waaL (O-antigen ligase), facilitate intracellular survival and resistance to phagocytosis and environmental stresses along with adhesion, invasion, colonization, motility, and biofilm formation [62][111]. Similarly, lpxM (myristoyl transferase), a gene involved in lipid A biosynthesis, plays a role in invasion, intracellular survival, colonization, and regulation of cytokine genes expression and nitric oxide production [64]. Whereas, sodA (superoxide dismutase) protects APEC from reactive oxygen species (ROS)-mediated host defense and promotes biofilm formation [63].
2.5. Toxins
Toxins are biological poisons that assist in the bacterial ability to invade and cause damage to the tissues [2][31]. Several genes encoding multiple types of toxins, hlyF, hlyA, hlyE (putative avian hemolysin), vat (vacuolating autotransporter toxin), sat (secreted autotransporter toxin), cdtB, cdtS (cytolethal distending factor), astA, EAST-1 (heat-stable enterotoxin), stx2f (shiga toxin variant), pic (serine protease autotransporter), espC (serine protease), and ace4/35 (acetylcholine esterase) have been reported in APEC [35][38][39][102][40][50][52][65][66][67][68][69]. These toxins also facilitate the colonization, motility, biofilm formation, agglutination, induction of vacuolization, and formation of outer membrane vesicles [65].
2.6. Other Virulence and Pathogenesis Factors
Other virulence and pathogenesis factors of APEC include the QS system, transcriptional regulators, two-component systems, secretion systems, and genes associated with bacterial metabolism [10][11][12][13][14][15][16][17][18]. These factors assist in different processes of APEC pathogenesis/infection, including adhesion, invasion, colonization, persistence, interbacterial competitions, resistance to host defenses, and modulation of host immune responses [10][11][12][13][14][15][16][17][18], thereby facilitating the APEC proliferation and establishment of disease in the host.
2.7. Genes Essential for Systemic Infections and Adaptation in Chickens
Identifying the genes essential for systemic infections and adaptation is crucial to develop rational treatments against the infections. Nelwike et al. (2012) [106] investigated the APEC genes induced during systemic infections in chickens using RIVET (recombination-based in vivo expression technology). Genes involved in metabolism, cell envelope and integrity, transport systems, and virulence (metH, lysA, pntA, purL, serS, ybjE, ycdK (rutC), wcaJ, gspL, sdsR, irp2, eitD, ylbE, yjiY, tkt1, and phage-related genes) were upregulated in APEC isolated from infected chickens. Similarly, Dozois et al. (2003) [107] studied the APEC genes expressed in infected tissues in chickens using SCOTS (selective capture of transcribed sequences) technology. Genes involved in adherence (pilN, pilQ, tsh, hpb, TcfD, Z5222), LPS synthesis (waaO, waaY), iron acquisition (iutA, iucA, iucD, iroC), plasmid function (ColE2, traK, traG, traT, sopA, psiA), phage-related (hkaG, hkbV, hkbQ, Z3370, Int), and of unknown functions (CC0532, TM0427, YPO3000, rhsH, RSp0733) were highly expressed in APEC infected tissues. On the other hand, Zhang et al. (2019) [108] identified essential genes for APEC adaptation in chickens using the TraDIS (transposon-directed insertion site sequencing) strategy. Genes involved in metabolism, transport, regulation and stress response, RNA processing and translation, cell division and DNA replication, cell envelope biogenesis, and unknown functions were essential to cause disease in chickens. Particularly, genes involved in biotin synthesis (bioABFCD), Rnf electron transport complex (rnfA, rfnE, and gene nth encoding endonuclease III), and cre two-component system (creABCD) were important for the adaptation of APEC in chickens. In another study, genes identified as upregulated through microarray analysis (yehD, potF, flgE, tyrR, and bfr) in APEC isolated from chicken showing swollen head syndrome were essential for adhesion, invasion, survival inside macrophages, and motility [37]. These studies provide insights on APEC pathophysiological processes during systemic infections in chickens.
Overall, multiple virulence and pathogenesis factors of APEC are involved in causing colibacillosis in poultry. As a result of the involvement of multiple virulence and pathogenesis factors, there is a hindrance in developing therapeutics broadly effective against APEC infections. In-depth understanding of these factors as well as unraveling the new factors will help to develop the effective therapeutics against colibacillosis in poultry. Furthermore, several of these factors have coordinated and overlapping functions, which necessitates a holistic strategy to formulate an ideal anti-APEC therapeutics. For instance, developing therapeutics targeting iron acquisition systems [112], QS system [113], bacterial metabolism [114], and secretion systems [115] can provide solutions to mitigate APEC infections in poultry in the future.
References
- Dho-Moulin, M.; Fairbrother, J.M. Avian pathogenic Escherichia coli (APEC). Vet. Res. 1999, 30, 299–316.
