Silicon nitride is a ceramic material with unique properties. These properties strongly encourage the use of monolithic silicon nitride and coatings as contemporary and future biomaterial for a variety of medical applications including spinal, orthopedic and dental implants, bone grafts and scaffolds, platforms for intelligent synthetic neural circuits, antibacterial and antiviral particles and coatings, optical biosensors, and nano-photonic waveguides for sophisticated medical diagnostic devices.
- silicon nitride
- structure
- properties
- processing
- coatings
- spinal implants
- arthroplastic implants
- bone scaffolds
- dental implants
- neural circuits
- biosensors
- medical diagnostics
1. Introduction
Recently, increasing interest has been devoted to silicon nitride, an advanced ceramic material that excels by high compressive and flexural strengths, comparatively high fracture toughness, very low friction coefficient, strong corrosion resistance, improved medical imaging ability based on radiolucency in the visible and near-infrared region, enhanced biocompatibility and osseoconductivity, antimicrobial activity, and microengineering capability conducive to integrate electronics and mechanics together with computational, chemical and optical elements. Owing to these properties, silicon nitride is now emerging as a novel and promising bioceramic material for a multitude of medical applications. These applications include wear-resistant bearings for hip- and knee endoprosthetic implants, novel dental implants, spinal intervertebral spacers, tissue engineering scaffolds, antibacterial and antiviral coatings, wave guides for medical diagnostics, microtubes for intelligent neural circuits, micro-spectroscopic imaging devices, photonic ICs, optical biosensors, and others. As an additional bonus, most recently silicon nitride has been found to be a powerful solid-state bioceramic deactivator of single-stranded RNA (ssRNA) viruses including the SARS-CoV-2 virus [1]. Developments are underway to commercialize antimicrobial ‘catch-and-kill’ face masks comprising silicon nitride-coated polypropylene fibers to protect against the COVID-19 pandemic [2]. In light of all these beneficial properties, silicon nitride may be correctly considered as close to ideal bioceramics.
2. Biomedical Applications
2.1. Intervertebral Spacers
Spinal implants are used to alleviate many forms of back pain and deformity including trauma-based injuries and disorders such as scoliosis, kyphosis, degenerative disc disease, and fracture. Their primary function is to help to fuse two vertebrae together and to replace natural disc material. The four types of surgery that utilize spinal implants are anterior, posterior, transforaminal lumbar interbody, and axial fusions.
Silicon nitride has been in clinical use for porous intervertebral spacers in spinal fusion surgery for over thirty years, without showing any undesirable long-term effects [3][4][5][6][7]. Silicon nitride possesses high strength, long-term resilience, mechanically reliable, and osseoconductive properties, partly based on a high proportion of covalent bonds. To achieve interbody fusion, the ability of silicon nitride to yield superior new bone ingrowth and osseointegration, along with its proven bacteriostatic properties and enhanced medical imaging, outperforms both conventional poly(ether ether ketone, PEEK) and titanium implants [8][9]. This is largely based on its pronounced hydrophilicity that attracts extracellular fluid rich in bone growth-mediating non-collagenous proteins, turning on osteoblasts and suppressing osteoclasts. Consequently, silicon nitride speeds up bone healing, bone fusion and thus, implant integration. The efficacy of silicon nitride for spinal reconstruction is supported by its strong bacterial resistance caused by the synergetic effects of surface chemistry, surface pH, texture, and electric charge [6].
Anterior cervical discectomy with fusion is a commonly applied surgical procedure to treat radicular arm pain. A study compared silicon nitride implants with PEEK cages filled with autograft harvested from osteophytes [10]. Patients treated with either silicon nitride or PEEK implants reported comparable recovery rates during follow-up interviews. No significant differences in clinical outcome were noticed at 24 months observation time. Fusion rates improved over time and were comparable between both groups. Figure 1 shows the Valeo CCsC® (SINTX Technologies, Salt Lake City, UT) silicon nitride cervical interbody fusion device used in the CASCADE trial with radiographic characteristics. The outer part of the device consists of dense silicon nitride, whereas the inset comprises cancellous structured ceramic (CSC) of the same composition, providing a scaffold for easy bone ingrowth [11].
