You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lily Guo and Version 1 by Luisa Silva.
Familial hypercholesterolaemia (FH) is a common inherited cause of premature cardiovascular disease, but the majority of patients remain undiagnosed.
- familial hypercholesterolaemia
- primary care
- genetics
1. Introduction
Familial hypercholesterolaemia (FH) is an autosomal-dominant disease and has long been recognized as a cause of premature coronary heart disease (CHD) [1]. It is associated with mutations in four genes: low-density lipoprotein (LDL) receptor, apolipoprotein B (Apo B), proprotein convertase subtilin/kexin 9 (PCSK9) and low-density lipoprotein receptor adaptor protein (LDLRAP) [2]. The majority of people with FH have the heterozygous form, with an estimated prevalence up to 1 in 200 [1,3][1][3]. In the most recent studies, compared to the general population, these patients have around a thirteen-fold increase in CHD mortality [1,4][1][4]. FH can also be inherited in an homozygous form, albeit much more rarely, with an estimated prevalence ranging from 1 in 160,000 to 1 in 1,000,000 individuals [2]. Further, a rarer autosomal-recessive form of the disease also exists [2]. Lipid-lowering treatment reduces CHD mortality by 44% [5]. However, up to 80% of individuals with FH remain undiagnosed and therefore untreated, resulting in major opportunities to prevent premature heart disease [1,3,6][1][3][6].
Several national guidelines on identifying FH have been published [7,8,9,10,11,12,13][7][8][9][10][11][12][13]. In these guidelines, confirmation of FH diagnosis involves assessment against one or more specified diagnostic criteria, such as the SimonBroome (SB) criteria [11], the USMedPed criteria [13], the Dutch Lipid Clinic Network (DLCN) criteria [14,15[14][15][16],16], and the Japanese criteria [8].
Currently, individuals with FH are found incidentally in usual practice. It has been suggested that a more systematic approach may help to identify more individuals in the non-specialist setting [15,17,18][15][17][18].
2. Study Selection
Two authors independently screened the titles and abstracts of the studies identified from the searches. The full texts of the potentially eligible studies were also screened independently by two different authors, and reasons for exclusion at the full text stage were noted. Any disagreements were resolved through discussion, or where necessary, with the assistance of a third author.
3. Selection of Articles
The search identified a total of 4638 citations, of which 32 citations were identified as potentially eligible based on title and abstract screening, representing 29 studies. Following full-text screening, three studies were eligible for inclusion into the review [27,28,29][19][20][21] (Figure 1: PRISMA diagram). Thus, 25 studies were excluded from the review at the full text stage. The reasons for exclusion were no baseline data of usual care (17 studies [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38]), ineligible participants (7 studies [47,48,49,50,51,52,53][39][40][41][42][43][44][45]) and ineligible condition (1 study [54][46]). There was also one ongoing study [55][47]. Details on the 17 studies excluded based on study design (no baseline data of usual care) are included as Supplementary Materials Table S2.
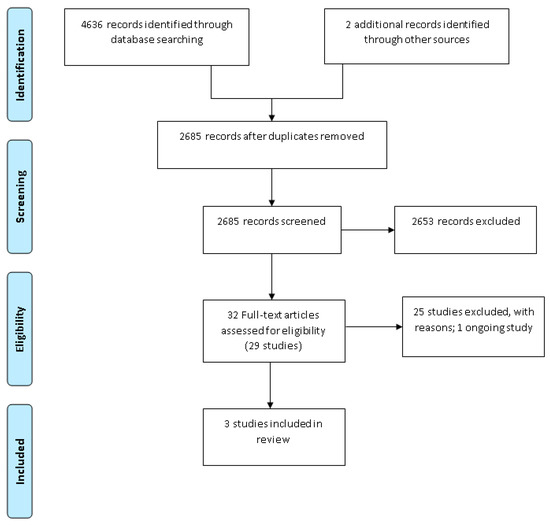

Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) diagram.
