Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Audrey Ferrand and Version 2 by Peter Tang.
Colorectal cancer (CRC) is the third most common cause of cancer-related death. Patients suffering inflammatory bowel disease have an increased risk of CRC. It is admitted that CRC found its origin within crypts of the colon mucosa, which host the intestinal stem cells (ISCs) responsible of the tissue renewal. ISC behavior is controlled by the fibroblasts that surround the crypt. During inflammation, the signals delivered by fibroblasts are altered, leading to stem cells’ dysregulation, possibly turning them into cancer-initiating cells.
- colon
- fibroblasts
- stroma
- colorectal cancer
- inflammation
- intestinal stem cells
1. Introduction
Despite its reputation as a curable disease, and a thorough characterization of the mutations involved in its adenoma–carcinoma sequence [1][2][3][1,2,3], colorectal cancer (CRC) remains worldwide the third most common cause of cancer-related death [4]. To date, the debate about the origin of the CRC stem cells remains largely open. Two theories have been proposed. The bottom-up theory, where the intestinal stem cells (ISCs) are the cells of cancer origin, and the top-down theory, where the cancer initiates in progenitors or differentiated cells. However, while the latter originated mainly from histopathological observations, recent reports strengthened the bottom-up theory. ISC-specific deletion of both adenomatous polyposis coli (APC) alleles using either Bmi1-, CD133-, or LGR5-Cre recombinase mice leads to a rapid full adenoma formation [5][6][7][5,6,7], while a similar APC deletion in late progenitors or differentiated cells only results in sporadic and slow-developing adenoma [7]. Therefore, the scientific community agrees on the idea that the crypt is the place where the cancer originates.
Indeed, the fact that almost all epithelial cells in the intestinal lining are replaced on a weekly basis puts great demands on the cellular organization of this tissue and represents, in consequence, a high risk of malignant conversion. The renewal of the intestinal epithelium is maintained by an ISC compartment that resides at the bottom of the crypt (Figure 1). It depends on the spatial organization of signals emanating from the supportive mesenchymal cells, as well as from differentiated epithelial progeny. However, the high number of patients developing CRC indicates that these regulatory mechanisms often fall short in protecting against malignant transformation. Fearon and Vogelstein have clearly demonstrated that CRC develops as a stepwise accumulation of genetic hits in specific genes and pathways [1]. The cancer stem cell theory refines this model and suggests that the actual tumorigenic capacity of individual cancer cells may be influenced by homeostatic signals derived from their microenvironment [8].
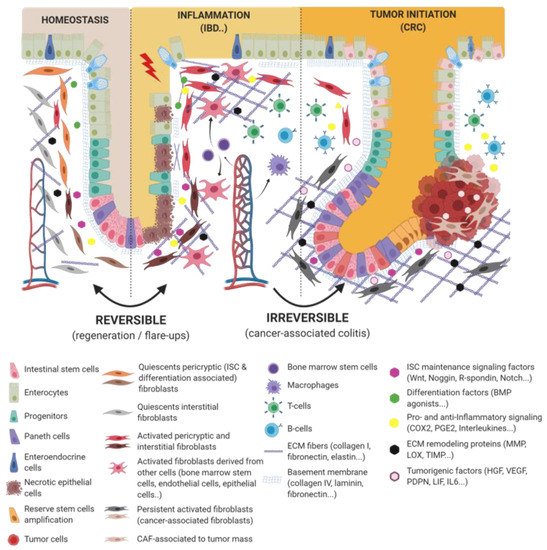
Figure 1. Epithelio-fibroblastic relationship in the physiological and injury context in intestinal tissue. Under the physiological context, normal fibroblasts form a very heterogeneous cell population that could be divide into two families: interstitial fibroblasts (extracellular matrix (ECM) production and regulation) and pericryptic fibroblasts (soluble factors secretion and ECM basement membrane production). Depending on pericryptic fibroblasts’ localization on the intestinal crypt compartment, they secrete (i) proliferating factors for intestinal stem cell (ISC) maintenance (wingless-related integration site (Wnt ligands, R-spondin, EGF, bone morphogenic protein (BMP) antagonists) and constitute an ISC niche or (ii) differentiating factors (BMP family). In the case of acute or chronic tissue injury, not only epithelial cells but also fibroblastic populations undergo a strong remodeling, resulting in ISC loss and/or inability to regenerate epithelium. Depending on the injury severity, resident fibroblasts can be lost or become transiently or definitively activated. Other cell types can differentiate and reach an activated-fibroblast phenotype, thus promoting epithelial regeneration, survival, and immune dialog. Activated fibroblasts can improve epithelial regeneration on a first intention (by increasing some ISC niche factors’ production) and then switch their phenotype to impaired ISC and epithelium renewal.