- Dziva, F.; Stevens, M.P. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008, 37, 355–366.
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118.
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932.
- Ghunaim, H.; Abu-Madi, M.A.; Kariyawasam, S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: Potentials and limitations. Vet. Microbiol. 2014, 172, 13–22.
- de Brito, B.G.; Gaziri, L.C.J.; Vidotto, M.C. Virulence Factors and Clonal Relationships among Escherichia coli Strains Isolated from Broiler Chickens with Cellulitis. Infect. Immun. 2003, 71, 4175.
- Lutful Kabir, S.M. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 2010, 7, 89–114.
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996.
- Collingwood, C.; Kemmett, K.; Williams, N.; Wigley, P. Is the Concept of Avian Pathogenic Escherichia coli as a Single Pathotype Fundamentally Flawed? Front. Vet. Sci. 2014, 1, 5.
- Palaniyandi, S.; Mitra, A.; Herren, C.D.; Zhu, X.; Mukhopadhyay, S. LuxS contributes to virulence in avian pathogenic Escherichia coli O78:K80:H9. Vet. Microbiol. 2013, 166, 567–575.
- Ma, J.; Bao, Y.; Sun, M.; Dong, W.; Pan, Z.; Zhang, W.; Lu, C.; Yao, H. Two functional type VI secretion systems in avian pathogenic Escherichia coli are involved in different pathogenic pathways. Infect. Immun. 2014, 82, 3867–3879.
- Wang, S.; Xu, X.; Liu, X.; Wang, D.; Liang, H.; Wu, X.; Tian, M.; Ding, C.; Wang, G.; Yu, S. Escherichia coli type III secretion system 2 regulator EtrA promotes virulence of avian pathogenic Escherichia coli. Microbiology 2017, 163, 1515–1524.
- Li, Q.; Yin, L.; Xue, M.; Wang, Z.; Song, X.; Shao, Y.; Liu, H.; Tu, J.; Qi, K. The transcriptional regulator PhoP mediates the tolC molecular mechanism on APEC biofilm formation and pathogenicity. Avian Pathol. 2020, 49, 211–220.
- Zhuge, X.; Tang, F.; Zhu, H.; Mao, X.; Wang, S.; Wu, Z.; Lu, C.; Dai, J.; Fan, H. AutA and AutR, Two Novel Global Transcriptional Regulators, Facilitate Avian Pathogenic Escherichia coli Infection. Sci. Rep. 2016, 6, 25085.
- Barbieri, N.L.; Vande Vorde, J.A.; Baker, A.R.; Horn, F.; Li, G.; Logue, C.M.; Nolan, L.K. FNR Regulates the Expression of Important Virulence Factors Contributing to the Pathogenicity of Avian Pathogenic Escherichia coli. Front. Cell Infect. Microbiol. 2017, 7, 265.
- Guerra, P.R.; Herrero-Fresno, A.; Pors, S.E.; Ahmed, S.; Wang, D.; Thofner, I.; Antenucci, F.; Olsen, J.E. The membrane transporter PotE is required for virulence in avian pathogenic Escherichia coli (APEC). Vet. Microbiol. 2018, 216, 38–44.
- De Paiva, J.B.; Leite, J.L.; da Silva, L.P.; Rojas, T.C.; de Pace, F.; Conceicao, R.A.; Sperandio, V.; da Silveira, W.D. Influence of the major nitrite transporter NirC on the virulence of a Swollen Head Syndrome avian pathogenic E. coli (APEC) strain. Vet. Microbiol. 2015, 175, 123–131.
- Jiang, F.; An, C.; Bao, Y.; Zhao, X.; Jernigan, R.L.; Lithio, A.; Nettleton, D.; Li, L.; Wurtele, E.S.; Nolan, L.K.; et al. ArcA Controls Metabolism, Chemotaxis, and Motility Contributing to the Pathogenicity of Avian Pathogenic Escherichia coli. Infect. Immun. 2015, 83, 3545–3554.
- Markland, S.M.; LeStrange, K.J.; Sharma, M.; Kniel, K.E. Old Friends in New Places: Exploring the Role of Extraintestinal E. coli in Intestinal Disease and Foodborne Illness. Zoonoses Public Health 2015, 62, 491–496.
- Bélanger, L.; Garenaux, A.; Harel, J.; Boulianne, M.; Nadeau, E.; Dozois, C.M. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med Microbiol. 2011, 62, 1–10.
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S.; et al. Escherichia coli ST131-H22 as a Foodborne Uropathogen. mBio 2018, 9.
- Tivendale, K.A.; Logue, C.M.; Kariyawasam, S.; Jordan, D.; Hussein, A.; Li, G.; Wannemuehler, Y.; Nolan, L.K. Avian-pathogenic Escherichia coli strains are similar to neonatal meningitis E. coli strains and are able to cause meningitis in the rat model of human disease. Infect. Immun. 2010, 78, 3412–3419.
