Chitosan is increasingly used for safe drug and nucleic acid delivery due to well-known properties such as bioadhesion, low toxicity, biodegradability and biocompatibility. Furthermore, chitosan derivatization can be easily performed to improve solubility and stability of chitosan-nucleic acid polyplexes, and enhance efficient target cell drug delivery, cell uptake, intracellular endosomal escape, unpacking and nuclear import of expression plasmids. This review focus attention on recent advances in chitosan-mediated gene delivery for fish biotechnology applications such as fish vaccination against bacterial and viral infection, control of gonadal development, and gene overexpression and silencing for overcoming metabolic limitations such as dependence on protein-rich diets and low glucose tolerance of farmed fish.
- chitosan
- gene delivery
- gene overxpression
- gene silencing
- fish biotechnology
Chitosan and its derivatives are widely used in aquaculture. Low toxicity, biodegradability, biocompatibility, bioadhesion and immunomodulatory properties make chitosan and its derivatives of increasing interest for the fish farming industry as dietary additives, non-viral vectors enabling fish vaccination and protection against diseases, control of gonadal development and for the gene therapy-based modulation of fish metabolism.
Chitosan and Its Derivatives as Dietary Additives
Dietary supplementation with chitosan and its derivatives has been shown to improve fish growth performance, non-specific immunity and antioxidant effects [1][2]. However, the strategy for chitosan dietary supplementation in fish requires extensive investigation, according to the species and the growth stage of fish.
Dietary Supplementation with Chitosan
The inclusion of chitosan as feed additive for fish has been receiving attention since the 1980s [3]. Shiau et al. reported that inclusion of dietary levels of chitosan from 2% to 10% for 28 days decreases the weight gain and increases the feed conversion ratio (FCR) in hybrid tilapia (
Oreochromis niloticus×
Oreochromis aureus) [89]. However, other studies performed in) [4]. However, other studies performed in
Oreochromis niloticus showed positive effects of chitosan on fish growth. Feed supplementation of tilapia with chitosan (0–8 g/kg dry diet) for 56 days led to the conclusion that 4 g/kg of chitosan was the optimal dose to promote the highest body weight gain (BWG) rate and specific growth rate (SGR) [90]. Similarly, chitosan supplementation at 5 g/kg diet for 60 days improved growth performance, BWG, SGR and FCR in tilapia [91]. The contradictory effects reported for chitosan on tilapia growth could be attributed to the fact that the studies were performed using different fish growth stages. Indeed, the initial weight of fish in the study by Shiau et al. was of 0.99 ± 0.01 g, while the latter two reports used a significantly higher initial body weight (50.1 ± 4.1 g and 39.3 ± 0.3 g, respectively).showed positive effects of chitosan on fish growth. Feed supplementation of tilapia with chitosan (0–8 g/kg dry diet) for 56 days led to the conclusion that 4 g/kg of chitosan was the optimal dose to promote the highest body weight gain (BWG) rate and specific growth rate (SGR) [5]. Similarly, chitosan supplementation at 5 g/kg diet for 60 days improved growth performance, BWG, SGR and FCR in tilapia [6]. The contradictory effects reported for chitosan on tilapia growth could be attributed to the fact that the studies were performed using different fish growth stages. Indeed, the initial weight of fish in the study by Shiau et al. was of 0.99 ± 0.01 g, while the latter two reports used a significantly higher initial body weight (50.1 ± 4.1 g and 39.3 ± 0.3 g, respectively).
In addition to the developmental stage and amount of dietary chitosan supplied, chitosan effects exerted on fish growth performance also seem to depend on the species [2]. According to the effect observed on SGR, the apparent digestibility coefficient of dry matter and the apparent digestibility coefficient of protein, 75 days of feeding on diets supplemented with 10–20 g chitosan/kg significantly reduced the growth performance of gibel carp (
Carassius gibelio) (initial body weight, 4.80 ± 0.01 g) [92]. However, the supply of 0–0.2 g chitosan/kg diet caused a dose dependent increase of the average daily weight and SGR in post-larvae sea bass () (initial body weight, 4.80 ± 0.01 g) [7]. However, the supply of 0–0.2 g chitosan/kg diet caused a dose dependent increase of the average daily weight and SGR in post-larvae sea bass (
Dicentrarchus labrax) [93]. Yan et al. also reported that dietary supplementation of 0%–5% chitosan improved growth performance by inducing dose dependent increases of BWG and SGR, while FCR decreased [94]. Similarly, 70 days of supplementation with 1–5 g chitosan/kg diet of loach fish () [8]. Yan et al. also reported that dietary supplementation of 0%–5% chitosan improved growth performance by inducing dose dependent increases of BWG and SGR, while FCR decreased [9]. Similarly, 70 days of supplementation with 1–5 g chitosan/kg diet of loach fish (
Misgurnus anguillicadatus) with an average body weight of 3.14 ± 0.05 g, significantly increased BWG, SGR and condition factor (CF), whereas it decreased FCR [95]. In contrast, Najafabad et al. found that Caspian kutum () with an average body weight of 3.14 ± 0.05 g, significantly increased BWG, SGR and condition factor (CF), whereas it decreased FCR [10]. In contrast, Najafabad et al. found that Caspian kutum (
Rutilus kutum) fingerlings (1.7 ± 0.15 g) supplied with 0–2 g chitosan/kg diet for 60 days showed no effect of final weight, SGR and condition factor [96].) fingerlings (1.7 ± 0.15 g) supplied with 0–2 g chitosan/kg diet for 60 days showed no effect of final weight, SGR and condition factor [11].
The positive effect of chitosan on the growth performance of some fish species might result from its role in nonspecific immunity. Chitosan acts as an immunostimulary drug through induction of nonspecific immunity in fish. In loach fish, the dietary supplement of chitosan increased the serum levels of factors considered as immune boosters, such as the content of immunoglobulin M (IgM), complement component 3 (C3) levels, the activity of lysozyme, acid phosphatase and alkaline phosphatase, as well as increased the survival rate after being challenged by
Aeromonas hydrophila [95]. In accordance with the immune boost, other investigations also showed immune reinforcement by chitosan, when fish were challenged by bacteria in regard to immunoglobulin content, serum lysozyme, bactericidal activity, immune-related gene expression, phagocytosis and respiratory burst activity [90,92,94,97]. Consistently, chitosan was shown to modify hematological parameters of fish, which are also considered important indicators of immunostimulation. In Asian seabass ([10]. In accordance with the immune boost, other investigations also showed immune reinforcement by chitosan, when fish were challenged by bacteria in regard to immunoglobulin content, serum lysozyme, bactericidal activity, immune-related gene expression, phagocytosis and respiratory burst activity [5][7][9][12]. Consistently, chitosan was shown to modify hematological parameters of fish, which are also considered important indicators of immunostimulation. In Asian seabass (
Lates calcarifer), chitosan supplement during 60 days at 5–20 g/kg diet increased red blood cells (RBC), white blood cells (WBC), total serum protein, albumin and globulin [98]. Supplementation with chitosan was reported also to increase RBC, WBC, haemoglobin, lymphocytes, monocytes, neutrophils and thrombocytes in mrigal carp (), chitosan supplement during 60 days at 5–20 g/kg diet increased red blood cells (RBC), white blood cells (WBC), total serum protein, albumin and globulin [13]. Supplementation with chitosan was reported also to increase RBC, WBC, haemoglobin, lymphocytes, monocytes, neutrophils and thrombocytes in mrigal carp (
Cirrhinus mrigala) and kelp grouper (
Epinephelus bruneus) [99,100,101].Concomitant to the effects on immunity, chitosan also elevates antioxidant responses in fish. In loach fish, the activity of phenoloxidase, superoxide dismutase (SOD) and glutathione peroxidase (GPx) increased after 12 weeks of chitosan supplementation [10]. Similarly, chitosan induced the activity of SOD and catalase (CAT) after 56 days of dietary supplementation in tilapia [5], and the mRNA levels of SOD, CAT, GPx and nuclear factor erythroid 2-related factor 2 [9]. The protective effect of chitosan from oxidative stress was also reported in olive flounder (
Paralichthys olivaceus) challenged with H
2O
2 [97]. The authors observed that chitosan-coated diets significantly narrowed the increase of protein carbonyl formation and DNA damage in the plasma.[12]. The authors observed that chitosan-coated diets significantly narrowed the increase of protein carbonyl formation and DNA damage in the plasma.
Dietary Supplementation with Chitosan Nanoparticles
Wang et al. reported that BWG significantly increased in tilapia (initial body weight, 23.6 ± 1.2 g) fed with chitosan nanoparticles (5 g/kg dry diet) [17]. Similar results were described by other authors. Chitosan nanoparticle intake increased final weight, weight gain, SGR and FCR in tilapia supplied for 45 days with 0–2 g/kg (initial body weight, 19.8 ± 0.6 g) and 70 days for 1–5 g/kg (initial body weight, 5.66 ± 0.02 g). In these reports, innate immunity was also enhanced and fish exhibited increased respiratory burst activity, lysozyme malondialdehyde, CAT and SOD activity, and hematological parameters such as RBC, hematocrit, hemoglobin, mean corpuscular volume, WBC and platelets [18][19]. Remarkably, optimal supplement of dietary chitosan nanoparticles to improve growth and immunity against pathogens may vary, according to parameters such as developmental growth stage and species.