4. Characteristics of Included Studies
The three included studies were published between 2013 and 2018 (basic details in Table 1, with full details in Supplementary Materials Table S3). Two studies [28,29][20][21] were carried out in the United Kingdom, and the remaining study [27][19] was conducted in Australia. All three primary care studies used an uncontrolled before-and-after study design. A total of 281,869 participants were involved in the studies, although the vast majority of participants were from one study [28][20]. The other two studies included a relatively small number of participants: 96 [27][19] and 118 [29][21].
Table 1. Summary of included studies.
| udy and Year | Design/ Setting |
Participants | Intervention | Outcomes | Comparisons | Main Results | ||
|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Absolute Difference (95% CI), n |
||||||
| Bell et al., 2013 | Uncontrolled BA study General practices in Western Australia |
96 Patients Gender: Female 68 (70.9%), Male 28 (29.1%) Age (years): mean ± SD [range]: 53.7 ± 10.7 [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,][25][26][27][28][29][30][31][32][33][34][35][52,[53,54,49][55,19][[38][39][40][41][42][43][44][45][56,36][57][48]20][21][22][23][2437]46][47][50][51] |
Interpretative comments added to lipid results | -FH diagnosis (Modified DLCN criteria) -LDL-c level -Referral to specialist |
No comments added to lipid results (standard/usual care) | Definite FH: 0/96 (0%) Possible FH: 0/96 (0%) |
Definite FH: 2/96 (2.08%) Possible FH: 2/96 (2.08%) |
Definite FH: 2.08% (–2.05 to 7.28%), n = 96 Possible FH: 2.08% (–2.05 to 7.28%), n = 96 |
| Green et al., 2016 | Uncontrolled BA study with two sequential interventions. General practices in South East England |
Approximately 290,000 patients Gender: not reported Age: In 2011, 37,200 people were aged >65 years and 4400 aged >85 years |
1: computer based reminder message 2: FH Nurse Advisor Programme—2-part process. Part 1 as above; Part 2 involved consultation with nurse to collect further information |
-FH diagnosis (Baseline: S-B; Post-interv: S-B and/or DLCN criteria) | Baseline prevalence of FH | EHR Search and reminder | ||
| Definite FH: 331/262,030 (0.13%) Possible FH: 12/262,030 (0.005%) |
Definite FH 354/199,346 (0.18%) Possible FH: 88/199,346 (0.04%) |
Definite FH: 0.05% (0.03 to 0.07%), n = 262,030 Possible FH: 0.04% (–0.03 to 0.05%), n = 262,030 |
||||||
| EHR search and reminder + nurse intervention | ||||||||
| Definite FH: 331/262,030 (0.13%) Possible FH: 12/262,030 (0.005%) |
Definite FH: 546/281,655 (0.19%) Possible FH: 147/281,655 (0.05%) |
Definite FH: 0.07% (0.05 to 0.09%), n = 262,030 Possible FH: 0.05% (0.04 to 0.06%), n = 262,030 |
||||||
| Weng et al., 2018 | Uncontrolled BA study Six General Practices in Central England |
831 identified, 118 patients medical records accessed Gender: Female 46 (39%), Male 72 (61%) Age (years) mean (SD): male 58 (9.0), female 56 (7.5) |
Combined approach: Opportunistic recruitment following computer-based reminder message with systematic postal recruitment of eligible patients | -FH diagnosis (S-B criteria) -Cholesterol -Statins prescribed |
Same 118 participants with Cholesterol ≥ 7.5 mmol/L after the release of the NICE FH guidelines | Definite FH: 0/118 (0%) Possible FH: 0/118 (0%) |
Definite FH: 2/118 (1.69%) Possible FH: 30/118 (25.42%) |
Definite FH: 1.69% (–1.69 to 5.97%), n = 118 Possible FH: 25.42% (17.75 to 33.97%), n = 118 |
The interventions assessed within the three included studies focused on systematically identifying participants through electronic health record (EHR) searches and using computer reminders; however, the modalities varied between studies. In two studies, the computer reminders were on-screen prompts for participants who had raised cholesterol recorded within their EHR [28,29][20][21]. The Weng study [29][21] also incorporated postal invitations to be assessed for FH by completing a family history questionnaire. Additionally, in the follow-up phase of the Green study [28][20], a combined intervention of on-screen prompts with an invitation for clinical assessment by a specialist FH nurse was also assessed. In the third study, the computer reminder was an on-screen prompt in the downloaded laboratory results [27][19].