- Agunos, A.; Léger, D.; Carson, C. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can. Vet. J. 2012, 53, 1289–1300.
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126.
- Gregersen, R.H.; Christensen, H.; Ewers, C.; Bisgaard, M. Impact of Escherichia coli vaccine on parent stock mortality, first week mortality of broilers and population diversity of E. coli in vaccinated flocks. Avian Pathol. 2010, 39, 287–295.
- Wang, S.; Peng, Q.; Jia, H.M.; Zeng, X.F.; Zhu, J.L.; Hou, C.L.; Liu, X.T.; Yang, F.J.; Qiao, S.Y. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017, 96, 2576–2586.
- Kaikabo, A.A.; AbdulKarim, S.M.; Abas, F. Evaluation of the efficacy of chitosan nanoparticles loaded PhiKAZ14 bacteriophage in the biological control of colibacillosis in chickens. Poult. Sci. 2017, 96, 295–302.
- Allan, B.; Wheler, C.; Koster, W.; Sarfraz, M.; Potter, A.; Gerdts, V.; Dar, A. In Ovo Administration of Innate Immune Stimulants and Protection from Early Chick Mortalities due to Yolk Sac Infection. Avian Dis. 2018, 62, 316–321.
- Peng, L.Y.; Yuan, M.; Cui, Z.Q.; Wu, Z.M.; Yu, Z.J.; Song, K.; Tang, B.; Fu, B.D. Rutin inhibits quorum sensing, biofilm formation and virulence genes in avian pathogenic Escherichia coli. Microb. Pathog. 2018, 119, 54–59.
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry. Int. J. Mol. Sci. 2016, 17, 603.
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10.
- Awad, A.M.; El-Shall, N.A.; Khalil, D.S.; Abd El-Hack, M.E.; Swelum, A.A.; Mahmoud, A.H.; Ebaid, H.; Komany, A.; Sammour, R.H.; Sedeik, M.E. Incidence, Pathotyping, and Antibiotic Susceptibility of Avian Pathogenic Escherichia coli among Diseased Broiler Chicks. Pathogens 2020, 9, 114.
- Mohamed, L.; Ge, Z.; Yuehua, L.; Yubin, G.; Rachid, K.; Mustapha, O.; Junwei, W.; Karine, O. Virulence traits of avian pathogenic (APEC) and fecal (AFEC) E. coli isolated from broiler chickens in Algeria. Trop. Anim. Health Prod. 2018, 50, 547–553.
- Azam, M.; Mohsin, M.; Sajjad Ur, R.; Saleemi, M.K. Virulence-associated genes and antimicrobial resistance among avian pathogenic Escherichia coli from colibacillosis affected broilers in Pakistan. Trop. Anim. Health Prod. 2019, 51, 1259–1265.
- Maciel, J.F.; Matter, L.B.; Trindade, M.M.; Camillo, G.; Lovato, M.; de Avila Botton, S.; Castagna de Vargas, A. Virulence factors and antimicrobial susceptibility profile of extraintestinal Escherichia coli isolated from an avian colisepticemia outbreak. Microb. Pathog. 2017, 103, 119–122.
- Silveira, F.; Maluta, R.P.; Tiba, M.R.; de Paiva, J.B.; Guastalli, E.A.; da Silveira, W.D. Comparison between avian pathogenic (APEC) and avian faecal (AFEC) Escherichia coli isolated from different regions in Brazil. Vet. J. 2016, 217, 65–67.
- De Paiva, J.B.; da Silva, L.P.; Casas, M.R.; Conceicao, R.A.; Nakazato, G.; de Pace, F.; Sperandio, V.; da Silveira, W.D. In vivo influence of in vitro up-regulated genes in the virulence of an APEC strain associated with swollen head syndrome. Avian Pathol. 2016, 45, 94–105.
- Wang, X.; Cao, C.; Huan, H.; Zhang, L.; Mu, X.; Gao, Q.; Dong, X.; Gao, S.; Liu, X. Isolation, identification, and pathogenicity of O142 avian pathogenic Escherichia coli causing black proventriculus and septicemia in broiler breeders. Infect. Genet. Evol. 2015, 32, 23–29.
- Aslam, M.; Toufeer, M.; Narvaez Bravo, C.; Lai, V.; Rempel, H.; Manges, A.; Diarra, M.S. Characterization of Extraintestinal Pathogenic Escherichia coli isolated from retail poultry meats from Alberta, Canada. Int. J. Food Microbiol. 2014, 177, 49–56.
- Zhuge, X.; Zhou, Z.; Jiang, M.; Wang, Z.; Sun, Y.; Tang, F.; Xue, F.; Ren, J.; Dai, J. Chicken-source Escherichia coli within phylogroup F shares virulence genotypes and is closely related to extraintestinal pathogenic E. coli causing human infections. Transbound Emerg. Dis. 2020.