Dietary supplementation of chitosan nanoparticles complexed with vitamin C and thymol is more effective in enhancing immunity than supplementation with the single additives. Dietary chitosan–vitamin C nanoparticles slightly improved growth performance of tilapia, while inducing the viscerosomatic index, therefore decreasing economic performance. However, when fish fed chitosan–vitamin C nanoparticles were challenged by imidacloprid-polluted water, chitosan–vitamin C supplementation significantly strengthened immunity and antioxidant activity, including the activity of lysozyme, glutathione reductase and CAT, C3 and immunoglobulins [20]. Growth effects of dietary supplementation with chitosan nanoparticles mixed with thymol, the most important phenolic compound in
Thymus vulgaris essential oil, were evaluated on hematological parameters, and the liver and kidney function in tilapia [106]. The results showed that chitosan–thymol nanoparticle supplementation increased feed efficiency and protein efficiency ratio, while it had moderated effects on final weight, weight gain and SGR. Nevertheless, chitosan–thymol produced a synergistic effect on lymphocytes and monocyte leukocytes. The use of chitosan nanoparticles as feed additive is limited by the fact that it can exhibit toxic effects at high levels. In this regard, chitosan nanoparticles significantly decreased hatching rate and survival rate of zebrafish (essential oil, were evaluated on hematological parameters, and the liver and kidney function in tilapia [21]. The results showed that chitosan–thymol nanoparticle supplementation increased feed efficiency and protein efficiency ratio, while it had moderated effects on final weight, weight gain and SGR. Nevertheless, chitosan–thymol produced a synergistic effect on lymphocytes and monocyte leukocytes. The use of chitosan nanoparticles as feed additive is limited by the fact that it can exhibit toxic effects at high levels. In this regard, chitosan nanoparticles significantly decreased hatching rate and survival rate of zebrafish (
Danio rerio) when the immersion concentration reached 20 and 30 μg/mL or higher [107,108].) when the immersion concentration reached 20 and 30 μg/mL or higher [22][23].
Dietary Supplementation with Chitin and Chitooligosaccharide
Meanwhile the inclusion of chitin in the diet has no significant effects on fish growth performance [24][25][26], chitooligosaccharide (COS) enhances growth performance parameters such as BWG, hepatosomatic and intestosomatic index, SGR and FCR in a number of fish species, including juvenile largemouth bass (
Micropterus salmoides) [112], striped catfish () [27], striped catfish (
Pangasianodon hypophthalmus) [113], Nile tilapia () [28], Nile tilapia (
Oreochromis niloticus) [114], tiger puffer () [29], tiger puffer (
Takifugu rubripes) [115], koi () [30], koi (
Cyprinus carpio koi) [116], and silverfish () [31], and silverfish (
Trachinotus ovatus) [117]. Similarly as in most fish species, dietary supplementation with low molecular weight and highly deacetylated COS enhances growth performance, innate immunity and digestive enzyme activity in Pacific white shrimp () [32]. Similarly as in most fish species, dietary supplementation with low molecular weight and highly deacetylated COS enhances growth performance, innate immunity and digestive enzyme activity in Pacific white shrimp (
Litopenaeus vannamei) [118]. However, the effect of dietary COS may depend on the species. In this regard, dietary COS supplementation was reported to cause not significant effects on weight gain, FCR and the survival rate in hybrid tilapia () [33]. However, the effect of dietary COS may depend on the species. In this regard, dietary [34] COS supplementation was reported to cause not significant effects on weight gain, FCR and the survival rate in hybrid tilapia (
Oreochromis niloticus×O. aureus) [109]. Similar results were reported for rainbow trout () [24]. Similar results were reported for rainbow trout (
Oncorhynchus mykiss) [119]. Incomplete intestinal development in early developmental stages may contribute to the lack of COS effect on growth performance observed in several fish species.) [35]. Incomplete intestinal development in early developmental stages may contribute to the lack of COS effect on growth performance observed in several fish species.
A number of studies showed that both chitin and COS can be potentially utilized as immunostimulants in fish. Respiratory burst activity, phagocytic activity and lysozyme activity, which are considered indicators of non-specific immunity, have been shown to be significantly stimulated by chitin and COS in a number of fish species, including juvenile largemouth bass (
Micropterus salmoides) [112], Nile tilapia () [27], Nile tilapia (
Oreochromis niloticus) [114], striped catfish () [29], striped catfish (
Pangasianodon hypophthalmus) [113] and mrigal carp () [28] and mrigal carp (
Cirrhina mrigala) [99]. Chitin and COS also induce other immunity parameters, such as nitric oxide production, inducible nitric oxide synthase (iNOS) activity and gene expression [112,120], leukocyte count [99,112,116] and complement activity [99,100].) [14]. Chitin and COS also induce other immunity parameters, such as nitric oxide production, inducible nitric oxide synthase (iNOS) activity and gene expression [27], leukocyte count [14][27][31] and complement activity [14][15].
Chitosan as a Carrier for Drug Delivery in Fish
Chitosan is nanoscale, biodegradable, biocompatible, hemocompatible, simple and mild for preparation conditions, and is highly efficient for drug loading. Therefore, chitosan has been used for loading a variety of bioactive compounds, such as vitamins, metal ions, inactivated pathogens for vaccines, proteins and nucleic acids in a variety of applications in fish farming. In addition, loading into chitosan can significantly boost the bioeffects of these compounds.
Chitosan Loading Chemical Compounds
The sustained release of compounds complexed with chitosan nanoparticles fulfills the requirements of artificial breeding in fish farming and enable delivery and cell uptake of compounds with low toxicity [36][37]. Chitosan nanoparticles loaded with vitamin C, an important but labile antioxidant, were proven to enhance sustained vitamin C release in the stomach, the intestine and in serum after oral administration in rainbow trout (
Oncorhynchus mykiss) [123]. Chitosan–vitamin C nanoparticles exhibited a markedly high antioxidant activity and no toxicity up to 2.5 mg/mL in the culture medium of ZFL cells, a zebrafish liver-derived cell line. In addition, chitosan–vitamin C nanoparticles showed the capability to penetrate the intestinal epithelium of) [38]. Chitosan–vitamin C nanoparticles exhibited a markedly high antioxidant activity and no toxicity up to 2.5 mg/mL in the culture medium of ZFL cells, a zebrafish liver-derived cell line. In addition, chitosan–vitamin C nanoparticles showed the capability to penetrate the intestinal epithelium of
Solea senegalensis [124]. Several studies evaluated chitosan nanoparticles loading aromatase inhibitors and eurycomanone, compounds that promote gonadal development. Chitosan-mediated delivery of aromatase inhibitors and eurycomanone prolonged serum presence, improved testicular development with lack of testicular toxicity, and led to higher serum concentrations of reproductive hormones [125,126,127,128].[39]. Several studies evaluated chitosan nanoparticles loading aromatase inhibitors and eurycomanone, compounds that promote gonadal development. Chitosan-mediated delivery of aromatase inhibitors and eurycomanone prolonged serum presence, improved testicular development with lack of testicular toxicity, and led to higher serum concentrations of reproductive hormones [40][41][42][43].
Chitosan Loading Metal Ions
Loading with chitosan facilitates delivery of metal ions that are micronutrients and antibacterial factors, such as selenium and silver, to fish in culture. Barakat et al. showed that chitosan–silver nanoparticles successfully treated European sea bass larvae infected with
Vibrio anguillarum. Chitosan–silver nanoparticles significantly decreased the bacterial number and improved fish survival [129]. In addition, dietary supplementation with chitosan–silver nanoparticles were shown to altering gut morphometry and microbiota in zebrafish. Feeding with chitosan–silver nanoparticles increased. Chitosan–silver nanoparticles significantly decreased the bacterial number and improved fish survival [44]. In addition, dietary supplementation with chitosan–silver nanoparticles were shown to altering gut morphometry and microbiota in zebrafish. Feeding with chitosan–silver nanoparticles increased
Fusobacteriaand
Bacteroidetes phyla, goblet cell density and villi height, while upregulated the expression of immune-related genes [130]. Similarly, chitosan–selenium nanoparticles had immunostimulary roles and increased disease resistance in zebrafish andphyla, goblet cell density and villi height, while upregulated the expression of immune-related genes [45]. Similarly, chitosan–selenium nanoparticles had immunostimulary roles and increased disease resistance in zebrafish and
Paramisgurnus dabryanus by improving the activity of lysozyme, acid phosphatase and alkaline phosphatase, phagocytic respiratory burst and splenocyte-responses towards concanavalin A [131,132].by improving the activity of lysozyme, acid phosphatase and alkaline phosphatase, phagocytic respiratory burst and splenocyte-responses towards concanavalin A [46][47].