In fact, CRC is a disease in which the homeostatic capacities of intestinal crypt stem cells are impaired. Indeed, under physiological conditions, the homeostasis and integrity of the crypt is tightly controlled by a very specific environment, namely the intestinal crypt niche [9]. This niche includes the basal lamina upon which reposes the crypt and the stroma surrounding the crypt and including the extracellular matrix and the stromal cells (fibroblasts; neurons; and glial, immune, and vascular cells). The intestinal stroma’s main functions toward the epithelium, beside immunity, are mechanical (support) and metabolic (nutrition and various exchanges). Among the stroma, the fibroblasts that sheathe the crypt are key regulators of the crypt cells by secreting factors such as wingless-related integration site (Wnt) ligands, R-spondin, Noggin, and bone morphogenetic protein (BMP) that control stem cell phenotype and differentiation processes along the crypt. Thus, in order to preserve a good control of the crypt homeostasis and integrity, and thus avoid any risk of tumoral transformation, the integrity of each partner, the niche as well as the crypt stem cells, has to be preserved. This implies that any alteration of one or another of the crypt or niche entities could in turn impact on the homeostasis of the colonic mucosa and, thus, favor cancer initiation [10].
To date, little is known on the mechanisms implicated in the stroma-dependent deregulation of the crypt stem cells. Compared to the healthy intestinal stroma, the inflammatory and the tumor stroma have distinct pathological features and are said to be “activated” with continuous remodeling. The predominant cell type in the stroma is the fibroblast. The functions of fibroblasts include the deposition of extracellular matrix (ECM), regulation of epithelial differentiation, regulation of inflammation, and involvement in wound healing (Figure 2). As the principal source of ECM constituents, fibroblasts are considered the main mediators of scar formation and tissue fibrosis. In an activated stroma state, activated fibroblasts present a different phenotype and secrete different types of chemokines, cytokines, proteases, and ECM proteins than in their normal non-activated conditions [11]. Activated fibroblasts are involved in the proliferation and dedifferentiation of tumor epithelial cells and can participate in tumor resistance for example by increasing the ECM stiffness around the tumor, or by upregulating the epithelial stemness capacities and favoring the epithelial–mesenchymal transition in vivo [11][12][13][11,12,13]. Thus, through the secretion of numerous factors, activated fibroblasts, including the cancer-associated fibroblasts (CAFs), contribute to the creation of an environment favoring the tumor development not only by regulating the reorganization of the connective tissue but also through tumor neo-angiogenesis allowing metastasis.
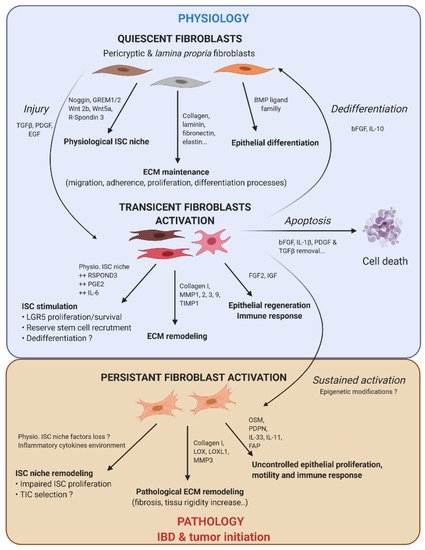
Figure 2. Fibroblast activation dynamic switch depending on the physiopathological context. Normal fibroblasts have a pivotal role under the physiological context to produce ECM protein, control ISC compartment proliferation/differentiation, and maintain intestinal tissue homeostasis. Fibroblasts are very plastic cells able to respond to their environment by becoming activated in case of injury to support ISC proliferation and renewal. To compensate eventual ISC loss, the intestinal epithelium can call on reserve stem cells, which can be recruited by stromal stimulation, in particular prostaglandin E2 (PGE2), or a dedifferentiation of secretory or fully differentiated progenitors, to join the compartment strain and thereby regenerate the epithelium. After epithelium repair, the activated fibroblasts can either become quiescent again, or regain their physiological function (by dedifferentiation), or die by apoptosis. However, if the injury persists and becomes chronic, the activated fibroblasts are locked into this state (possibly due to changes in the epigenetic status). This is where activated fibroblasts can play an important role in the emergence or initiation of new pathologies such as cancer.