- Li, Y.; Wang, H.; Ren, J.; Chen, L.; Zhuge, X.; Hu, L.; Li, D.; Tang, F.; Dai, J. The YfcO fimbriae gene enhances adherence and colonization abilities of avian pathogenic Escherichia coli in vivo and in vitro. Microb. Pathog. 2016, 100, 56–61.
- Verma, R.; Rojas, T.C.; Maluta, R.P.; Leite, J.L.; da Silva, L.P.; Nakazato, G.; Dias da Silveira, W. Fimbria-Encoding Gene yadC Has a Pleiotropic Effect on Several Biological Characteristics and Plays a Role in Avian Pathogenic Escherichia coli Pathogenicity. Infect. Immun. 2016, 84, 187–193.
- Zhuge, X.; Wang, S.; Fan, H.; Pan, Z.; Ren, J.; Yi, L.; Meng, Q.; Yang, X.; Lu, C.; Dai, J. Characterization and functional analysis of AatB, a novel autotransporter adhesin and virulence factor of avian pathogenic Escherichia coli. Infect. Immun. 2013, 81, 2437–2447.
- Zhu-Ge, X.K.; Pan, Z.H.; Tang, F.; Mao, X.; Hu, L.; Wang, S.H.; Xu, B.; Lu, C.P.; Fan, H.J.; Dai, J.J. The effects of upaB deletion and the double/triple deletion of upaB, aatA, and aatB genes on pathogenicity of avian pathogenic Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 10639–10654.
- Ali, A.; Kolenda, R.; Khan, M.M.; Weinreich, J.; Li, G.; Wieler, L.H.; Tedin, K.; Roggenbuck, D.; Schierack, P. Novel Avian Pathogenic Escherichia coli Genes Responsible for Adhesion to Chicken and Human Cell Lines. Appl. Environ. Microbiol. 2020, 86.
- Wang, S.; Niu, C.; Shi, Z.; Xia, Y.; Yaqoob, M.; Dai, J.; Lu, C. Effects of ibeA deletion on virulence and biofilm formation of avian pathogenic Escherichia coli. Infect. Immun. 2011, 79, 279–287.
- Wang, S.; Shi, Z.; Xia, Y.; Li, H.; Kou, Y.; Bao, Y.; Dai, J.; Lu, C. IbeB is involved in the invasion and pathogenicity of avian pathogenic Escherichia coli. Vet. Microbiol. 2012, 159, 411–419.
- Wang, S.; Bao, Y.; Meng, Q.; Xia, Y.; Zhao, Y.; Wang, Y.; Tang, F.; ZhuGe, X.; Yu, S.; Han, X.; et al. IbeR facilitates stress-resistance, invasion and pathogenicity of avian pathogenic Escherichia coli. PLoS ONE 2015, 10, e0119698.
- Pilatti, L.; Boldrin de Paiva, J.; Rojas, T.C.; Leite, J.L.; Conceicao, R.A.; Nakazato, G.; Dias da Silveira, W. The virulence factor ychO has a pleiotropic action in an Avian Pathogenic Escherichia coli (APEC) strain. BMC Microbiol. 2016, 16, 35.
- Thomrongsuwannakij, T.; Blackall, P.J.; Djordjevic, S.P.; Cummins, M.L.; Chansiripornchai, N. A comparison of virulence genes, antimicrobial resistance profiles and genetic diversity of avian pathogenic Escherichia coli (APEC) isolates from broilers and broiler breeders in Thailand and Australia. Avian Pathol. 2020, 49, 457–466.
- Tu, J.; Xue, T.; Qi, K.; Shao, Y.; Huang, B.; Wang, X.; Zhou, X. The irp2 and fyuA genes in High Pathogenicity Islands are involved in the pathogenesis of infections caused by avian pathogenic Escherichia coli (APEC). Pol. J. Vet. Sci. 2016, 19, 21–29.
- Xu, X.; Sun, Q.; Zhao, L. Virulence Factors and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. J. Vet. Res. 2019, 63, 317–320.
- Varga, C.; Brash, M.L.; Slavic, D.; Boerlin, P.; Ouckama, R.; Weis, A.; Petrik, M.; Philippe, C.; Barham, M.; Guerin, M.T. Evaluating Virulence-Associated Genes and Antimicrobial Resistance of Avian Pathogenic Escherichia coli Isolates from Broiler and Broiler Breeder Chickens in Ontario, Canada. Avian Dis. 2018, 62, 291–299.
- Paixao, A.C.; Ferreira, A.C.; Fontes, M.; Themudo, P.; Albuquerque, T.; Soares, M.C.; Fevereiro, M.; Martins, L.; Correa de Sa, M.I. Detection of virulence-associated genes in pathogenic and commensal avian Escherichia coli isolates. Poult. Sci. 2016, 95, 1646–1652.