Chitosan Loading Inactivated Pathogens
Vaccines against pathogens is a major challenge in aquaculture. In this regard, chitosan can be used as proper carrier and adjuvant to enhance effectiveness of vaccination. A number of inactivated bacteria and virus have been evaluated with chitosan or its derivatives as adjuvant against infections in fish. Vaccines, such as inactivated
Edwardsiella ictaluriand infectious spleen and kidney necrosis virus, have been tested with chitosan in yellow catfish (
Pelteobagrus fulvidraco) and Chinese perch (
Siniperca chuasi), respectively. Chitosan enhanced incorporation into the host cells and improved fish survival rate and immune response, increasing IgM content, lysozyme activity and mRNA levels of interleukin (IL)-1β, IL-2 and interferon (IFN)-γ2 [133,134]. A mixture of COS and inactivated), respectively. Chitosan enhanced incorporation into the host cells and improved fish survival rate and immune response, increasing IgM content, lysozyme activity and mRNA levels of interleukin (IL)-1β, IL-2 and interferon (IFN)-γ2 [48][49]. A mixture of COS and inactivated
Vibrio anguillarumvaccine significantly reduced zebrafish mortality against
Vibro anguillarum [135], while COS combined with inactivated[50], while COS combined with inactivated
Vibrio harveyialso markedly increased survival rate, IgM and the expression of immune-related genes, such as IL-1β, IL-16, tumor necrosis factor-alpha (TNF-α) and major histocompatibility complex class I alpha (MHC-Iα), in the grouper ♀
Epinephelus fuscoguttatus×♂
Epinephelus lanceolatus [136]. Similarly, rainbow trout ([51]. Similarly, rainbow trout (
Oncorhynchus mykiss) immunized against bacterial infection (
Lactococcus garvieaeand
Streptococcus iniae) through chitosan–alginate coated vaccination exhibited a higher survival rate, immune-related gene expression, and antibody titer than fish submitted to non-coated vaccination [137].) through chitosan–alginate coated vaccination exhibited a higher survival rate, immune-related gene expression, and antibody titer than fish submitted to non-coated vaccination [52].
Olive flounder (
Paralichthys olivaceus) vaccinated against inactivated viral haemorrhagic septicaemia virus encapsulated with chitosan through oral and immersion routes showed effective immunization in the head kidney, which is considered as the primary organ responsible for the initiation of adaptive immunity in fish, skin and intestine, which are regarded as the main sites for antigen uptake and mucosal immunity. Additionally to upregulation of IgM, immunoglobulin T (IgT), polymeric Ig receptor (pIgR), MHC-I, major histocompatibility complex class II (MHC-II) and IFN-γ in the three tissues, caspase 3 was also highly induced 48 h post-challenge, suggesting cytotoxicity due to rapid T-cell response and impairment of viral proliferation [138].) vaccinated against inactivated viral haemorrhagic septicaemia virus encapsulated with chitosan through oral and immersion routes showed effective immunization in the head kidney, which is considered as the primary organ responsible for the initiation of adaptive immunity in fish, skin and intestine, which are regarded as the main sites for antigen uptake and mucosal immunity. Additionally to upregulation of IgM, immunoglobulin T (IgT), polymeric Ig receptor (pIgR), MHC-I, major histocompatibility complex class II (MHC-II) and IFN-γ in the three tissues, caspase 3 was also highly induced 48 h post-challenge, suggesting cytotoxicity due to rapid T-cell response and impairment of viral proliferation [53].
Coating chitosan with membrane vesicles from pathogens such as
Piscirickettsia salmoniswas also shown to be an effective strategy to induce immune response in zebrafish (
Danio rerio) and upregulation of CD 4, CD 8, MHC-I, macrophage-expressed 1, tandem duplicate 1 (Mpeg1.1), TNFα, IL-1β, IL-10, and IL-6 [139].) and upregulation of CD 4, CD 8, MHC-I, macrophage-expressed 1, tandem duplicate 1 (Mpeg1.1), TNFα, IL-1β, IL-10, and IL-6 [54].
Chitosan Loading Proteins
Effectiveness of fish vaccination against infections can be also improved with antigenic proteins derived from bacteria and virus. For example, chitosan nanoparticles encapsulated with the recombinant outer membrane protein A of
Edwardsiella tardawas used for oral vaccination of fringed-lipped peninsula carp (
Labeo fimbriatus). Treated fish showed significant higher levels of post-vaccination antibody in circulation and survival rate against
Edwardsiella tarda [140]. In another study, oral vaccination with alginate-chitosan microspheres encapsulating the recombinant protein serine-rich repeat (rSrr) of[55]. In another study, oral vaccination with alginate-chitosan microspheres encapsulating the recombinant protein serine-rich repeat (rSrr) of
Streptococcus iniaewere evaluated and the results showed that lysozyme activity and immune-related genes were induced, leading to a 60% increased survival rate of channel catfish (
Ictalurus punctatus) against
Streptococcus iniae infection [141]. In grass carp (infection [56]. In grass carp (
Ctenopharyngodon idella), chitosan was also used for carrying the immunomodulatory factor IFN-γ2. Treatment with chitosan–
Ctenopharyngodon idella IFN-γ2 highly upregulated inflammatory factors, leading to severe inflammatory damage in the intestine, hepatopancreas and decreased survival rate [142].IFN-γ2 highly upregulated inflammatory factors, leading to severe inflammatory damage in the intestine, hepatopancreas and decreased survival rate [57].
Chitosan Loading Nucleic Acids
Compared to chitosan-based gene delivery in other organisms, gene therapy methodologies using chitosan for improving desirable traits in farmed fish have great potential for development (
Figure 1b). A number of studies addressed the characterization of factors that can influence the efficiency of chitosan loading and nucleic acid release, such as the average diameter, zeta potential and encapsulation efficiency of chitosan–DNA microspheres or nanospheres.
Table 1 summarizes chitosan–plasmid DNA encapsulation efficiency and changes in particle diameter and zeta potential before and after encapsulation for fish biotechnology studies. Existing data show that the diameter of chitosan nanospheres before loading DNA mostly ranged from ~30 to ~230 nm, while encapsulation with plasmid DNA led to ~40–190 nm diameter increase. The zeta potential indicates the surface charge on the particles. A higher positive zeta potential suggests higher stability of nanoparticles in the suspension [143]. The zeta potential before loading plasmid DNA were ~25–33 mV, which mostly tended to decrease to ~14–18 mV. The exception was reported by Rather et al., who found that zeta potential of chitosan nanospheres increased ~6 mV following DNA encapsulation [144]. DNA encapsulation efficiency was generally higher than 80%, which indicates that chitosan is capable to load a high mass of DNA, which in turn may benefit many applications in aquaculture.summarizes chitosan–plasmid DNA encapsulation efficiency and changes in particle diameter and zeta potential before and after encapsulation for fish biotechnology studies. Existing data show that the diameter of chitosan nanospheres before loading DNA mostly ranged from ~30 to ~230 nm, while encapsulation with plasmid DNA led to ~40–190 nm diameter increase. The zeta potential indicates the surface charge on the particles. A higher positive zeta potential suggests higher stability of nanoparticles in the suspension [58]. The zeta potential before loading plasmid DNA were ~25–33 mV, which mostly tended to decrease to ~14–18 mV. The exception was reported by Rather et al., who found that zeta potential of chitosan nanospheres increased ~6 mV following DNA encapsulation [59]. DNA encapsulation efficiency was generally higher than 80%, which indicates that chitosan is capable to load a high mass of DNA, which in turn may benefit many applications in aquaculture.
Characteristics of chitosan–plasmid DNA polyplexes for studies performed in fish.
| Preloading Diameter (nm) | Postloading Diameter (nm) | Preloading Zeta Potential (mV) | Postloading Zeta Potential (mV) | Encapsulation Efficiency | References |
|---|---|---|---|---|---|
| - | <10,000 | - | - | 94.5% | [145][60] |
| 30–60 | - | - | - | - | [146][61] |
| - | 200 | - | - | 91.5% | [147][62] |
| - | - | - | - | 83.6% | [148][63] |
| 193 ± 53 1 | 246 ± 74 1 | 32.0 ± 1.0 1 | 14.4 ± 1.3 1 | - | [80][64] |
| - | 146 ± 2 2 | - | 24.3 ± 0.5 2 | 92.8% ± 1.4% 2 | [149][65] |
| - | 133 | - | 34.3 | 63% | [150][66] |
| - | 50-200 | - | - | 97.5% | [151][67] |
| 87 | 156 | 30.3 | 36.5 | 60% | [144][59] |
| - | 743 | - | - | 98.6% | [152][68] |
| 135 | - | 26.7 | - | 86% | [153][69] |
| - | - | - | - | 84.2% | [154][70] |
| 224 ± 62 1 | Similar to preloading diameter | 33.0 ± 1.2 1 | 14.4 ± 1.3 1 | - | [81][71] |
| - | 750–950 | - | - | 98.6% | [155][72] |
| 116 | 306 | 24.7 | 18.0 | - | [156][73] |
| 231 ± 18 2 | 272 ± 36 2 | 31.2 ± 1.5 2 | 14.1 ± 2.3 2 | - | [157][74] |
| - | 267 | - | 27.1 | 87.4% | [158][75] |
Mean ± SD;
2 mean ± SEM.mean ± SEM.
