The difficulty of sample pretreatment/preparation in food testing is much higher than that for human biological samples. For example, whole blood analysis and diagnosis tests usually require only centrifugation to separate the blood cells and plasma for detection. By contrast, when detecting acidic preservatives in food samples, for example, it is necessary to homogenize the sample first and then extract the test substance by steam distillation before detection can be performed. As a result, sample processing in traditional food detection methods usually involves a high cost, a long process, and the use of multiple equipment. This section therefore reviews the pretreatment methods proposed in the literature for milk sample analysis using compact and low-cost microfluidic devices. Broadly speaking, pretreatment methods on microfluidic platforms fall into three main categories, namely separation, extraction, and concentration/amplification.
1. Sample Separation Microfluidic Devices
In food testing, the sample usually has a complex composition consisting of fats, proteins, and many other components. As a result, it is generally necessary to separate the sample prior to the detection process in order to avoid possible interference with the detection of the target analyte. Singh et al. [1] presented a hand-held microfluidic device incorporating a gold-coated glass slide for the separation of microbes from model solutions and complex matrices. The feasibility of the proposed device was demonstrated by capturing the microbial cells in a yoghurt sample. The results showed that the device captured up to 99.3% of the cells within 60 min. Many other researchers have presented microfluidic platforms for the separation of milk samples in recent years, including antibiotics and toxins [2], quinolone drugs [3], and foodborne bacteria [4]. For instance, Jung et al. [4] developed a magnetophoresis-based microfluidic device for the rapid separation and concentration of foodborne bacteria consisting of a polyethylene tube wrapped around a magnet (see
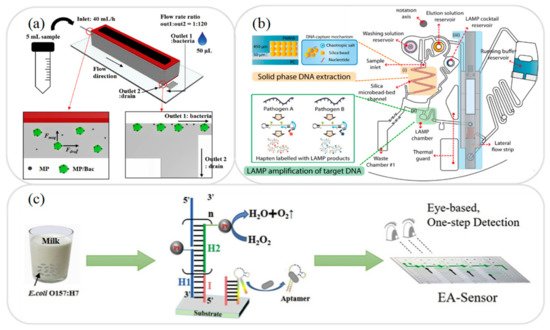
In food testing, the sample usually has a complex composition consisting of fats, proteins, and many other components. As a result, it is generally necessary to separate the sample prior to the detection process in order to avoid possible interference with the detection of the target analyte. Singh et al. [33] presented a hand-held microfluidic device incorporating a gold-coated glass slide for the separation of microbes from model solutions and complex matrices. The feasibility of the proposed device was demonstrated by capturing the microbial cells in a yoghurt sample. The results showed that the device captured up to 99.3% of the cells within 60 min. Many other researchers have presented microfluidic platforms for the separation of milk samples in recent years, including antibiotics and toxins [34], quinolone drugs [35], and foodborne bacteria [36]. For instance, Jung et al. [36] developed a magnetophoresis-based microfluidic device for the rapid separation and concentration of foodborne bacteria consisting of a polyethylene tube wrapped around a magnet (see a). It was shown that an efficient sample separation performance could be achieved even under high flow rates simply by increasing the length of the tube. For example, when applied to the analysis of milk and homogenized cabbage samples, the device showed a separation efficiency of more than 92% at a flow rate of 40 mL/h.
Figure 1.
(
a) Schematic illustration showing working principle of magnetophoresis-based microfluidic device for sample separation and concentration during pretreatment process. Reprinted with permission from ref. [4]. Copyright 2020 Elsevier. (
) Schematic illustration showing working principle of magnetophoresis-based microfluidic device for sample separation and concentration during pretreatment process. Reprinted with permission from ref. [36]. Copyright 2020 Elsevier. ( b) Schematic illustration showing working principle of extraction and amplification of DNA on microfluidic device. Reprinted with permission from ref. [5]. Copyright 2017 Elsevier. (
) Schematic illustration showing working principle of extraction and amplification of DNA on microfluidic device. Reprinted with permission from ref. [39]. Copyright 2017 Elsevier. ( c) Schematic illustration showing HCR amplification of bacteria on microfluidic device. Reprinted with permission from ref. [6]. Copyright 2020 Elsevier.
) Schematic illustration showing HCR amplification of bacteria on microfluidic device. Reprinted with permission from ref. [47]. Copyright 2020 Elsevier.
2. Sample Extraction Microfluidic Devices
In developing microfluidic devices for the analysis of food samples, one of the key requirements is that of developing miniaturized extraction techniques compatible with lab-on-a-chip and lab-on-paper platforms. The literature contains many proposals for the extraction of various substances in milk samples, including antibiotics [7] and foodborne pathogens [8][5][9][10]. For example, Park et al. [5] presented an integrated rotary microfluidic system for performing the extraction and amplification of foodborne pathogens. As shown in
In developing microfluidic devices for the analysis of food samples, one of the key requirements is that of developing miniaturized extraction techniques compatible with lab-on-a-chip and lab-on-paper platforms. The literature contains many proposals for the extraction of various substances in milk samples, including antibiotics [37] and foodborne pathogens [38,39,40,41]. For example, Park et al. [39] presented an integrated rotary microfluidic system for performing the extraction and amplification of foodborne pathogens. As shown in b, the system was fabricated using PC (polycarbonate) and PMMA substrates and was designed to perform three functions, namely solid-phase DNA extraction, loop-mediated isothermal amplification (LAMP), and lateral flow strip paper detection. In the proposed device, the extraction step was performed using a solid phase DNA extraction method based on the use of silica bead as a solid matrix. The experimental results showed that the proposed platform was capable of detecting
Salmonella (S.) Typhimurium
and
Vibrio (V.) parahaemolyticus
bacteria in contaminated milk samples with a LOD of 50 CFU (colony-forming unit) within 80 min.
3. Sample Amplification Microfluidic Devices
In food analysis samples, the target analyte is typically present in only very small quantities. Consequently, some form of sample amplification system must be employed in order to improve the LOD performance. Several microfluidic platforms with integrated amplification systems have been proposed for the detection and analysis of milk based on polymerase chain reaction (PCR) [11][12][13], LAMP [14][15], hybridization chain reaction (HCR) [6], and catalyzed hairpin assembly (CHA) [16]. For example, Li et al. [6] presented an HCR amplification microfluidic aptasensor for the detection of
In food analysis samples, the target analyte is typically present in only very small quantities. Consequently, some form of sample amplification system must be employed in order to improve the LOD performance. Several microfluidic platforms with integrated amplification systems have been proposed for the detection and analysis of milk based on polymerase chain reaction (PCR) [42,43,44], LAMP [45,46], hybridization chain reaction (HCR) [47], and catalyzed hairpin assembly (CHA) [48]. For example, Li et al. [47] presented an HCR amplification microfluidic aptasensor for the detection of E. coli
O157:H7 in milk samples. As shown in
c, an HCR initiator and aptamer were deposited on the substrate, and the stronger binding ability of the pathogen with the aptamer was then exploited to dissociate the aptamer from the initiator and trigger a series of hybridization events to achieve sample amplification. It was shown that the aptasensor had good specificity for the detection of
E. coli
O157:H7 and amplified the signal by around 100 times compared to that of the original (non-amplified) sample.