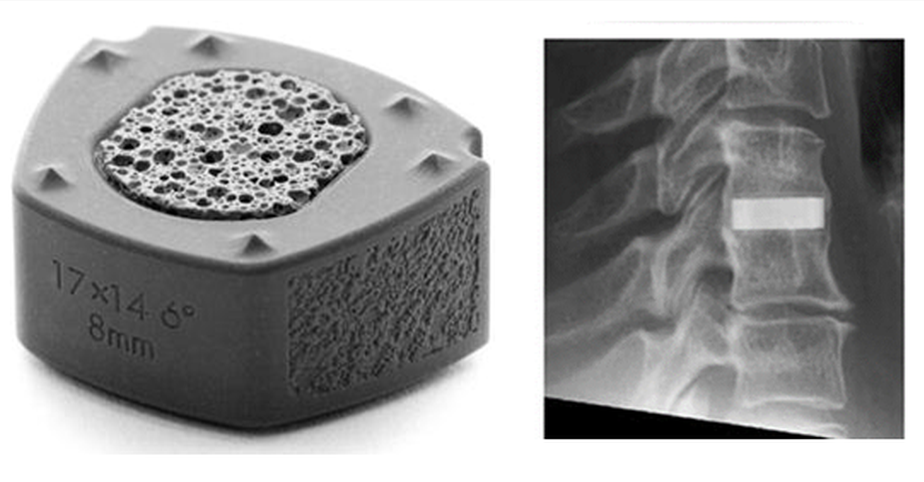 Figure 1. Silicon nitride cervical interbody fusion device (Valeo CCsC ®, left) with the radiographic image of the implanted device (right) [10]. © Creative Commons Attribution 4.0 International License.
Figure 1. Silicon nitride cervical interbody fusion device (Valeo CCsC ®, left) with the radiographic image of the implanted device (right) [10]. © Creative Commons Attribution 4.0 International License.
2.2. Knee- and Hip Endoprosthetic Implants
Silicon nitride compacts can be polished to a high surface finish to provide an exceptionally smooth, wear-resistant, and tribological superior surface. This renders the material ideal for articulating applications including bearings for knee and hip endoprostheses [12][13][14] as well as acetabular cups. Salient materials properties conducive to supporting bearing applications are phase stability [15], wear resistance [15][16], strength and fracture toughness [17], hydrophilicity [18], favorable medical imaging capability [6], and unsurpassed bacterial resistance [8][19]. Figure 21 shows a selection of silicon nitride compacts produced by SINTX Technologies of Salt Lake City, UT, USA, the only vertically integrated silicon nitride medical device manufacturer worldwide [20].

Figure 21. Various silicon nitride compacts produced by SINTX Technologies, Salt Lake City, UT, including ball bearings (left), an acetabular cup (center), an intervertebral spacer with a CSC core (foreground, center), and spinal interbody fusion devices (right) [20]. © Creative Commons Attribution 4.0 International License.
2.3. Bone Grafts and Scaffolds
Bone grafting is a surgical procedure that uses transplanted bone to repair and rebuild diseased or damaged bones. In the clinical practice, bone grafts present limitations that include harvesting morbidity, insufficient bioactivity, and concern about the transmission of diseases [5]. Silicon nitride bone scaffolds and bone fusion devices [21] excel by high and reliable mechanical strength, biocompatibility, and antibiotic capability, resulting in a bone healing sequence comparable to hydroxylapatite [22]. However, there is a caveat. Using silicon nitride for permanent scaffolds is counter-indicated by its apparent lack of in vivo resorbability, a necessary and sufficient requirement for the replacement of implant material by natural bone. In contrast, non-stoichiometric SiNx coatings very slowly dissolve in simulated body fluid at a rate of about 0.3 nm/day [23].