- Sabri, M.; Caza, M.; Proulx, J.; Lymberopoulos, M.H.; Bree, A.; Moulin-Schouleur, M.; Curtiss, R., 3rd; Dozois, C.M. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain chi7122. Infect. Immun. 2008, 76, 601–611.
- Azam, M.; Mohsin, M.; Johnson, T.J.; Smith, E.A.; Johnson, A.; Umair, M.; Saleemi, M.K.; Sajjad Ur, R. Genomic landscape of multi-drug resistant avian pathogenic Escherichia coli recovered from broilers. Vet. Microbiol. 2020, 247, 108766.
- Mu, X.; Gao, R.; Xiao, W.; Gao, Q.; Cao, C.; Xu, H.; Gao, S.; Liu, X. EntE, EntS and TolC synergistically contributed to the pathogenesis of APEC strain E058. Microb. Pathog. 2020, 141, 103990.
- Hejair, H.M.A.; Ma, J.; Zhu, Y.; Sun, M.; Dong, W.; Zhang, Y.; Pan, Z.; Zhang, W.; Yao, H. Role of outer membrane protein T in pathogenicity of avian pathogenic Escherichia coli. Res. Vet. Sci. 2017, 115, 109–116.
- Song, X.; Qiu, M.; Jiang, H.; Xue, M.; Hu, J.; Liu, H.; Zhou, X.; Tu, J.; Qi, K. ybjX mutation regulated avian pathogenic Escherichia coli pathogenicity though stress-resistance pathway. Avian Pathol. 2020, 49, 144–152.
- Song, X.; Hou, M.; Tu, J.; Xue, M.; Shao, Y.; Jiang, H.; Liu, H.; Xue, T.; Wang, G.; Qi, K. Outer membrane proteins YbjX and PagP co-regulate motility in Escherichia coli via the bacterial chemotaxis pathway. Res. Vet. Sci. 2019, 125, 279–284.
- Nielsen, D.W.; Ricker, N.; Barbieri, N.L.; Allen, H.K.; Nolan, L.K.; Logue, C.M. Outer membrane protein A (OmpA) of extraintestinal pathogenic Escherichia coli. BMC Res. Notes 2020, 13, 51.
- Zuo, J.; Tu, C.; Wang, Y.; Qi, K.; Hu, J.; Wang, Z.; Mi, R.; Yan, H.; Chen, Z.; Han, X. The role of the wzy gene in lipopolysaccharide biosynthesis and pathogenesis of avian pathogenic Escherichia coli. Microb. Pathog. 2019, 127, 296–303.
- Gao, Q.; Xia, L.; Wang, X.; Ye, Z.; Liu, J.; Gao, S. SodA Contributes to the Virulence of Avian Pathogenic Escherichia coli O2 Strain E058 in Experimentally Infected Chickens. J. Bacteriol. 2019, 201.
- Xu, H.; Ling, J.; Gao, Q.; He, H.; Mu, X.; Yan, Z.; Gao, S.; Liu, X. Role of the lpxM lipid A biosynthesis pathway gene in pathogenicity of avian pathogenic Escherichia coli strain E058 in a chicken infection model. Vet. Microbiol. 2013, 166, 516–526.
- Murase, K.; Martin, P.; Porcheron, G.; Houle, S.; Helloin, E.; Penary, M.; Nougayrede, J.P.; Dozois, C.M.; Hayashi, T.; Oswald, E. HlyF Produced by Extraintestinal Pathogenic Escherichia coli Is a Virulence Factor That Regulates Outer Membrane Vesicle Biogenesis. J. Infect. Dis. 2016, 213, 856–865.
- Zhao, S.; Wang, C.L.; Chang, S.K.; Tsai, Y.L.; Chou, C.H. Characterization of Escherichia coli Isolated from Day-old Chicken Fluff in Taiwanese Hatcheries. Avian Dis. 2019, 63, 9–16.
- Ibrahim, R.A.; Cryer, T.L.; Lafi, S.Q.; Basha, E.A.; Good, L.; Tarazi, Y.H. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Vet. Res. 2019, 15, 159.
- Sgariglia, E.; Aconiti Mandolini, N.; Napoleoni, M.; Medici, L.; Fraticelli, R.; Conquista, M.; Gianfelici, P.; Staffolani, M.; Fisichella, S.; Capuccella, M.; et al. Antibiotic resistance pattern and virulence genesin avian pathogenic Escherichia coli (APEC) from different breeding systems. Vet. Ital. 2019, 55, 26–33.
- Dou, X.; Gong, J.; Han, X.; Xu, M.; Shen, H.; Zhang, D.; Zhuang, L.; Liu, J.; Zou, J. Characterization of avian pathogenic Escherichia coli isolated in eastern China. Gene 2016, 576, 244–248.