Chitosan-encapsulated DNA is more stable in vivo, exhibit sustained-release and increased cell uptake than naked DNA. Taken together, these factors confer chitosan-delivered DNA a particular expression profile regarding tissue distribution, persistence of expression and abundance in fish. Sáez et al. found that intramuscular injection led to a restricted expression to adjacent tissues of both chitosan-encapsulated DNA and naked DNA, while the oral administration of chitosan-encapsulated DNA, largely used for fish vaccination studies, showed enhanced expression not only in the intestine, but also in the liver of gilthead sea bream (
Sparus aurata) [152,155]. Furthermore, oral administration of chitosan nanoparticles loaded with pCMVβ, a plasmid encoding for) [68][72]. Furthermore, oral administration of chitosan nanoparticles loaded with pCMVβ, a plasmid encoding for
Escherichia coliβ-galactosidase, enabled sustained detection of the exogenous plasmid and bacterial β-galactosidase activity in the liver and the intestine of
Sparus aurata juveniles up to 60 days posttreatment [152].juveniles up to 60 days posttreatment [68].
Through the immersion route, Rao et al. showed that chitosan-coated DNA was confined to the surface area of rohu (
Labeo rohita), i.e., gill, intestine and skin-muscle, while no detection was observed in the kidney and the liver. Naked DNA was undetectable due to degradation [158]. Oral delivery seems to have a wider distribution of chitosan-encapsulated DNA, being found in the stomach, spleen, intestine, gill, muscle, liver, heart and kidney [148,154,159]. Chitosan-encapsulated DNA has longer and more abundant presence than naked DNA after administration. For example, Rajesh Kumar et al. showed that antibody in serum from fish immunized with a chitosan–DNA vaccine was 30% higher than naked DNA after 21 days of oral immunity [160]. The presence of DNA vaccine was reported more than 90 days after oral administration of chitosan–DNA [145]. Additionally, Rather et al. reported that chitosan–DNA induced 2-fold longer and higher peak abundant expression of downstream genes than naked DNA [144].), i.e., gill, intestine and skin-muscle, while no detection was observed in the kidney and the liver. Naked DNA was undetectable due to degradation [75]. Oral delivery seems to have a wider distribution of chitosan-encapsulated DNA, being found in the stomach, spleen, intestine, gill, muscle, liver, heart and kidney [63][70][76]. Chitosan-encapsulated DNA has longer and more abundant presence than naked DNA after administration. For example, Rajesh Kumar et al. showed that antibody in serum from fish immunized with a chitosan–DNA vaccine was 30% higher than naked DNA after 21 days of oral immunity [77]. The presence of DNA vaccine was reported more than 90 days after oral administration of chitosan–DNA [60]. Additionally, Rather et al. reported that chitosan–DNA induced 2-fold longer and higher peak abundant expression of downstream genes than naked DNA [59].
Chitosan-Based Applications in Fish Biotechnology and Gene Therapy
In recent years, chitosan has been increasingly used for drug and gene delivery in fish biotechnology. Most of the studies used chitosan-based systems to improve oral vaccination, control of gonadal development, and the modification of fish intermediary metabolism.
Fish Vaccination
DNA vaccines delivered by chitosan significantly increase relative percent survival of fish at a range of 45%–82% against bacterial and viral infection [67][73]. Higher doses of chitosan–DNA vaccines resulted in concomitant increase of fish relative percent survival from ~47% to ~70% [71]. In addition, DNA vaccination with chitosan stimulated expression of immune-related genes. Zheng et al. reported upregulation of the expression of immune-related genes, such as interferon-induced GTP-binding protein Mx2 (MX2), IFN, chemokine receptor (CXCR), T-cell receptor (TCR), MHC-Iα and MHC-IIα, 7 days after oral vaccination against reddish body iridovirus in turbot (
Scophthalmus maximus). A 10-fold higher expression of TNF-α gene expression was found in the hindgut [149].). A 10-fold higher expression of TNF-α gene expression was found in the hindgut [65].
In addition to the short-term modification of the expression levels of immune-related genes, the administration of chitosan–DNA vaccines also promote a sustained effect after treatment. Valero et al. found that European sea bass (
Dicentrarchus labrax) orally vaccinated with chitosan-encapsulated DNA against nodavirus failed to induce circulating IgM. However, the expression of genes involved in cell-mediated cytotoxicity (TCRβ and CD8α) and the interferon pathway (IFN, MX and IFN-γ) were upregulated. Three months following vaccination, challenged fish exhibited partial protection with retarded onset of fish death and lower cumulative mortality [151]. Kole et al. immunized rohu () orally vaccinated with chitosan-encapsulated DNA against nodavirus failed to induce circulating IgM. However, the expression of genes involved in cell-mediated cytotoxicity (TCRβ and CD8α) and the interferon pathway (IFN, MX and IFN-γ) were upregulated. Three months following vaccination, challenged fish exhibited partial protection with retarded onset of fish death and lower cumulative mortality [68]. Kole et al. immunized rohu (
Labeo rohita) with chitosan nanoparticles complexed with a bicistronic DNA plasmid encoding the antigen
Edwardsiella tardaglyceraldehyde 3-phosphate dehydrogenase and the immune adjuvant gene
Labeo rohita IFN-γ [156]. Follow-up of the expression of immune-related genes in the the kidney, liver and spleen showed maximal upregulation of IgHC (IgM heavy chain), iNOS, toll like receptor 22 (TLR22), nucleotide binding and oligomerization domain-1 (NOD1) and IL-1β at 14 days post immunization. The authors also confirmed that oral and immersion vaccination with chitosan–DNA nanoparticles enhances the fish immune response to a greater extent than intramuscular injection of naked DNA. In another study, the oral vaccination of rainbow trout fry with chitosan–TPP nanoparticles complexed with pcDNA3.1-VP2, showed that the expression of genes related with innate immune response, IFN-1 and MX, reached maximal values at 3 days postvaccination and 7 days after boosting (22 days postvaccination), while, with regard to genes involved in the adaptative immune response, CD4 peaked at 15 days postvaccination and IgM and IgT at 30 days postvaccination [154].IFN-γ [73]. Follow-up of the expression of immune-related genes in the the kidney, liver and spleen showed maximal upregulation of IgHC (IgM heavy chain), iNOS, toll like receptor 22 (TLR22), nucleotide binding and oligomerization domain-1 (NOD1) and IL-1β at 14 days post immunization. The authors also confirmed that oral and immersion vaccination with chitosan–DNA nanoparticles enhances the fish immune response to a greater extent than intramuscular injection of naked DNA. In another study, the oral vaccination of rainbow trout fry with chitosan–TPP nanoparticles complexed with pcDNA3.1-VP2, showed that the expression of genes related with innate immune response, IFN-1 and MX, reached maximal values at 3 days postvaccination and 7 days after boosting (22 days postvaccination), while, with regard to genes involved in the adaptative immune response, CD4 peaked at 15 days postvaccination and IgM and IgT at 30 days postvaccination [70].
Control of Gonadal Development
Chitosan nanoparticles have been used for drug delivery in studies aiming proper gonadal development in fish farming. Bhat et al. administered chitosan conjugated with salmon luteinizing hormone-releasing hormone (sLHRH) into walking catfish (
Clarias batrachus) to promote gonadal development. Chitosan-conjugated sLHRH and naked sLHRH exerted similar effects: upregulation of Sox9 expression in the gonads and increase of circulating steroid hormonal levels, testosterone and 11-ketotestosterone in males and testosterone and 17β-estradiol in females. However, sLHRH conjugation with chitosan induced sustained and controlled release of the hormones with maximal levels observed in the last sampling point of the experiment (36 h posttreatment), while naked sLHRH peaked circulating steroid hormones at 12 h posttreatment [150]. Similarly, compared to the administration of naked kisspeptin-10, intramuscular injection of chitosan-encapsulated kisspeptin-10 in immature female) to promote gonadal development. Chitosan-conjugated sLHRH and naked sLHRH exerted similar effects: upregulation of Sox9 expression in the gonads and increase of circulating steroid hormonal levels, testosterone and 11-ketotestosterone in males and testosterone and 17β-estradiol in females. However, sLHRH conjugation with chitosan induced sustained and controlled release of the hormones with maximal levels observed in the last sampling point of the experiment (36 h posttreatment), while naked sLHRH peaked circulating steroid hormones at 12 h posttreatment [66]. Similarly, compared to the administration of naked kisspeptin-10, intramuscular injection of chitosan-encapsulated kisspeptin-10 in immature female
Catla catla caused a delayed but greater increase of gonadotropin-releasing hormone, luteinizing hormone and follicle-stimulating hormone expression, as well as circulating levels of 11-ketotestosterone and 17β-estradiol [144].caused a delayed but greater increase of gonadotropin-releasing hormone, luteinizing hormone and follicle-stimulating hormone expression, as well as circulating levels of 11-ketotestosterone and 17β-estradiol [59].