2. From Normal to Activated Fibroblasts
2.1. Fibroblasts Act as ISCs’ Nanny
From an overall perspective, fibroblasts within the colon stroma are either sparsely disseminated within the ECM or sheathing the crypt (Figure 3). Increasing numbers of studies portray fibroblasts as crucial players in the ISC niche. These subepithelial fibroblasts are very heterogeneous. Along the crypt, we could distinguish at least two main peri-epithelial fibroblast populations. The subepithelial fibroblasts located around the bottom of the crypt are an important source of Wnt ligands, which regulate ISC renewal, and BMP antagonists, blocking the differentiation process. Fibroblasts located at the top of the crypt are associated with the epithelial differentiation process by inducing BMP pathway activation [14][15][14,15]. Using in situ hybridization and RT-PCR on human colon tissue, Kosinski et al. observed that BMP antagonists such as Noggin, Gremlin 1, Gremlin 2, and Chordin-like 1 are expressed by myofibroblasts expressing vimentin (VIM) and alpha smooth muscle actin (αSMA) and located at proximity of the bottom of the crypt [16]. αSMA+ cells also expressed Wnt2b and Wnt5a [17]. However, using single-molecule-RNA fluorescence in situ hybridization (FISH), authors demonstrated that αSMA− cells strongly expressed those isoforms. These last years, numerous studies, performed on either the mouse or human small intestine or colon, aimed to decipher pericryptic fibroblasts’ heterogeneity by identifying many other non-myofibroblast markers present in the ISC niche. These include Foxl1 [18][19][18,19], Gli1 [20], platelet-derived growth factor receptor (PDGFR) α [21][22][21,22], CD90 [23], or gp38 [24][25][24,25]. Precisely, Stzepourginski et al. observed that a population of mesenchymal cells identified as gp38+ (first fibroblast marker described in literature also known as podoplanin, (PDPN)), in combination with CD34+ but αSMA−, are the main producers of Wnt2b, as well as R-spondin 1, and Gremlin 1 (GREM1), an inhibitor of the pro-differentiative BMP pathway [25]. In addition to αSMA and vimentin, PDGFRα has been proposed as a marker of subepithelial pericryptic fibroblasts in mice and humans [26]. In fact, combinations of cells positive for PDGFRα, gp38, Gli1, and CD90 cells were shown to support mice intestinal organoid growth in co-culture in contrast to the PDGFRα+, gp38+, Gli1+ but CD90- population [23]. Using genetic ablation of porcupine acyltransferase (porcn) in PDGFRα+ pericryptal stromal cells, resulting in the loss of global epithelial Wnt secretion, Greicius et al. proposed those cells as the major in vivo source of Wnts in the murine intestine [21]. They also demonstrate that PDGFRα+ cells provide R-spondin 3, an important co-activator of Wnt/β-catenin signaling in ISC. Recently, using single-cell RNA-seq and whole-mount high-resolution microscopy, McCarthy et al. identified three distinct pericryptic mesenchymal cell types in the mouse small intestine based on the level of expression of PDGFRα. They identify a low PDGFRα subpopulation (called trophocytes) expressing CD81 at the crypt bottom that supports ISC proliferation in organoid co-culture assay. RNA-seq data reveal that contrary to villus-associated telocytes highly expressing PDGFRα, trophocytes suppress BMP signaling via high GREM1 expression levels while promoting ISC proliferation by expressing Wnt ligands (Wnt2b and 9a) and RSPOND 1, 2, and 3 genes [22]. These data confirmed by Brugger et al. [27], taken as a whole, show that the characterization of the fibroblast populations involved in the ISC niche still remains largely incomplete, with some publications even reporting contradictory findings.
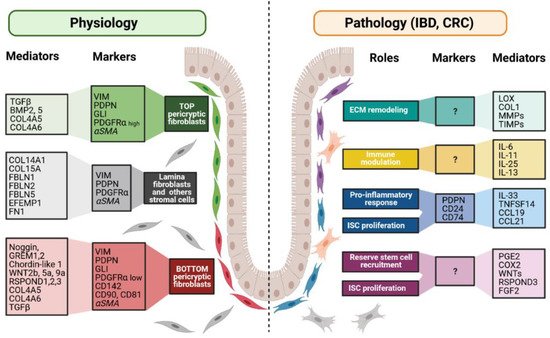
Figure 3. Fibroblast markers and mediators under physiological and pathological contexts. Fibroblasts can be divided into different populations depending on their locations, functions along the crypt axis, and pathophysiological status. More and more studies try to present a panel of markers that can be used to decipher those populations. Although none of them are actually specific of a subpopulation, in combination, they allow the discrimination of the different subpopulations, namely top pericryptic-, bottom pericryptic- and lamina propria-associated fibroblasts. However, discrepancies exist for example concerning alpha smooth muscle actin (αSMA), which can be found, or not, on the different populations. Under the pathological context, the discrimination of the diverse fibroblasts populations is increasingly complex, with no markers being currently known to clearly identify the ones implicated in the various pathological processes. Markers and mediators listed in this figure are used as examples and are not exhaustive.