MC3T3-E1 cells were used to study the osteoblastic differentiation and mineralization on sterile samples of silicon nitride, and compared them to samples of titanium and PEEK, standard materials for bone scaffolds [24]. The study reported a more profound and faster ECM deposition and mineralization on Si3N4 surface as compared to titanium and PEEK. Results further indicated the upregulation of osteogenic transcription factors such as RUNX2 and osterix (SP7), as well as collagen type I and osteocalcin. Hence, silicon nitride rapidly conducts mineralized tissue formation via extracellular matrix deposition and biomarker expression in murine calvarial pre-osteoblast cells.
2.4. Dental Implants
Silicon nitride is an emerging material for dental restoration and dental implant application. In addition to its excellent structural, mechanical, thermal, tribological and biocompatible properties, silicon nitride is a potent antibacterial material. Dental implants made from silicon nitride are thought to exhibit favorable mechanical properties compared to classic titanium alloy implants [25]. Hence, in view of scant available pre-clinical data, silicon nitride appears to have the essential characteristics to be a strong candidate for dental implants’ material. This novel ceramic has a surface with potentially antimicrobial properties, and if this is confirmed in future research, it could be of great interest for oral use [26][27]. However, at this point in time, there are no clinical studies and trials are yet to confirm the positive changes silicon nitride may elicit as a dental implant material. Further research and clinical trials into the in vivo performance of antibacterial surface coatings such as enhancing bone regeneration around the implant surface or reducing the risk of periimplantitis [25].
An earlier study [28] investigated the potential use of porous silicon nitride for all-ceramic dental restorations as a core material and results were compared with those of a commercial ZrO2 ceramic. The color of silicon nitride could be tailored by the porosity introduced and a color shade suitable for restorative applications was obtained. The flexural strength of Si3N4 was found to be 418 MPa despite an open porosity of nearly 10.5%. This rather high strength may be related to the strong neck formation between β-Si3N4 grains, the intertwined distribution of these grains, and the crack deflection potential of rod-like β-Si3N4. The Vickers hardness of Si3N4 was found to be 10.9 MPa whereas ZrO2 had 13.7 MPa, which reduces the risk of the wear of opposing natural teeth. Shear bond