The three studies made a diagnosis of definite, probable and possible FH based on either the Simon-Broome criteria [28,29][20][21] or a modified version of the DLCN criteria (where only participants with LDL-C ≥ 6.5 mmol/L were selected) [27][19]. However, in the follow-up phase of the Green study [28][20], a diagnosis of FH was based on using a combination of the Simon-Broome and/or DLCN criteria.
The overall risk of bias for all three of the included studies was low [27[19][21],29], including the EHR reminder phase in Green [28][20]; however, for the combined intervention of computer on-screen prompts and FH nurse intervention in the follow-up phase of the Green study [28][20], the overall risk of bias was moderate to account for the risk of attrition bias due to missing outcome data for some participants (data could not be extracted for 62,684 out of 262,030 participants) (Supplementary Materials Table S3).
4.1. Diagnosis of Definite FH
All included studies reported the number of participants identified with definite FH. In the Green study, systematically identifying participants in the EHRs using on-screen prompts found a small absolute improvement in the proportion of participants diagnosed with FH with the prevalence increasing from 0.13% to 0.18% over the two years of the intervention period (SB criteria: MD 0.05%, 95% CI 0.03 to 0.07%) [28][20]. Additionally, during the follow-up phase of this study, combining on-screen prompts in the EHR with an invitation to an assessment with a specialist FH nurse, led to a further marginal increase in prevalence of definite FH to 0.19% (SB criteria: MD 0.07%, 95% CI 0.05 to 0.09%).
In the Weng study, it was found that systematically identifying participants in EHRs using on-screen prompts and postal invitations to complete family history questionnaires may slightly increase the number of patients diagnosed with definite FH (SB criteria: 0 at baseline versus 2 diagnoses 6 months post-intervention; MD 1.69%, 95% CI –1.69% to 5.97%) [29][21].
Similarly, in the Bell study, it was found that systematically identifying participants in EHRs using on-screen prompts within the laboratory results may slightly increase the number of diagnoses with definite FH (modified DLCN criteria: 0 versus 2 diagnoses 4 months post-intervention; MD 2.08%, 95% CI –2.05% to 7.28%) [27][19]. Both diagnoses in this study had an identifiable LDL-receptor gene mutation on genetic testing.
4.2. Diagnosis of Possible and Probable FH
Systematically identifying participants in EHRs, using on-screen prompts, found a small absolute improvement in the proportion of participants diagnosed with possible FH from the baseline (SB criteria: MD 0.04%, 95% CI 0.03% to 0.05%) [28][20]. Additionally, combining on-screen prompts in the EHR with an invitation for a specialist FH nurse assessment had an added small absolute improvement in the diagnosis of possible FH from the baseline (SB criteria: MD 0.02%, 95% CI 0.02% to 0.03%) [28][20].
In contrast, using on-screen prompts in EHRs, combined with postal invitations to complete family history questionnaires, resulted in a 25% absolute improvement in the identification of possible FH (SB criteria: 0 at baseline versus 30 cases post-intervention; MD 25.42%, 95% CI 17.75% to 33.97%) [29][21].
However, systematically identifying participants in EHRs using on-screen prompts in the laboratory results did not increase the diagnosis of probable FH (modified DLCN criteria: MD 2.08%, 95% CI –2.05% to 7.28%) [27][19].