- Zuo, J.; Yin, H.; Hu, J.; Miao, J.; Chen, Z.; Qi, K.; Wang, Z.; Gong, J.; Phouthapane, V.; Jiang, W.; et al. Lsr operon is associated with AI-2 transfer and pathogenicity in avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 109.
- Cui, Z.Q.; Wu, Z.M.; Fu, Y.X.; Xu, D.X.; Guo, X.; Shen, H.Q.; Wei, X.B.; Yi, P.F.; Fu, B.D. Autoinducer-2 of quorum sensing is involved in cell damage caused by avian pathogenic Escherichia coli. Microb. Pathog. 2016, 99, 247–252.
- Wu, X.; Lv, X.; Lu, J.; Yu, S.; Jin, Y.; Hu, J.; Zuo, J.; Mi, R.; Huang, Y.; Qi, K.; et al. The role of the ptsI gene on AI-2 internalization and pathogenesis of avian pathogenic Escherichia coli. Microb. Pathog. 2017, 113, 321–329.
- Xu, D.; Zuo, J.; Chen, Z.; Lv, X.; Hu, J.; Wu, X.; Qi, K.; Mi, R.; Huang, Y.; Miao, J.; et al. Different activated methyl cycle pathways affect the pathogenicity of avian pathogenic Escherichia coli. Vet. Microbiol. 2017, 211, 160–168.
- Yi, Z.; Wang, D.; Xin, S.; Zhou, D.; Li, T.; Tian, M.; Qi, J.; Ding, C.; Wang, S.; Yu, S. The CpxR regulates type VI secretion system 2 expression and facilitates the interbacterial competition activity and virulence of avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 40.
- Matter, L.B.; Ares, M.A.; Abundes-Gallegos, J.; Cedillo, M.L.; Yanez, J.A.; Martinez-Laguna, Y.; De la Cruz, M.A.; Giron, J.A. The CpxRA stress response system regulates virulence features of avian pathogenic Escherichia coli. Environ. Microbiol. 2018, 20, 3363–3377.
- Wang, S.; Dai, J.; Meng, Q.; Han, X.; Han, Y.; Zhao, Y.; Yang, D.; Ding, C.; Yu, S. DotU expression is highly induced during in vivo infection and responsible for virulence and Hcp1 secretion in avian pathogenic Escherichia coli. Front. Microbiol. 2014, 5, 588.
- de Pace, F.; Boldrin de Paiva, J.; Nakazato, G.; Lancellotti, M.; Sircili, M.P.; Guedes Stehling, E.; Dias da Silveira, W.; Sperandio, V. Characterization of IcmF of the type VI secretion system in an avian pathogenic Escherichia coli (APEC) strain. Microbiology 2011, 157, 2954–2962.
- Ding, X.; Zhang, Q.; Wang, H.; Quan, G.; Zhang, D.; Ren, W.; Liao, Y.; Xia, P.; Zhu, G. The different roles of hcp1 and hcp2 of the type VI secretion system in Escherichia coli strain CE129. J. Basic Microbiol. 2018, 58, 938–946.
- Song, X.; Hou, M.; Jiang, H.; Shen, X.; Xue, M.; Shao, Y.; Wang, L.; He, Q.; Zheng, L.; Tu, J.; et al. Hcp2a of type VI secretion system contributes to IL8 and IL1β expression of chicken tracheal epithelium by affecting APEC colonization. Res. Vet. Sci. 2020, 132, 279–284.
- Xue, M.; Xiao, Y.; Fu, D.; Raheem, M.A.; Shao, Y.; Song, X.; Tu, J.; Xue, T.; Qi, K. Transcriptional Regulator YqeI, Locating at ETT2 Locus, Affects the Pathogenicity of Avian Pathogenic Escherichia coli. Animals 2020, 10, 1658.
- Wang, S.; Liu, X.; Xu, X.; Yang, D.; Wang, D.; Han, X.; Shi, Y.; Tian, M.; Ding, C.; Peng, D.; et al. Escherichia coli Type III Secretion System 2 ATPase EivC Is Involved in the Motility and Virulence of Avian Pathogenic Escherichia coli. Front. Microbiol. 2016, 7, 1387.
- Tu, J.; Huang, B.; Zhang, Y.; Xue, T.; Li, S.; Qi, K. Modulation of virulence genes by the two-component system PhoP-PhoQ in avian pathogenic Escherichia coli. Pol. J. Vet. Sci. 2016, 19, 31–40.
- Yin, L.; Li, Q.; Xue, M.; Wang, Z.; Tu, J.; Song, X.; Shao, Y.; Han, X.; Xue, T.; Liu, H.; et al. The role of the phoP transcriptional regulator on biofilm formation of avian pathogenic Escherichia coli. Avian Pathol. 2019, 48, 362–370.