With the ultimate goal of controlling gonadal development in fish, chitosan was also assayed for gene delivery. In walking catfish (
Clarias batrachus), intramuscular administration of chitosan nanoparticles conjugated with an expression plasmid encoding steroidogenic acute regulatory protein (StAR), a major regulator of steroidogenesis, also resulted in long-lasting stimulatory effects than administration of the naked plasmid construct on the expression of key genes in reproduction, cytochrome P450 (CYP) 11A1, CYP17A1, CYP19A1, 3β-hydroxysteroid dehydrogenase and 173β-hydroxysteroid dehydrogenase [153].), intramuscular administration of chitosan nanoparticles conjugated with an expression plasmid encoding steroidogenic acute regulatory protein (StAR), a major regulator of steroidogenesis, also resulted in long-lasting stimulatory effects than administration of the naked plasmid construct on the expression of key genes in reproduction, cytochrome P450 (CYP) 11A1, CYP17A1, CYP19A1, 3β-hydroxysteroid dehydrogenase and 173β-hydroxysteroid dehydrogenase [69].
Control of Fish Metabolism
Chitosan has been used for enhancing fish digestibility, the absorption of food constituents and increasing the utilization of dietary carbohydrate in carnivorous fish. To supplement exogenous proteolytic enzymes and thus facilitate protein digestion and amino acid absorption, Kumari et al. orally administered chitosan–TPP nanoparticles encapsulating trypsin to
Labeo rohita over 45 days. Treatment with chitosan–TPP–trypsin enhanced nutrient digestibility, intestinal protease activity and transamination activity, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), in the liver and the muscle [161].over 45 days. Treatment with chitosan–TPP–trypsin enhanced nutrient digestibility, intestinal protease activity and transamination activity, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), in the liver and the muscle [78].
The substitution of dietary protein by cheaper nutrients with reduced environmental impact in farmed fish is a challenging trend for sustainable aquaculture [79]. However, the metabolic features of fish, particularly carnivorous fish, constrain the replacement of dietary protein by other nutrients in aquafeeds. Carnivorous fish exhibit a preferential use of amino acids as fuel and gluconeogenic substrates, and thus require high levels of dietary protein for optimal growth. Instead, carbohydrates are metabolized markedly slower than in mammals, and give rise to prolonged hyperglycemia [80][81]. The essential role of the liver in controlling the intermediary metabolism makes this organ an ideal target for investigating and modifying the glucose tolerance of farmed fish.
To overcome metabolic limitations of carnivorous fish, in recent years we synthesized chitosan–TPP nanoparticles, complexed with plasmid DNA, to induce in vivo transient overexpression and the silencing of target genes in the liver of gilthead sea bream (
Sparus aurata). With the aim of decreasing the use of amino acids for gluconeogenic purposes and improving carbohydrate metabolism in the liver, chitosan–TPP nanoparticles complexed with a plasmid overexpressing a shRNA designed to silence the expression of cytosolic ALT (cALT) were intraperitoneally administered to
Sparus aurata juveniles. Seventy-two hours posttreatment, a significant decrease in cALT1 mRNA levels, immunodetectable ALT and ALT activity was observed in the liver of treated fish. Knockdown of cALT expression to ~63%–70% of the values observed in control fish significantly increased the hepatic activity of key enzymes in glycolysis, 6-phosphofructo 1-kinase (PFK1) and pyruvate kinase, and protein metabolism, glutamate dehydrogenase (GDH). In addition to showing efficient gene silencing after administration of chitosan–TPP–DNA nanoparticles, the findings supported evidence that the downregulation of liver transamination increased the use of dietary carbohydrates to obtain energy, and thus made it possible to spare protein in carnivorous fish [80].juveniles. Seventy-two hours posttreatment, a significant decrease in cALT1 mRNA levels, immunodetectable ALT and ALT activity was observed in the liver of treated fish. Knockdown of cALT expression to ~63%–70% of the values observed in control fish significantly increased the hepatic activity of key enzymes in glycolysis, 6-phosphofructo 1-kinase (PFK1) and pyruvate kinase, and protein metabolism, glutamate dehydrogenase (GDH). In addition to showing efficient gene silencing after administration of chitosan–TPP–DNA nanoparticles, the findings supported evidence that the downregulation of liver transamination increased the use of dietary carbohydrates to obtain energy, and thus made it possible to spare protein in carnivorous fish [64].
Following the same methodology, we showed that the shRNA-mediated knockdown of GDH significantly decreased GDH mRNA and immunodetectable levels in the liver, which, in turn, reduced GDH activity to ~53%. Downregulation of GDH decreased liver glutamate, glutamine and 2-oxoglutarate, as well as the hepatic activity of AST, while it increased 2-oxoglutarate dehydrogenase activity and the PFK1/fructose-1,6-bisphosphatase (FBP1) activity ratio. Therefore, by reducing hepatic transdeamination and gluconeogenesis, the knockdown of GDH could impair the use of amino acids as gluconeogenic substrates and facilitate the metabolic use of dietary carbohydrates [71].
With the aim of inducing a multigenic action leading to a stronger protein-sparing effect,
Sparus aurata were intraperitoneally injected with chitosan–TPP nanoparticles complexed with a plasmid expressing the N-terminal nuclear fragment of hamster SREBP1a, a transcription factor that—in addition to exhibiting strong transactivating capacity of genes required for fatty acid, triglycerides and cholesterol synthesis—previous reports showed can also transactivate the promoter of genes encoding key enzymes in hepatic glycolysis, glucokinase (GK) and 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase (PFKFB1) in fish [165,166]. Overexpression of exogenous SREBP1a in the liver ofwere intraperitoneally injected with chitosan–TPP nanoparticles complexed with a plasmid expressing the N-terminal nuclear fragment of hamster SREBP1a, a transcription factor that—in addition to exhibiting strong transactivating capacity of genes required for fatty acid, triglycerides and cholesterol synthesis—previous reports showed can also transactivate the promoter of genes encoding key enzymes in hepatic glycolysis, glucokinase (GK) and 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase (PFKFB1) in fish [82][83]. Overexpression of exogenous SREBP1a in the liver of
Sparus aurataenhanced the expression of glycolytic enzymes GK and PFKFB1, decreased the activity of the gluconeogenic enzyme FBP1 and increased the mRNA levels of key enzymes in fatty acid synthesis, elongation and desaturation (acetyl-CoA carboxylase 1, acetyl-CoA carboxylase 2, elongation of very long chain fatty acids protein 5, fatty acid desaturase 2), as well as induced NADPH formation (glucose 6-phophate dehydrogenase) and cholesterol synthesis (3-hydroxy-3-methylglutaryl-coenzyme A reductase). As a result, chitosan-mediated SREBP1a overexpression caused a multigenic action that enabled the conversion of dietary carbohydrates into lipids (
Figure 7), leading to increased circulating levels of triglycerides and cholesterol in carnivorous fish [157].1), leading to increased circulating levels of triglycerides and cholesterol in carnivorous fish [74].
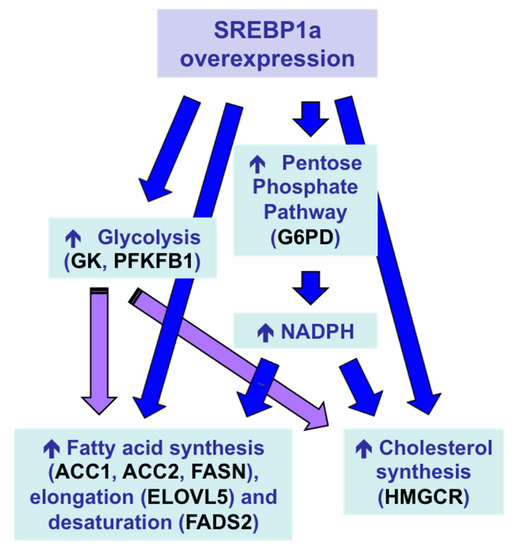
Multigenic action and metabolic effects in the liver of
Sparus aurata after intraperitoneal administration of chitosan–TPP–DNA nanoparticles to overexpress exogenous SREBP1a [157]. ACC1, acetyl-CoA carboxylase 1; ACC2, acetyl-CoA carboxylase 2; ELOVL5, elongation of very long chain fatty acids protein 5; FADS2, fatty acid desaturase 2; G6PD, glucose 6-phophate dehydrogenase; GK, glucokinase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; PFKFB1, 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase.after intraperitoneal administration of chitosan–TPP–DNA nanoparticles to overexpress exogenous SREBP1a [74]. ACC1, acetyl-CoA carboxylase 1; ACC2, acetyl-CoA carboxylase 2; ELOVL5, elongation of very long chain fatty acids protein 5; FADS2, fatty acid desaturase 2; G6PD, glucose 6-phophate dehydrogenase; GK, glucokinase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; PFKFB1, 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase.
References
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review. Res. Vet. Sci. 2019, 126, 68–82.
- Abdel-Ghany, H.M.; Salem, M.E. Effects of dietary chitosan supplementation on farmed fish; a review. Rev. Aquac. 2020, 12, 438–452.
- Kono, M.; Matsui, T.; Shimizu, C.; Effect of chitin, chitosan, and cellulose as deit supplements on the growth of cultured fish. Bull. Jpn. Soc. Sci. Fish. 1987, 53, 125–129.