4.3. Adverse Events Associated with the Intervention
None of the included studies reported adverse events.
4.4. Cholesterol Levels
In the Weng study, there was no significant reduction in total cholesterol in the intervention group compared to the baseline (total cholesterol: MD –0.16, 95% CI –0.78 to 0.46) [29][21]. There were mixed results for LDL cholesterol in the Weng study, with no significant changes (LDL-C: MD –0.12, 95% CI –0.81 to 0.57) [29][21]. However, the Bell study found a significant decrease 12 months after intervention (LDL-C: MD –3.00, 95% CI –3.42 to –2.58) [27][19].
4.5. Cardiovascular Mortality and Morbidity
None of the included studies reported this outcome measure.
4.6. Lipid-Lowering Treatment
Using on-screen prompts in EHRs, combined with postal invitations to complete family history questionnaires, resulted in an absolute increase of 19% in the prescribing of statins (MD 18.75%, 95% CI 8.9% to 35.3%), and an absolute increase of 9% in the prescribing of high potency statins (MD 9.38%, 95% CI 4.% to 24.2%), compared to the baseline [29][21].
4.7. Referral to a Specialist Service
One study reported that systematically identifying participants in EHRs using on-screen prompts in the laboratory result resulted in 4% (4/96) of participants being referred to a specialist service; however, at baseline, outcome data were not reported [27][19].
4.8. Adverse Self-Reported Psychological Effects
None of the included studies reported this outcome measure.
References
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490.
- Singh, S.; Bittner, V. Familial Hypercholesterolaemia—Epidemiology, Diagnosis and Screening. Curr. Atheroscler. Rep. 2015, 17, 3.
- Wald, D.S.; Bestwick, J.P.; Morris, J.K.; Whyte, K.; Jenkins, L.; Wald, N.J. Child–Parent Familial Hypercholesterolemia Screening in Primary Care. N. Engl. J. Med. 2016, 375, 1628–1637.
- Demott, K.; Nherera, L.; Shaw, E.J.; Minhas, R.; Humphries, S.E.; Kathoria, M.; Ritchie, G.; Nunes, V.; Davies, D.; Lee, P.; et al. Clinical Guidelines and Evidence Review for Familial Hypercholesterolaemia: The Identification and Management of Adults and Children with Familial Hypercholesterolaemia; National Collaborating Centre for Primary Care and Royal College of General Practitioners: London, UK, 2008.
- Besseling, J.; Reitsma, J.B.; Hovingh, G.K.; Hutten, A. Predicting the presence of a mutation resulting in familial hypercholesterolemia-development of a prediction model in a cohort of 64,000 subjects. Circulation 2014, 130 (Suppl. 2), A16172.
- Qureshi, N.; Humphries, S.E.; Seed, M.; Rowlands, P.; Minhas, R. Identification and management of familial hypercholesterolaemia: What does it mean to primary care? Br. J. Gen. Pr. 2009, 59, 773–778.
- Goldberg, A.; Hopkins, M.D.; Toth, P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; De Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5 (Suppl. 3), 133–140.
- Harada-Shiba, M.; Arai, H.; Okamura, T.; Yokote, K.; Oikawa, S.; Nohara, A.; Okada, T.; Ohta, T.; Bujo, H.; Watanabe, M.; et al. Multicenter study to determine the diagnosis criteria of heterozygous familial hypercholesterolemia in Japan. J. Atheroscler. Thromb. 2012, 19, 1019–1026.
- Hata, Y.; Mabuchi, H.; Saito, Y.; Itakura, H.; Egusa, G.; Ito, H.; Teramoto, T.; Tsushima, M.; Tada, N.; Oikawa, S.; et al. Report of the Japan Atherosclerosis Society (JAS) Guideline for Diagnosis and Treatment of Hyperlipidemia in Japanese Adults. J. Atheroscler. Thromb. 2002, 9, 1–27.