strength to dentin was tested and indicated low values for silicon nitride without a coupling agent (2.24 ± 1.15 MPa), which strongly increased by adding a silane coupling agent to 8.44 ± 2.98 MPa, suggesting that the usage of coupling agents for Si3N4 is essential. The radiolucent behavior of Si3N4 will enable for both the restorations and the surrounding tissues to be imaged using plain radiography (Figure 32). Hence, this study shows that with the tailored manufacturing methods including additive manufacturing, silicon nitride can be considered an effective dental restorative material.
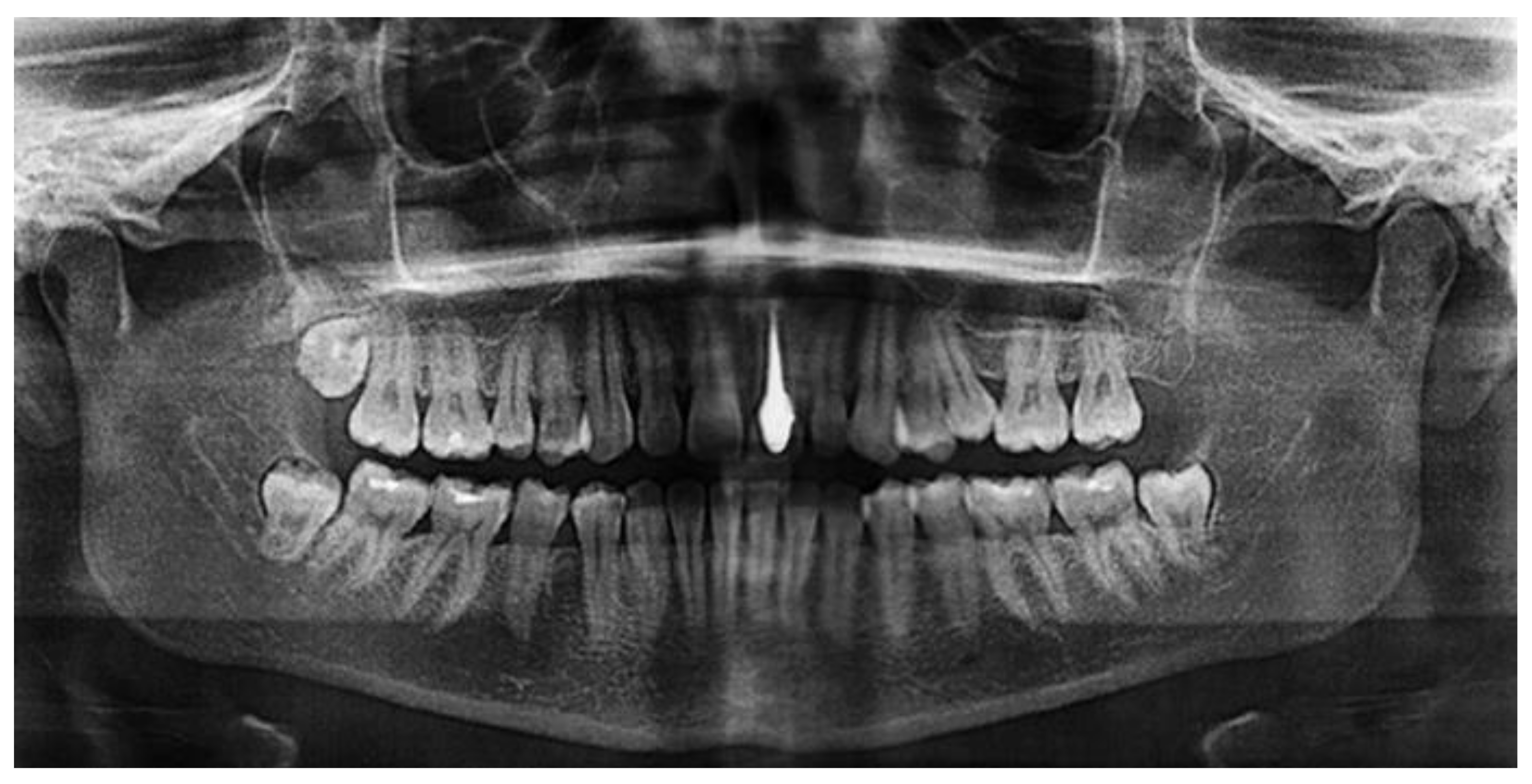
Figure 32. Silicon nitride as a material for dental core restoration, revealing radiolucency [29]. © Creative Commons Attribution 4.0 International License.
2.5. Intelligent Synthetic Neural Circuits
Synthetic regulatory circuits encoded in RNA rather than DNA could provide a means to control cell behavior while avoiding potentially harmful genomic integration in therapeutic applications. Post-transcriptional circuits have been developed using RNA-binding proteins that can be wired in a plug-and-play fashion to create networks of higher complexity [30]. Application of this research in the realm of future information technology include signal transmission platforms for artificial intelligence (AI) algorithms with unparalleled computing speed based on novel synthetic neural circuits.
A neuron cell culturing platform consisting of arrays of ordered silicon nitride microtubes (2.7–4.4 μm in diameter) was formed by strain-induced self-rolled-up nano-membrane (s-RUM) technology using ultrathin (<40 nm) silicon nitride films deposited on transparent substrates such as glass [31]. These microtubes presented strong guidance for fast growing primary cortical neurons, with a coaxially confined configuration resembling that of myelin sheaths. Neurons revealed a dramatically enhanced growth rate inside the microtube compared to regions outside the microtubes. The acceleration and precise guidance provided by the silicon nitride microtube array was attributed to 3D-adhesion and electrostatic interaction with the silicon nitride microtubes, respectively. The microtubes not only provide structure for the neural network and guiding connections, but also accelerate the growth of axons, an important feature as time is crucial for restoring severed connections in the case of spinal cord injury or limb reattachment. A time-lapse sequence of a cortical neuron rapidly growing from inside a microtube through neighboring microtubes can be watched on the Internet [32].