- Shiau, S.-Y.; Yu, Y.-P.; Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus×O. aureus. Aquaculture 1999, 179, 439–446.
- Shengjun Wu; The growth performance, body composition and nonspecific immunity of Tilapia (Oreochromis niloticus) affected by chitosan. International Journal of Biological Macromolecules 2020, 145, 682-685, 10.1016/j.ijbiomac.2019.12.235.
- Sabreen E. Fadl; Ghada A. El-Gammal; Walied S. Abdo; Mohamed Barakat; Osama A. Sakr; Eldsokey Nassef; Doaa M. Gad; Hamdy S. El-Sheshtawy; Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia ( Oreochromis niloticus ) as well as the resistance to Streptococcus agalactiae infection. Aquaculture Research 2019, 51, 1120-1132, 10.1111/are.14458.
- Y. Chen; X. Zhu; Y. Yang; D. Han; J. Jin; S. Xie; Effect of dietary chitosan on growth performance, haematology, immune response, intestine morphology, intestine microbiota and disease resistance in gibel carp (Carassius auratus gibelio). Aquaculture Nutrition 2014, 20, 532-546, 10.1111/anu.12106.
- Heba S. El-Sayed; Khouloud M. Barakat; Effect of dietary chitosan on challenged Dicentrarchus labrax post larvae with Aeromonas hydrophila. Russian Journal of Marine Biology 2016, 42, 501-508, 10.1134/s1063074016060043.
- J. Yan; C. Guo; Mahmoud A. O. Dawood; J. Gao; Effects of dietary chitosan on growth, lipid metabolism, immune response and antioxidant-related gene expression inMisgurnus anguillicaudatus. Beneficial Microbes 2017, 8, 439-449, 10.3920/bm2016.0177.
- Jing Chen; Li Chen; Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnus anguillicadatus).. Carbohydrate Polymers 2019, 225, 115227, 10.1016/j.carbpol.2019.115227.
- Masume Kamali Najafabad; Mohammad Reza Imanpoor; Vahid Taghizadeh; Alireza Alishahi; Effect of dietary chitosan on growth performance, hematological parameters, intestinal histology and stress resistance of Caspian kutum (Rutilus frisii kutum Kamenskii, 1901) fingerlings. Fish Physiology and Biochemistry 2016, 42, 1063-1071, 10.1007/s10695-016-0197-3.
- Kalpa W. Samarakoon; Seon-Heui Cha; Ji-Hyeok Lee; You-Jin Jeon; The Growth, Innate Immunity and Protection against H2O2-Induced Oxidative Damage of a Chitosan-Coated Diet in the Olive Flounder Paralichthys olivaceus. Fisheries and aquatic sciences 2013, 16, 149-158, 10.5657/fas.2013.0149.
- Ritesh Ranjan; Kurcheti Pani Prasad; T Vani; Rajesh Kumar; Effect of dietary chitosan on haematology, innate immunity and disease resistance of Asian seabass Lates calcarifer (Bloch). Aquaculture Research 2012, 45, 983-993, 10.1111/are.12050.
- Shanthi Mari, L.S.; Jagruthi, C.; Anbazahan, S.M.; Yogeshwari, G.; Thirumurugan, R.; Arockiaraj, J.; Mariappan, P.; Balasundaram, C.; Harikrishnan, R. Protective effect of chitin and chitosan enriched diets on immunity and disease resistance in Cirrhina mrigala against Aphanomyces invadans. Fish Shellfish Immunol. 2014, 39, 378–385. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.-S.; Balasundaram, C.; Heo, M.-S. Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection. Aquaculture 2012, 326–329, 46–52. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.-S.; Balasundaram, C.; Heo, M.-S. Dietary supplementation with chitin and chitosan on haematology and innate immune response in Epinephelus bruneus against Philasterides dicentrarchi. Exp. Parasitol. 2012, 131, 116–124.
- Yanbo Wang; Jianrong Li; Effects of chitosan nanoparticles on survival, growth and meat quality of tilapia, Oreochromis nilotica. Nanotoxicology 2010, 5, 425-431, 10.3109/17435390.2010.530354.
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258.
- Abd El-Naby, F.S.; Naiel, M.A.E.; Al-Sagheer, A.A.; Negm, S.S. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2019, 501, 82–89.
- Naiel, M.A.E.; Ismael, N.E.M.; Abd El-hameed, S.A.A.; Amer, M.S.; The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture 2020, 523, 735219.
- Asmaa S. Abd El-Naby; Adham Al-Sagheer; Samar S. Negm; Mohammed A.E. Naiel; Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577, 10.1016/j.aquaculture.2019.734577.
- Gao, J.-Q.; Hu, Y.L.; Wang, Q.; Han, F.; Shao, J.Z. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359.
- Nikapitiya, C.; Dananjaya, S.H.S.; De Silva, B.C.J.; Heo, G.-J.; Oh, C.; De Zoysa, M.; Lee, J. Chitosan nanoparticles: A positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 76, 240–246.
- Qin, C.; Zhang, Y.; Liu, W.; Xu, L.; Yang, Y.; Zhou, Z. Effects of chito-oligosaccharides supplementation on growth performance, intestinal cytokine expression, autochthonous gut bacteria and disease resistance in hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Fish Shellfish Immunol. 2014, 40, 267–274.
- Gopalakannan, A.; Arul, V. Immunomodulatory effects of dietary intake of chitin, chitosan and levamisole on the immune system of Cyprinus carpio and control of Aeromonas hydrophila infection in ponds. Aquaculture 2006, 255, 179–187.
- Karlsen, Ø.; Amlund, H.; Berg, A.; Olsen, R.E. The effect of dietary chitin on growth and nutrient digestibility in farmed Atlantic cod, Atlantic salmon and Atlantic halibut. Aquac. Res. 2017, 48, 123–133.
- Shi-Mei Lin; Yu Jiang; Yong-Jun Chen; Li Luo; Sompong Doolgindachbaporn; Bundit Yuangsoi; Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish & Shellfish Immunology 2017, 70, 40-47, 10.1016/j.fsi.2017.08.035.
- Ngoc Duy Nguyen; Phu Van Dang; Anh Quoc Le; Thi Kim Lan Nguyen; Duy Hai Pham; Nguyen Van Nguyen; Quoc Hien Nguyen; Effect of oligochitosan and oligo-β-glucan supplementation on growth, innate immunity, and disease resistance of striped catfish (Pangasianodon hypophthalmus). Biotechnology and Applied Biochemistry 2017, 64, 564-571, 10.1002/bab.1513.
- Xiao Meng; Jiting Wang; Wenju Wan; Mengmeng Xu; Tingting Wang; Influence of low molecular weight chitooligosaccharides on growth performance and non-specific immune response in Nile tilapia Oreochromis niloticus. Aquaculture International 2017, 25, 1265-1277, 10.1007/s10499-017-0112-7.
- P. Su; Y. Han; C. Jiang; Y. Ma; J. Pan; S. Liu; T. Zhang; Effects of chitosan-oligosaccharides on growth performance, digestive enzyme and intestinal bacterial flora of tiger puffer (Takifugu rubripesTemminck et Schlegel, 1850). Journal of Applied Ichthyology 2017, 33, 458-467, 10.1111/jai.13282.
- Shimei Lin; Shuhong Mao; Yong Guan; Lin Luo; Li Luo; Yu Pan; Effects of dietary chitosan oligosaccharides and Bacillus coagulans on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Aquaculture 2012, 342, 36-41, 10.1016/j.aquaculture.2012.02.009.
- Lin, S.; Mao, S.; Guan, Y.; Lin, X.; Luo, L.; Dietary administration of chitooligosaccharides to enhance growth, innate immune response and disease resistance of Trachinotus ovatus. Fish Shellfish Immunol. 2012, 32, 909–913.
- Liu, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P.; Effects of chitooligosaccharides supplementation with different dosages, molecular weights and degrees of deacetylation on growth performance, innate immunity and hepatopancreas morphology in Pacific white shrimp (Litopenaeus vannamei). Carbohydr. Polym. 2019, 226, 115254.
- Lichun Liu; Yang Zhou; Xiaoheng Zhao; Hong Wang; Li Wang; Gailing Yuan; Muhammad Asim; Weimin Wang; Lingbing Zeng; Xiaoling Liu; et al.Li Lin Oligochitosan stimulated phagocytic activity of macrophages from blunt snout bream (Megalobrama amblycephala) associated with respiratory burst coupled with nitric oxide production. Developmental & Comparative Immunology 2014, 47, 17-24, 10.1016/j.dci.2014.06.005.
- Luo, L.; Cai, X.; He, C.; Xue, M.; Wu, X.; Cao, H.; Immune response, stress resistance and bacterial challenge in juvenile rainbow trouts Oncorhynchus mykiss fed diets containing chitosan-oligosaccharides. Curr. Zool. 2009, 55, 416–422.
- Fernández-Díaz, C.; Coste, O.; Malta, E. Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immunostimulant effect in Solea senegalensis macrophages. Algal Res. 2017, 26, 135–142.