- National Institute for Health and Care Excellence. Familial Hypercholesterolaemia: Identification and Management: Update Clinical Guideline 71. 2019. Available online: (accessed on 17 June 2020).
- Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ 1991, 303, 893.
- Sullivan, D.; Watts, G.; Hamilton, I. Guidelines for the Diagnosis and Management of Familial Hypercholesterolaemia; Cardiac Society of Australia and New Zealand: Sydney, Australia, 2013; Available online: (accessed on 27 September 2020).
- Williams, R.R.; Hunt, S.C.; Schumacher, M.; Hegele, R.A.; Leppert, M.F.; Ludwig, E.H.; Hopkins, P.N. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am. J. Cardiol. 1993, 72, 171–176.
- Defesche, J.C.; Lansberg, P.J.; Umans-Eckenhausen, M.A.; Kastelein, J.J. Advanced method for the identification of patients with familial hypercholesterolaemia. Semin. Vasc. Med. 2004, 4, 59–65.
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wilkund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for management of dyslipidaemias. Eur. Heart J. 2011, 32, 1769–1818.
- Watts, G.F.; Sullivan, D.R.; Poplawski, N.; van Bockxmeer, F.; Hamilton-Craig, I.; Clifton, P.M.; O’Brien, R.; Bishop, W.; George, P.; Barter, P.J.; et al. Familial hypercholesterolaemia: A model of care for Australasia. Atheroscler. Suppl. 2011, 12, 221–263.
- Gidding, S.S.; Champagne, M.A.; de Ferranti, S.D.; Defesche, J.; Ito, M.K.; Knowles, J.W.; McCrindle, B.; Raal, F.; Rader, D.; Santos, R.D.; et al. The agenda for familial hypercholesterolaemia: A scientific statement from the American Heart Association. Circulation 2015, 132, 2167–2192.
- Vallejo-Vaz, A.J.; Kondapally Seshasai, S.R.; Cole, D.; Hovingh, G.K.; Kastelein, J.J.; Mata, P.; Raal, F.J.; Santos, R.D.; Soran, H.; Watts, G.F.; et al. Familial hypercholesterolaemia: A global call to arms. Atherosclerosis 2015, 243, 257–259.
- Bell, D.A.; Bender, R.; Hooper, A.J.; McMahon, J.; Edwards, G.; Van Bockxmeer, F.M.; Watts, G.F.; Burnett, J.R. Impact of interpretative commenting on lipid profiles in people at high risk of familial hypercholesterolaemia. Clin. Chim. Acta 2013, 422, 21–25.
- Green, P.H.; Neely, D.; Humphries, S.E.; Saunders, T.; Gray, V.; Gordon, L.; Payne, J.; Carter, S.; Neuwirth, C.; Rees, A.; et al. Improving detection of familial hypercholesterolaemia in primary care using electronic audit and nurse-led clinics. J. Eval. Clin. Pr. 2016, 22, 341–348.
- Weng, S.; Kai, J.; Tranter, J.; Leonardi-Bee, J.; Qureshi, N. Improving identification and management of familial hypercholesterolaemia in primary care: Pre- and post-intervention study. Atherosclerosis 2018, 274, 54–60.
- Bell, D.A.; Hooper, A.J.; Bender, R.; McMahon, J.; Edwards, G.; Van Bockxmeer, F.M.; Watts, G.F.; Burnett, J.R. Opportunistic screening for familial hypercholesterolaemia via a community laboratory. Ann. Clin. Biochem. Int. J. Lab. Med. 2012, 49, 534–537.
- Bell, D.; Hooper, A.; Edwards, G.; Southwell, L.; Pang, J.; Van Bockxmeer, F.; Watts, G.; Burnett, J. Impact of Telephoning the Requestors of Individuals Found to be at High Risk of Familial Hypercholesterolaemia. Hear. Lung Circ. 2013, 22, S230.