2.6. Antibacterial and Antiviral Particles and Coatings
The efficacy of silicon nitride as a ‘killer ceramics’ for Gram-negative bacteria such as Porphyromonas gingivalis was found to be the result of chemically-driven mechanisms related to the peculiar pH-dependent surface chemistry of silicon nitride [19]. In an alkaline pH environment, a buffering effect controlled by the release of ammonium (NH4+) ions causes the lysis of bacterial proteins as confirmed by conventional fluorescence and in situ Raman microprobe spectroscopies. The formation of peroxinitrite within the bacteria leads to the degradation of nucleic acid, and reductions in phenylalanine content and liquid concentration were observed. Experiments confirmed that the modification of the surface chemistry of silicon nitride by chemical etching or thermal oxidation influenced the peroxinitrite formation and thus, affected bacteria metabolism.
Polyethylene coated by pulsed laser technique with silicon nitride powder in a nitrogen gas atmosphere showed antibacterial properties in vitro against S. epidermidis. The coating sensibly reduced the amount of living bacteria when compared to the uncoated polymer. Osteoconductivity was also tested in vitro using SaOS-2 osteosarcoma cells. The presence of the silicon nitride coating resulted in an increased amount of hydroxyapatite deposited from extracellular fluid (ECF). Hence, the coating of polyethylene with silicon nitride may lead to the improved performance of orthopedic endoprosthetic medical devices [33].
2.7. Medical Diagnostics
Cellular sampling and characterization include the culture, differentiation and fixing of cells on silicon nitride substrates for imaging and mapping across the electromagnetic spectrum, from X-ray fluorescence (XRF) to X-ray absorption near edge spectroscopy (XANES) to visible and infrared microspectroscopies [34]. Cells were found to adhere strongly to silicon nitride surfaces, allowing for investigation of visually displayed proliferative and phenotypic growth.
Lasers for medical application operate in a wide range of the electromagnetic spectrum, from X-ray up to UV, continue in the visible and near-infrared to mid-IR. These electromagnetic waves need to be transmitted from the laser source to the target tissue by a flexible device, called a waveguide, which will enable the easy manipulation of the laser beam in a medical diagnostic setting [35]. Novel integrated nano-photonic sensing devices operating in the visible and near-infrared regions with drastically reduced propagation losses were developed based on silicon nitride waveguides [36]. Silicon nitride is frequently chosen as the passive waveguide material due to its several advantages compared to silicon, including higher transparency at wavelengths below 1.1 μm, ultra-low two-photon absorption effect at telecommunication wavelengths, and ultimately, smaller propagation losses. These properties have made silicon nitride a widely used platform in silicon-integrated photonic applications [37].
References
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Zhu, W.; Marin, E.; McEntire, B.J.; Bal, B.S.; Mazda, O. Silicon nitride: A potent solid-state bioceramic inactivator of ssRNA viruses. Sci. Rep. 2021, 11, 2977.
- Spine Firm Jumps into Virus-Killing Mask Market. Available online: (accessed on 13 March 2021).
- Sorrell, C.; Hardcastle, P.H.; Druitt, R.K.; Howlett, C.R.; McCartney, E.R. Results of 15-years clinical study of reaction-bonded silicon nitride intervertebral spacers. In Proceedings of the 7th World Biomaterial Congress, Sydney, Australia, 17–21 May 2004; Australian Society for Biomaterials: Sydney, Australia, 2004; p. 1872.
- Guedes e Silva, C.C.; König, B., Jr.; Carbonari, M.J.; Yoshimoto, M.; Allegrini, S., Jr.; Bressiani, J.C. Bone growth around silicon nitride implants—An evaluation by scanning electron microscopy. Mater. Character. 2008, 59, 1339–1341.