- Yu, L.; Wang, H.; Han, X.; Li, W.; Xue, M.; Qi, K.; Chen, X.; Ni, J.; Deng, R.; Shang, F.; et al. The two-component system, BasSR, is involved in the regulation of biofilm and virulence in avian pathogenic Escherichia coli. Avian Pathol. 2020, 49, 532–546.
- Xue, M.; Raheem, M.A.; Gu, Y.; Lu, H.; Song, X.; Tu, J.; Xue, T.; Qi, K. The KdpD/KdpE two-component system contributes to the motility and virulence of avian pathogenic Escherichia coli. Res. Vet. Sci. 2020, 131, 24–30.
- Gao, Q.; Su, S.; Li, X.; Wang, H.; Liu, J.; Gao, S. Transcriptional analysis of RstA/RstB in avian pathogenic Escherichia coli identifies its role in the regulation of hdeD-mediated virulence and survival in chicken macrophages. Vet. Microbiol. 2020, 241, 108555.
- Gao, Q.; Ye, Z.; Wang, X.; Mu, X.; Gao, S.; Liu, X. RstA is required for the virulence of an avian pathogenic Escherichia coli O2 strain E058. Infect. Genet. Evol. 2015, 29, 180–188.
- Herren, C.D.; Mitra, A.; Palaniyandi, S.K.; Coleman, A.; Elankumaran, S.; Mukhopadhyay, S. The BarA-UvrY two-component system regulates virulence in avian pathogenic Escherichia coli O78:K80:H9. Infect. Immun. 2006, 74, 4900–4909.
- Wiebe, H.; Gurlebeck, D.; Gross, J.; Dreck, K.; Pannen, D.; Ewers, C.; Wieler, L.H.; Schnetz, K. YjjQ Represses Transcription of flhDC and Additional Loci in Escherichia coli. J. Bacteriol. 2015, 197, 2713–2720.
- Yu, L.; Li, W.; Qi, K.; Wang, S.; Chen, X.; Ni, J.; Deng, R.; Shang, F.; Xue, T. McbR is involved in biofilm formation and H2O2 stress response in avian pathogenic Escherichia coli X40. Poult. Sci. 2019, 98, 4094–4103.
- Gao, Q.; Xu, H.; Wang, X.; Zhang, D.; Ye, Z.; Gao, S.; Liu, X. RfaH promotes the ability of the avian pathogenic Escherichia coli O2 strain E058 to cause avian colibacillosis. J. Bacteriol. 2013, 195, 2474–2480.
- Zhuge, X.; Sun, Y.; Jiang, M.; Wang, J.; Tang, F.; Xue, F.; Ren, J.; Zhu, W.; Dai, J. Acetate metabolic requirement of avian pathogenic Escherichia coli promotes its intracellular proliferation within macrophage. Vet. Res. 2019, 50, 31.
- Hejair, H.M.A.; Zhu, Y.; Ma, J.; Zhang, Y.; Pan, Z.; Zhang, W.; Yao, H. Functional role of ompF and ompC porins in pathogenesis of avian pathogenic Escherichia coli. Microb. Pathog. 2017, 107, 29–37.
- Li, D.; Chen, Y.; Qian, X.; Liu, Y.; Ren, J.; Xue, F.; Sun, J.; Tang, F.; Dai, J. orf20 in prophage phiv142-3 contributes to the adhesion and colonization ability of avian pathogenic Escherichia coli strain DE142 by affecting the formation of flagella and I fimbriae. Vet. Microbiol. 2019, 235, 301–309.
- Li, D.; Tang, F.; Xue, F.; Ren, J.; Liu, Y.; Yang, D.; Dai, J. Prophage phiv142-3 enhances the colonization and resistance to environmental stresses of avian pathogenic Escherichia coli. Vet. Microbiol. 2018, 218, 70–77.
- Liu, Y.; Gong, Q.; Qian, X.; Li, D.; Zeng, H.; Li, Y.; Xue, F.; Ren, J.; Zhu Ge, X.; Tang, F.; et al. Prophage phiv205-1 facilitates biofilm formation and pathogenicity of avian pathogenic Escherichia coli strain DE205B. Vet. Microbiol. 2020, 247, 108752.
- Verma, R.; Rojas, T.C.G.; Maluta, R.P.; Leite, J.L.; Nakazato, G.; de Silveira, W.D. Role of hypothetical protein YicS in the pathogenicity of Avian Pathogenic Escherichia coli in vivo and in vitro. Microbiol. Res. 2018, 214, 28–36.
- Liu, H.; Chen, L.; Si, W.; Wang, C.; Zhu, F.; Li, G.; Liu, S. Physiology and pathogenicity of cpdB deleted mutant of avian pathogenic Escherichia coli. Res. Vet. Sci. 2017, 111, 21–25.
- Lamarche, M.G.; Dozois, C.M.; Daigle, F.; Caza, M.; Curtiss, R., 3rd; Dubreuil, J.D.; Harel, J. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect. Immun. 2005, 73, 4138–4145.