- Wisdom, K.S.; Bhat, I.A.; Chanu, T.I.; Kumar, P.; Pathakota, G.-B.; Nayak, S.K.; Walke, P.; Sharma, R. Chitosan grafting onto single-walled carbon nanotubes increased their stability and reduced the toxicity in vivo (catfish) model. Int. J. Biol. Macromol. 2020, 155, 697–707.
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.R.; Farahmand, H.; Koshio, S.; Dorkoosh, F.A.; Elsabee, M.Z.; Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss)Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohydr. Polym. 2011, 86, 142–146.
- Eduardo Jiménez Fernández; Angels Ruyra; Nerea Roher; Eugenia Zuasti; Carlos Infante; Catalina Fernández-Díaz; Nanoparticles as a novel delivery system for vitamin C administration in aquaculture. Aquaculture 2014, 432, 426-433, 10.1016/j.aquaculture.2014.03.006.
- Bhat, I.A.; Nazir, M.I.; Ahmad, I.; Pathakota, G.-B.; Chanu, T.I.; Goswami, M.; Sundaray, J.K.; Sharma, R. Fabrication and characterization of chitosan conjugated eurycomanone nanoparticles: In vivo evaluation of the biodistribution and toxicity in fish. Int. J. Biol. Macromol. 2018, 112, 1093–1103.
- Wisdom, K.S.; Bhat, I.A.; Kumar, P.; Pathan, M.K.; Chanu, T.I.; Walke, P.; Sharma, R. Fabrication of chitosan nanoparticles loaded with aromatase inhibitors for the advancement of gonadal development in Clarias magur (Hamilton, 1822). Aquaculture 2018, 497, 125–133.
- Bhat, I.A.; Ahmad, I.; Mir, I.N.; Yousf, D.J.; Ganie, P.A.; Bhat, R.A.H.; Gireesh-Babu, P.; Sharma, R. Evaluation of the in vivo effect of chitosan conjugated eurycomanone nanoparticles on the reproductive response in female fish model. Aquaculture 2019, 510, 392–399.
- Bhat, I.A.; Ahmad, I.; Mir, I.N.; Bhat, R.A.H.; P, G.-B.; Goswami, M.; Sundaray, J.K.; Sharma, R. Chitosan-eurycomanone nanoformulation acts on steroidogenesis pathway genes to increase the reproduction rate in fish. J. Steroid Biochem. Mol. Biol. 2019, 185, 237–247.
- Khouloud M. Barakat; Heba S. El-Sayed; Yousry M. Gohar; Protective effect of squilla chitosan–silver nanoparticles for Dicentrarchus labrax larvae infected with Vibrio anguillarum. International Aquatic Research 2016, 8, 179-189, 10.1007/s40071-016-0133-2.
- R.M.C. Udayangani; S.H.S. Dananjaya; Chamilani Nikapitiya; Gang-Joon Heo; Jehee Lee; Mahanama De Zoysa; Metagenomics analysis of gut microbiota and immune modulation in zebrafish ( Danio rerio ) fed chitosan silver nanocomposites. Fish & Shellfish Immunology 2017, 66, 173-184, 10.1016/j.fsi.2017.05.018.
- Xia, I.F.; Cheung, J.S.; Wu, M.; Wong, K.-S.; Kong, H.-K.; Zheng, X.-T.; Wong, K.-H.; Kwok, K.W. Dietary chitosan-selenium nanoparticle (CTS-SeNP) enhance immunity and disease resistance in zebrafish. Fish Shellfish Immunol. 2019, 87, 449–459.
- Victor, H.; Zhao, B.; Mu, Y.; Dai, X.; Wen, Z.; Gao, Y.; Chu, Z. Effects of Se-chitosan on the growth performance and intestinal health of the loach Paramisgurnus dabryanus (Sauvage). Aquaculture 2019, 498, 263–270.
- Zhang, J.; Fu, X.; Zhang, Y.; Zhu, W.; Zhou, Y.; Yuan, G.; Liu, X.; Ai, T.; Zeng, L.; Su, J. Chitosan and anisodamine improve the immune efficacy of inactivated infectious spleen and kidney necrosis virus vaccine in Siniperca chuatsi. Fish Shellfish Immunol. 2019, 89, 52–60.
- Zhu, W.; Zhang, Y.; Zhang, J.; Yuan, G.; Liu, X.; Ai, T.; Su, J. Astragalus polysaccharides, chitosan and poly(I:C) obviously enhance inactivated Edwardsiella ictaluri vaccine potency in yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 2019, 87, 379–385.
- Xiaohong Liu; Hua Zhang; Yuan Gao; Yang Zhang; Haizhen Wu; Yuanxing Zhang; Efficacy of chitosan oligosaccharide as aquatic adjuvant administrated with a formalin-inactivated Vibrio anguillarum vaccine. Fish & Shellfish Immunology 2015, 47, 855-860, 10.1016/j.fsi.2015.10.012.
- Guangben Wei; Shuanghu Cai; Yuanzhi Wu; Shaohong Ma; Yucong Huang; Immune effect of Vibrio harveyi formalin-killed cells vaccine combined with chitosan oligosaccharide and astragalus polysaccharides in ♀Epinephelus fuscoguttatus×♂Epinephelus lanceolatus. Fish & Shellfish Immunology 2020, 98, 186-192, 10.1016/j.fsi.2020.01.015.
- Mostafa Halimi; Mojtaba Alishahi; Mohammadreza Abbaspour; Masoud Ghorbanpoor; Mohammad Reza Tabandeh; Valuable method for production of oral vaccine by using alginate and chitosan against Lactococcus garvieae/Streptococcus iniae in rainbow trout (Oncorhynchus mykiss). Fish & Shellfish Immunology 2019, 90, 431-439, 10.1016/j.fsi.2019.05.020.
- Sajal Kole; Syed Shariq Nazir Qadiri; Su-Mi Shin; Wi-Sik Kim; Jehee Lee; Sung-Ju Jung; Nanoencapsulation of inactivated-viral vaccine using chitosan nanoparticles: Evaluation of its protective efficacy and immune modulatory effects in olive flounder (Paralichthys olivaceus) against viral haemorrhagic septicaemia virus (VHSV) infection.. Fish & Shellfish Immunology 2019, 91, 136-147, 10.1016/j.fsi.2019.05.017.
- Julia Tandberg; Leidy Lagos; Erik Ropstad; Gro Smistad; Marianne Hiorth; Hanne C. Winther-Larsen; The Use of Chitosan-Coated Membrane Vesicles for Immunization Against Salmonid Rickettsial Septicemia in an Adult Zebrafish Model. Zebrafish 2018, 15, 372-381, 10.1089/zeb.2017.1556.
- Saurabh Dubey; Kiran Avadhani; Srinivas Mutalik; Sangeetha Madambithara Sivadasan; Biswajit Maiti; Shivani Kallappa Girisha; Moleyur Nagarajappa Venugopal; Stephen Mutoloki; Øystein Evensen; Indrani Karunasagar; et al.Hetron Mweemba Munang’Andu Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus). Vaccines 2016, 4, 40, 10.3390/vaccines4040040.
- Erlong Wang; Xingli Wang; Kaiyu Wang; Jie He; Ling Zhu; Yang He; Defang Chen; Ping Ouyang; Yi Geng; Xiaoli Huang; et al.Weimin Lai Preparation, characterization and evaluation of the immune effect of alginate/chitosan composite microspheres encapsulating recombinant protein of Streptococcus iniae designed for fish oral vaccination. Fish & Shellfish Immunology 2018, 73, 262-271, 10.1016/j.fsi.2017.12.034.
- Tong Chen; Yazhen Hu; Jiancheng Zhou; Shengbiao Hu; Xun Xiao; Xiaoling Liu; Jianguo Su; Gailing Yuan; Chitosan reduces the protective effects of IFN-γ2 on grass carp (Ctenopharyngodon idella) against Flavobacterium columnare infection due to excessive inflammation. Fish & Shellfish Immunology 2019, 95, 305-313, 10.1016/j.fsi.2019.10.034.
- Deepak Sharma; Dipika Maheshwari; Gilphy Philip; Ravish Rana; Shanu Bhatia; Manisha Singh; Reema Gabrani; Sanjeev Sharma; Javed Ali; Rakesh Kumar Sharma; et al.Shweta Dang Formulation and Optimization of Polymeric Nanoparticles for Intranasal Delivery of Lorazepam Using Box-Behnken Design: In Vitro and In Vivo Evaluation. BioMed Research International 2014, 2014, 1-14, 10.1155/2014/156010.
- M.A. Rather; I.A. Bhat; P. Gireesh-Babu; A. Chaudhari; J.K. Sundaray; Rupam Sharma; G.B. Pathakota; Molecular characterization of kisspeptin gene and effect of nano–encapsulted kisspeptin-10 on reproductive maturation in Catla catla. Domestic Animal Endocrinology 2016, 56, 36-47, 10.1016/j.domaniend.2016.01.005.
- Jiyuan Tian; Juan Yu; Xiuqin Sun; Chitosan microspheres as candidate plasmid vaccine carrier for oral immunisation of Japanese flounder (Paralichthys olivaceus). Veterinary Immunology and Immunopathology 2008, 126, 220-229, 10.1016/j.vetimm.2008.07.002.