- Anderson, M.C.; Olsen, R. Bone ingrowth into porous silicon nitride. J. Biomed. Mater. Res. Part A 2009, 92, 1598–1605.
- Anderson, M.; Bernero, J.; Brodke, D. Medical Imaging Characteristics of Silicon Nitride Ceramic: A New Material for Spinal Arthroplasty Implants. In Proceedings of the 8th Annual Spine Arthroplasty Society Glob. Symposium, Miami Beach, FL, USA, 6–9 May 2008; p. 547.
- Taylor, R.M.; Bernero, J.P.; Patel, A.A.; Brodke, D.S.; Khandkar, A.C. Silicon nitride—A new material for spinal implants. J. Bone Jt. Surg. 2010, 92 (Suppl. 1), 133.
- Bal, B.S.; Gorth, D.J.; Puckett, S.; Webster, T.J.; Rahaman, M.; Ercan, B. Decreased bacteria activity on Si3N4 surfaces compared with PEEK or titanium. Int. J. Nanomed. 2012, 7, 4829–4840.
- Webster, T.; Patel, A.; Rahaman, M.; Bal, B.S. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454.
- Arts, M.P.; Wolfs, J.F.C.; Corbin, T.P. Porous silicon nitride spacers versus PEEK cages for anterior cervical discectomy and fusion: Clinical and radiological results of a single-blinded randomized controlled trial. Eur. Spine J. 2017, 26, 2372–2379.
- Arts, M.P.; Wolfs, J.F.; Corbin, T.P. The CASCADE trial: Effectiveness of ceramic versus PEEK cages for anterior cervical discectomy with interbody fusion; protocol of a blinded randomized controlled trial. BMC Musculoskelet. Disord. 2013, 14, 244.
- Zhou, Y.; Ohashi, M.; Tomita, N.; Ikeuchi, K.; Takashima, K. Study on the possibility of silicon nitride—Silicon nitride as a material for hip prostheses. Mater. Sci. Eng. C 1997, 5, 125–129.
- Mazzocchi, M.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part I: Processing, microstructure, mechanical properties, cytotoxicity. J. Mater. Sci. Mater. Med. 2008, 19, 2881–2887.
- Mazzocchi, M.; Gardini, D.; Traverso, P.L.; Faga, M.G.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part II: Chemical stability and wear resistance in body environment. J. Mater. Sci. Mater. Med. 2008, 19, 2889–2901.
- Bal, B.S.; Khandkar, A.; Lakshminarayanan, R.; Clarke, I.; Hoffman, A.A.; Rahaman, M.N. Testing of silicon nitride ceramic bearings for total hip arthroplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 447–454.
- McEntire, B.J.; Bal, B.S.; Lakshminarayanan, R.; Bock, R. Silicon nitride bearings for total joint arthroplasty. Bone Joint J. 2016, 98 (Suppl. 1), 34.
- Bal, B.; Rahaman, M. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898.
- Bock, R.M.; McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Boffelli, M.; Pezzotti, G. Surface modulation of silicon nitride ceramics for orthopaedic applications. Acta Biomater. 2015, 26, 318–330.
- Pezzotti, G.; Bock, R.M.; McEntire, B.J.; Jones, E.; Boffelli, M.; Zhu, W.; Baggio, G.; Boschetto, F.; Puppulin, L.; Adachi, T.; et al. Silicon Nitride Bioceramics Induce Chemically Driven Lysis inPorphyromonas gingivalis. Langmuir 2016, 32, 3024–3035.
- The Story of Silicon Nitride. Orthopaedic Product News. Available online: (accessed on 19 March 2021).
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1329–1343.
- Pezzotti, G.; McEntire, B.J.; Bock, R.; Boffelli, M.; Zhu, W.; Vitale, E.; Puppulin, L.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Silicon Nitride: A Synthetic Mineral for Vertebrate Biology. Sci. Rep. 2016, 6, 31717.
- Petterson, M. Silicon Nitride for Total Hip Replacement. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2015.