- Bell, D.A.; Kirke, A.B.; Barbour, R.; Southwell, L.; Pang, J.; Burrows, S.; Watts, G.F. Can Patients be Accurately Assessed for Familial Hypercholesterolaemia in Primary Care? Hear. Lung Circ. 2014, 23, 1153–1157.
- Bell, D.A.; Edwards, G.; Hooper, A.J.; McMahon, J.; Van Bockxmeer, F.M.; Watts, G.F.; Burnett, J.R. The potential role of an expert computer system to augment the opportunistic detection of individuals with familial hypercholesterolaemia from a community laboratory. Clin. Chim. Acta 2015, 448, 18–21.
- Bender, R.; Edwards, G.; McMahon, J.; Hooper, A.J.; Watts, G.F.; Burnett, J.R.; Bell, D.A. Interpretative comments specifically suggesting specialist referral increase the detection of familial hypercholesterolaemia. Pathology 2016, 48, 463–466.
- Benn, M.; Watts, G.F.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Familial hypercholesterolaemia in the Danish general population: Prevalence, coronary artery disease, and cholesterol-lowering medication. J. Clin. Endocrinol. Metab. 2012, 97, 3956–3964.
- Casula, M.; Catapano, A.L.; Bernardi, L.R.; Visconti, M.; Aronica, A. Detection of familial hypercholesterolaemia in patients from a general practice database. Atheroscler. Suppl. 2017, 29, 25–30.
- Elis, A.; Leventer-Roberts, M.; Bachrach, A.; Lieberman, N.; Durst, R.; Knobler, H.; Balicer, R. The characteristics of patients with possible familial hypercholesterolemia—screening a large payer/provider healthcare delivery system. QJM 2020, 113, 411–417.
- Gray, J.; Jaiyeola, A.; Whiting, M.; Modell, M.; Wierzbicki, A.S. Identifying patients with familial hypercholesterolaemia in primary care: An informatics-based approach in one primary care centre. Heart 2008, 94, 754–758.
- Jayne, Z.; Lungley, J.; Harvey, D.; Stuart, A.; Nair, D. Specialist Familial Hypercholesterolaemia (FH) nurses in primary care for identification of FH index cases. Atherosclerosis 2016, 245, e250.
- Kirke, A.B.; Barbour, R.A.; Burrows, S.; Bell, D.A.; Vickery, A.W.; Emery, J.; Watts, G.F. Systematic Detection of Familial Hypercholesterolaemia in Primary Health Care: A Community Based Prospective Study of Three Methods. Hear. Lung Circ. 2015, 24, 250–256.
- Qureshi, N.; Weng, S.; Tranter, J.; El-Kadiki, A.; Kai, J. Feasibility of improving identification of familial hypercholesterolaemia in general practice: Intervention development study. BMJ Open 2016, 6, e011734.
- Safarova, M.S.; Liu, H.; Kullo, I.J. Rapid identification of familial hypercholesterolaemia from electronic health records: The SEARCH study. J. Clin. Lipidol. 2016, 10, 1230–1239.
- Shipman, K.; Ganeshamoorthy, S.; Labib, M. Audit of the diagnosis of familial hypercholesterolaemia in primary care. Atherosclerosis 2014, 236, e306.
- Troeung, L.; Arnold-Reed, D.; Ping-Delfos, W.C.S.; Watts, G.F.; Pang, J.; Lugonja, M.; Bulsara, M.; Mortley, D.; James, M.; Brett, T. A new electronic screening tool for identifying risk of familial hypercholesterolaemia in general practice. Heart 2016, 102, 855–861.
- Vickery, A.W.; Ryan, J.; Pang, J.; Garton-Smith, J.; Watts, G.F. Increasing the Detection of Familial Hypercholesterolaemia Using General Practice Electronic Databases. Hear. Lung Circ. 2017, 26, 450–454.