- S. Vimal; G. Taju; K.S.N. Nambi; S. Abdul Majeed; V. Sarath Babu; M. Ravi; A. S. Sahul Hameed; Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquaculture 2012, 358, 14-22, 10.1016/j.aquaculture.2012.06.012.
- L Li; S-L Lin; Z-G Liu; L Deng; Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in black seabreamAcanthopagrus schlegeliiBleeker to protect fromVibrio parahaemolyticus. Journal of Fish Diseases 2013, 36, 987-995, 10.1111/jfd.12032.
- S. Vimal; S. Abdul Majeed; K.S.N. Nambi; N. Madan; M.A. Farook; C. Venkatesan; G. Taju; S. Venu; R. Subburaj; A.R. Thirunavukkarasu; et al.A. S. Sahul Hameed Delivery of DNA vaccine using chitosan–tripolyphosphate (CS/TPP) nanoparticles in Asian sea bass, Lates calcarifer (Bloch, 1790) for protection against nodavirus infection. Aquaculture 2014, 420, 240-246, 10.1016/j.aquaculture.2013.11.017.
- Juan D. González; Jonás Ismael Silva Marrero; Isidoro Metón; Albert Caballero-Solares; Ivan Viegas; Felipe Fernandez; Montserrat Miñarro; Anna Fàbregas; Josep R. Ticó; John G. Jones; et al.Isabel V. Baanante Chitosan-Mediated shRNA Knockdown of Cytosolic Alanine Aminotransferase Improves Hepatic Carbohydrate Metabolism. Marine Biotechnology 2015, 18, 85-97, 10.1007/s10126-015-9670-8.
- Fengrong Zheng; Hongzhan Liu; Xiuqin Sun; Yongqiang Zhang; Baiyu Zhang; Zhaojun Teng; Yongjiang Hou; Bo Wang; Development of oral DNA vaccine based on chitosan nanoparticles for the immunization against reddish body iridovirus in turbots ( Scophthalmus maximus ). Aquaculture 2016, 452, 263-271, 10.1016/j.aquaculture.2015.11.013.
- Irfan Ahmad Bhat; Mohd Ashraf Rather; Ratnadeep Saha; Gireesh Babu Pathakota; Annam Pavan-Kumar; Rupam Sharma; Anam Pavan Kumar; Expression analysis of Sox9 genes during annual reproductive cycles in gonads and after nanodelivery of LHRH in Clarias batrachus. Research in Veterinary Science 2016, 106, 100-106, 10.1016/j.rvsc.2016.03.022.
- Yulema Valero; Elham Awad; Francesco Buonocore; Marta Arizcun; M. Ángeles Esteban; J. Meseguer; Elena Chaves-Pozo; Alberto Cuesta; An oral chitosan DNA vaccine against nodavirus improves transcription of cell-mediated cytotoxicity and interferon genes in the European sea bass juveniles gut and survival upon infection. Developmental & Comparative Immunology 2016, 65, 64-72, 10.1016/j.dci.2016.06.021.
- M.I. Sáez; A.J. Vizcaíno; F.J. Alarcón; T.F. Martínez; Comparison of lacZ reporter gene expression in gilthead sea bream (Sparus aurata) following oral or intramuscular administration of plasmid DNA in chitosan nanoparticles. Aquaculture 2017, 474, 1-10, 10.1016/j.aquaculture.2017.03.023.
- Pravesh Kumar Rathor; Irfan Ahmad Bhat; Mohd Ashraf Rather; P. Gireesh-Babu; Kundan Kumar; Suresh Babu Padinhate Purayil; Rupam Sharma; Steroidogenic acute regulatory protein (StAR) gene expression construct: Development, nanodelivery and effect on reproduction in air-breathing catfish, Clarias batrachus. International Journal of Biological Macromolecules 2017, 104, 1082-1090, 10.1016/j.ijbiomac.2017.06.104.
- Sohrab Ahmadivand; Mehdi Soltani; Mahdi Behdani; Øystein Evensen; Ehsan Alirahimi; Reza Hassanzadeh; Ellahe Soltani; Oral DNA vaccines based on CS-TPP nanoparticles and alginate microparticles confer high protection against infectious pancreatic necrosis virus (IPNV) infection in trout. Developmental & Comparative Immunology 2017, 74, 178-189, 10.1016/j.dci.2017.05.004.
- Carlos Gaspar; Jonás Ismael Silva Marrero; Anna Fàbregas; Montserrat Miñarro; Josep R. Ticó; Isabel V. Baanante; Isidoro Metón; Administration of chitosan-tripolyphosphate-DNA nanoparticles to knockdown glutamate dehydrogenase expression impairs transdeamination and gluconeogenesis in the liver. Journal of Biotechnology 2018, 286, 5-13, 10.1016/j.jbiotec.2018.09.002.
- M.I. Sáez; A.J. Vizcaíno; F.J. Alarcón; T.F. Martínez; Feed pellets containing chitosan nanoparticles as plasmid DNA oral delivery system for fish: In vivo assessment in gilthead sea bream ( Sparus aurata ) juveniles. Fish & Shellfish Immunology 2018, 80, 458-466, 10.1016/j.fsi.2018.05.055.
- Sajal Kole; Ranjeeta Kumari; Deepika Anand; Saurav Kumar; Rupam Sharma; Gayatri Tripathi; M. Makesh; K.V. Rajendran; Megha Kadam Bedekar; Nanoconjugation of bicistronic DNA vaccine against Edwardsiella tarda using chitosan nanoparticles: Evaluation of its protective efficacy and immune modulatory effects in Labeo rohita vaccinated by different delivery routes. Vaccine 2018, 36, 2155-2165, 10.1016/j.vaccine.2018.02.099.
- Jonás Ismael Silva Marrero; Juliana Villasante; Ania Rashidpour; Mariana Palma; Anna Fàbregas; María Pilar Almajano; Ivan Viegas; John G. Jones; Montserrat Miñarro; Josep R. Ticó; et al.Isabel V. BaananteIsidoro Metón The Administration of Chitosan-Tripolyphosphate-DNA Nanoparticles to Express Exogenous SREBP1a Enhances Conversion of Dietary Carbohydrates into Lipids in the Liver of Sparus aurata.. Biomolecules 2019, 9, 297, 10.3390/biom9080297.
- B. Madhusudhana Rao; Sajal Kole; P. Gireesh-Babu; Rupam Sharma; Gayatri Tripathi; Megha Kadam Bedekar; Evaluation of persistence, bio-distribution and environmental transmission of chitosan/PLGA/pDNA vaccine complex against Edwardsiella tarda in Labeo rohita. Aquaculture 2019, 500, 385-392, 10.1016/j.aquaculture.2018.10.042.
- Erwin A. Ramos; Jenne Liza V. Relucio; Celia Aurora T. Torres-Villanueva; Gene Expression in Tilapia Following Oral Delivery of Chitosan-Encapsulated Plasmid DNA Incorporated into Fish Feeds. Marine Biotechnology 2005, 7, 89-94, 10.1007/s10126-004-3018-0.
- Parameswaran Vijayakumar; V.P. Ishaq Ahmed; V. Parameswaran; R. Sudhakaran; V. Sarath Babu; A. S. Sahul Hameed; Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio (Listonella) anguillarum. Fish & Shellfish Immunology 2008, 25, 47-56, 10.1016/j.fsi.2007.12.004.
- Rakhi Kumari; Subodh Gupta; Arvind R. Singh; Shajahan Ferosekhan; Dushyant Kothari; Asim Kumar Pal; Sanjay Balkrishna Jadhao; Chitosan Nanoencapsulated Exogenous Trypsin Biomimics Zymogen-Like Enzyme in Fish Gastrointestinal Tract. PLOS ONE 2013, 8, e74743, 10.1371/journal.pone.0074743.
- Rosamond L. Naylor; Ronald W. Hardy; Dominique Bureau; Alice Chiu; Matthew Elliott; Anthony P. Farrell; Ian Forster; Delbert M. Gatlin; Rebecca J. Goldburg; Katheline Hua; et al.Peter D. Nichols Feeding aquaculture in an era of finite resources. Proceedings of the National Academy of Sciences 2009, 106, 15103-15110, 10.1073/pnas.0905235106.
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B. 2012, 182, 1015–1045.
- Rashidpour, A.; Silva-Marrero, J.I.; Seguí, L.; Baanante, I.V.; Metón, I. Metformin counteracts glucose-dependent lipogenesis and impairs transdeamination in the liver of gilthead sea bream (Sparus aurata). Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R265–R273.
- Metón, I.; Egea, M.; Anemaet, I.G.; Fernández, F.; Baanante, I.V. Sterol regulatory element binding protein-1a transactivates 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene promoter. Endocrinology 2006, 147, 3446–3456.
- Egea, M.; Metón, I.; Córdoba, M.; Fernández, F.; Baanante, I.V. Role of Sp1 and SREBP-1a in the insulin-mediated regulation of glucokinase transcription in the liver of gilthead sea bream (Sparus aurata). Gen. Comp. Endocrinol. 2008, 155, 359–367.