- Ahuja, N.; Awad, K.R.; Brotto, M.; Aswath, P.B.; Varanasi, V. A comparative study on silicon nitride, titanium and polyether ether ketone on mouse pre-osteoblast cells. Med. Devices Sens. 2020.
- Raza, S.M.; Khurshid, Z.; Zafar, M.S.; Najeeb, S.; Ul Yaqin, S.A. Silicon nitride (SiN): An emerging material for dental implant applications. In Dental Implants: Materials, Coatings, Surface Modifications and Interfaces with Oral Tissue; Zafar, M.S., Khurshid, Z., Khan, A.S., Najeeb, S., Sefat, F., Eds.; Series in Biomaterials; Woodhead Publ. Ltd.: Sawston, Cambridge, UK, 2020; pp. 287–299.
- Badran, Z.; Struillou, X.; Hughes, F.J.; Soueidan, A.; Hoornaert, A.; Ide, M. Silicon Nitride (Si3N4) Implants: The Future of Dental Implantology? J. Oral Implant. 2017, 43, 240–244.
- Özdoğan, M.S.; Güngörmüş, M.; Çelik, A.; Topateş, G. Silicon nitride ceramic for all-ceramic dental restorations. Dent. Mater. J. 2020, 39, 1080–1086.
- Wananuruksawong, R.; Jinawath, S.; Padipatvuthikul, P.; Wasanapiarnpong, T. Fabrication of Silicon Nitride Dental Core Ceramics with Borosilicate Veneering material. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 192010.
- Is silicon Nitride a Superior Biomaterial? Available online: (accessed on 22 March 2021).
- Wroblewska, L.; Kitada, T.; Endo, K.; Siciliano, V.; Stillo, B.; Saito, H.; Weiss, R. Mammalian synthetic circuits with RNA binding proteins for RNA-only delivery. Nat. Biotechnol. 2015, 33, 839–841.
- Froeter, P.; Huang, Y.; Cangellaris, O.V.; Huang, W.; Dent, E.W.; Gillette, M.U.; Williams, J.C.; Li, X. Toward Intelligent Synthetic Neural Circuits: Directing and Accelerating Neuron Cell Growth by Self-Rolled-Up Silicon Nitride Microtube Array. ACS Nano 2014, 8, 11108–11117.
- Microtubes Create Cozy Space for Neurons to Grow, and Grow Fast. Available online: (accessed on 20 March 2012).
- Zanocco, M.; Marin, E.; Boschetto, F.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Zhu, W.; McEntire, B.J.; Bal, B.S.; Ashida, R.; et al. Surface Functionalization of Polyethylene by Silicon Nitride Laser Cladding. Appl. Sci. 2020, 10, 2612.
- Carter, E.A.; Rayner, B.S.; McLeod, A.I.; Wu, L.E.; Marshall, C.P.; Levina, A.; Aitken, J.B.; Witting, P.K.; Lai, B.; Cai, Z.; et al. Silicon nitride as a versatile growth substrate for microspectroscopic imaging and mapping of individual cells. Mol. BioSyst. 2010, 6, 1316–1322.
- Gannot, I.; Ben-David, M. Optical fibers and waveguides for medical applications. In Biomedical Photonics Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 229–251.
- Hainberger, R.; Muellner, P.; Melni, E.; Mutinati, G.; Eggeling, M.; Maese-Novo, A.; Vogelbacher, F.; Kraft, J.; Koppitsch, G.; Meinhardt, G.; et al. Silicon nitride waveguide integration platform for medical diagnostic application. In Proceedings of the 2016 Progress in Electromagnetic Research Symposium, Shanghai, China, 8–11 August 2016; p. 781.
- Rönn, J.; Zhang, W.; Autere, A.; Leroux, X.; Pakarinen, L.; Alonso-Ramos, C.; Säynätjoki, A.; Lipsanen, H.; Vivien, L.; Cassan, E.; et al. Ultra-high on-chip optical gain in erbium-based hybrid slot waveguides. Nat. Commun. 2019, 10, 432.