- Zamora, A.; Masana, L.; Comas-Cufi, M.; Vila, A.; Plana, N.; Garcia-Gil, M.; Alves-Cabratosa, L.; Marrugat, J.; Roman, I.; Ramos, R. Familial hypercholesterolaemia in a European Mediterranean population—Prevalence and clinical data from 2.5 million primary care patients. J. Clin. Lipidol. 2017, 11, 1013–1022.
- Amor-Salamanca, A.; Castillo, S.; Gonzalez-Vioque, E.; Dominguez, F.; Quintana, L.; Lluís-Ganella, C.; Escudier, J.M.; Ortega, J.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Genetically Confirmed Familial Hypercholesterolemia in Patients with Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2017, 70, 1732–1740.
- Benlian, P.; Turquet, A.; Carrat, F.; Amsellem, S.; Sanchez, L.; Briffaut, D.; Girardet, J.P. Diagnosis scoring for clinical identification of children with heterozygous familial hypercholesterolaemia. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 456–463.
- ClinicalTrials.gov. IN-TANDEM Familial Hypercholesterolemia Pilot Study; National Library of Medicine (US): Bethesda, MD, USA, 2018. Available online: (accessed on 27 September 2020).
- ClinicalTrials.gov. Prevalence of Familial Hypercholesterolaemia (FH) in Italian Patients with Coronary Artery Disease; National Library of Medicine (US): Bethesda, MD, USA, 2018. Available online: (accessed on 27 September 2020).
- ClinicalTrials.gov. A Prospective Pilot Study of Screening out Rate and Clinical Management of Familial Hypercholesterolemia; National Library of Medicine (US): Bethesda, MD, USA, 2019. Available online: (accessed on 27 September 2020).
- Nanchen, D.; Gencer, B.; Auer, R.; Räber, L.; Stefanini, G.G.; Klingenberg, R.; Schmied, C.M.; Cornuz, J.; Muller, O.; Vogt, P.; et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur. Hear. J. 2015, 36, 2438–2445.
- Steyn, K.; Fourie, J.M.; Shepherd, J. Detection and measurement of hypercholesterolaemia in South Africans attending general practitioners in private practice—The cholesterol monitor. S. Afr. Med. J. 1998, 88, 1569–1574.
- Aref-Eshghi, E.; Oake, J.; Godwin, M.; Aubrey-Bassler, K.; Duke, P.; Mahdavian, M.; Asghari, S. Identification of Dyslipidemic Patients Attending Primary Care Clinics Using Electronic Medical Record (EMR) Data from the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) Database. J. Med. Syst. 2017, 41, 45.
- E Arnold-Reed, D.; Brett, T.; Troeung, L.; Vickery, A.; Garton-Smith, J.; Bell, D.; Pang, J.; Grace, T.; Bulsara, C.; Li, I.; et al. Detection and management of familial hypercholesterolaemia in primary care in Australia: Protocol for a pragmatic cluster intervention study with pre-post intervention comparisons. BMJ Open 2017, 7, e017539.
- Scottish Intercollegiate Guidelines Network. Available online: (accessed on 27 September 2020).
- Sterne, J.A.; Higgins, J.P.; Reeves, B.C.; on behalf of the development group for ROBINS-1. ROBINS-1: A Tool for Assessing Risk of Bias in Non-Randomized Studies of Interventions (Version 7). Available online: (accessed on 5 January 2017).
- Lan, N.S.; Martin, A.C.; Brett, T.; Watts, G.F.; Bell, D.A. Improving the detection of familial hypercholesterolaemia. Pathology 2019, 51, 213–221.
- Weng, S.; Kai, J.; Akyea, R.; Qureshi, N. Detection of familial hypercholesterolaemia: External validation of the FAMCAT clinical case-finding algorithm to identify patients in primary care. Lancet Public Health 2019, 4, e256–e264.
More