- Mu, X.; Huan, H.; Xu, H.; Gao, Q.; Xiong, L.; Gao, R.; Gao, S.; Liu, X. The transfer-messenger RNA-small protein B system plays a role in avian pathogenic Escherichia coli pathogenicity. J. Bacteriol. 2013, 195, 5064–5071.
- Vanderkelen, L.; Ons, E.; Van Herreweghe, J.M.; Callewaert, L.; Goddeeris, B.M.; Michiels, C.W. Role of lysozyme inhibitors in the virulence of avian pathogenic Escherichia coli. PLoS ONE 2012, 7, e45954.
- Delannoy, S.; Schouler, C.; Souillard, R.; Yousfi, L.; Le Devendec, L.; Lucas, C.; Bougeard, S.; Keita, A.; Fach, P.; Galliot, P.; et al. Diversity of Escherichia coli strains isolated from day-old broiler chicks, their environment and colibacillosis lesions in 80 flocks in France. Vet. Microbiol. 2021, 252, 108923.
- Mbanga, J.; Nyararai, Y.O. Virulence gene profiles of avian pathogenic Escherichia coli isolated from chickens with colibacillosis in Bulawayo, Zimbabwe. Onderstepoort J. Vet. Res. 2015, 82, e1–e8.
- Hayashi, W.; Tanaka, H.; Taniguchi, Y.; Iimura, M.; Soga, E.; Kubo, R.; Matsuo, N.; Kawamura, K.; Arakawa, Y.; Nagano, Y.; et al. Acquisition of mcr-1 and Cocarriage of Virulence Genes in Avian Pathogenic Escherichia coli Isolates from Municipal Wastewater Influents in Japan. Appl. Environ. Microbiol. 2019, 85.
- Saha, O.; Hoque, M.N.; Islam, O.K.; Rahaman, M.M.; Sultana, M.; Hossain, M.A. Multidrug-Resistant Avian Pathogenic Escherichia coli Strains and Association of Their Virulence Genes in Bangladesh. Microorganisms 2020, 8, 1135.
- Tuntufye, H.N.; Lebeer, S.; Gwakisa, P.S.; Goddeeris, B.M. Identification of Avian pathogenic Escherichia coli genes that are induced in vivo during infection in chickens. Appl. Environ. Microbiol. 2012, 78, 3343–3351.
- Dozois, C.M.; Daigle, F.; Curtiss, R., 3rd. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 2003, 100, 247–252.
- Zhang, H.; Chen, X.; Nolan, L.K.; Zhang, W.; Li, G. Identification of Host Adaptation Genes in Extraintestinal Pathogenic Escherichia coli during Infection in Different Hosts. Infect. Immun. 2019, 87.
- Zhang, Z.; Jiang, S.; Liu, Y.; Sun, Y.; Yu, P.; Gong, Q.; Zeng, H.; Li, Y.; Xue, F.; Zhuge, X.; et al. Identification of ireA, 0007, 0008, and 2235 as TonB-dependent receptors in the avian pathogenic Escherichia coli strain DE205B. Vet. Res. 2020, 51, 5.
- Li, Y.; Dai, J.; Zhuge, X.; Wang, H.; Hu, L.; Ren, J.; Chen, L.; Li, D.; Tang, F. Iron-regulated gene ireA in avian pathogenic Escherichia coli participates in adhesion and stress-resistance. BMC Vet. Res. 2016, 12, 167.
- Han, Y.; Han, X.; Wang, S.; Meng, Q.; Zhang, Y.; Ding, C.; Yu, S. The waaL gene is involved in lipopolysaccharide synthesis and plays a role on the bacterial pathogenesis of avian pathogenic Escherichia coli. Vet. Microbiol. 2014, 172, 486–491.
- Forsyth, V.S.; Himpsl, S.D.; Smith, S.N.; Sarkissian, C.A.; Mike, L.A.; Stocki, J.A.; Sintsova, A.; Alteri, C.J.; Mobley, H.L.T. Optimization of an Experimental Vaccine To Prevent Escherichia coli Urinary Tract Infection. mBio 2020, 11, e00555-20.
- Linciano, P.; Cavalloro, V.; Martino, E.; Kirchmair, J.; Listro, R.; Rossi, D.; Collina, S. Tackling Antimicrobial Resistance with Small Molecules Targeting LsrK: Challenges and Opportunities. J. Med. Chem. 2020, 63, 15243–15257.
- Murima, P.; McKinney, J.D.; Pethe, K. Targeting Bacterial Central Metabolism for Drug Development. Chem. Biol. 2014, 21, 1423–1432.
- Marshall, N.C.; Finlay, B.B. Targeting the type III secretion system to treat bacterial infections. Expert Opin. Ther. Targets 2014, 18, 137–152.