2.2. Fibroblasts Activation: When the Nanny Turns into the Nurse
Because of their major participation in the integrity of the ISC niche, fibroblasts have a leading role in intestinal epithelial lining repair in case of an injury and/or in an inflammatory context. Fibroblasts implicated in the establishment of a normal ISC niche play a concomitant role under the chronic inflammatory context to regenerate intestinal epithelium. Degirmenci et al. found that the Gli1+ stromal population (i.e., identified by authors to contribute to ISC niche) is increased in mice after dextran sulfate sodium (DSS) treatment compared to control. Gli1+ population enrichment improves RSPOND3 secretion and promotes ISC proliferation for epithelium healing [20]. Moreover, Harnack et al. demonstrated that, in addition to the physiological maintenance of the LGR5+ ISC in physiological conditions, under stress conditions, RSPOND3 acts via the LGR4 receptor expressed by progenitor and some specialized colonic cells to reactivate the expression of Wnt target genes to allow the regenerative process [28][30]. Moreover, to ensure wound healing, fibroblasts acquire an activated phenotype induced by various stimuli such as TGFβ [29][30][31,32] and PDGF [31][33], arising when tissue damage occurs and mainly released by epithelial cells or infiltrated monocytes and macrophages.
Activated fibroblasts are present under physiological conditions in normal colonic mucosa [32][34]; however, the population enrichment occurring during inflammation or tissue injury raises the question of the cellular origin of these newly activated fibroblasts. While it is well established that the activation of the resident fibroblasts is a great purveyor of activated fibroblasts, it has been shown that bone marrow stem cells can be another source [33][34][35][35,36,37]. Indeed, bone-marrow-derived fibroblasts from male mice can be detected via the detection of the presence of Y chromosomes, in the entire small intestine and colon subepithelial mucosa of the female recipients 7 days post-engraftment [33][35]. After trinitrobenzene sulfonic acid (TNBS)-induced colitis, bone-marrow-derived activated fibroblasts were significantly increased in the inflamed areas [34][36]. Moreover, this treatment can also induce an epithelial-to-mesenchymal transition (EMT), showing that the intestinal epithelial cells can provide new fibroblasts contributing to the fibrosis [36][38].
Compared to quiescent resident fibroblasts, activated fibroblasts display an altered protein expression profile allowing them to intervene in immune response, ECM remodeling, and epithelium regeneration (Figure 2). For example, normal adult human colonic subepithelial myofibroblasts, a subtype of activated fibroblasts expressing αSMA, express COX 1 and 2 and prostaglandin E2 (PGE2), known to be involved in the inflammatory response [37][39]. An increased COX2 expression is observed in response to IL-1α stimulation in human intestinal myofibroblasts and is frequently observed during acute and chronic intestinal inflammation [38][40]. This increase is mediated by the activation of multiple parallel signaling pathways implicating nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs), extracellular signal-regulated protein kinase-1 or -2 (ERK-1/2), p38, c-Jun NH2-terminal kinase (JNK), stress-activated protein kinase (SAPK), and protein kinase C (PKC) [38][40]. The COX2–PGE2 pathway has a critical role in epithelium regeneration following DSS-induced colitis in mice via the activation of tumor progression locus-2 (Tpl2) kinase [39][41], a pro-inflammatory mediator, whose downregulation is genetically linked to inflammatory bowel diseases (IBD) [40][42]. Indeed, Tpl2 ablation in intestinal myofibroblasts results in enhanced ulceration and impaired compensatory crypt cells proliferation. Moreover, following epithelial injury and ISC loss, the stromal source of PGE2 has been proposed to play a crucial role in the recruitment of the epithelial reserve stem cells via the PTGER4 receptor combined with the activation of the Yes-associated protein (YAP) pathway to replenish the LGR5 active stem cell and, thus, improve the regeneration process [41][43]. These data show that under a regenerative context, the fibroblast-secreted PGE2 (in addition to RSPOND3) induces two epithelial responses to fully regenerate the intestinal lining: a dedifferentiation process and reserve stem cell recruitment, both allowing repopulation of the LGR5+ ISC compartment.
3. When the Good Guys Turn into Bad Guys
3.1. Wound Ending: How to Avoid the Drift?
Activated fibroblasts are key regulators of the immune response. They secrete a plethora of cytokines and pro-inflammatory molecules involved in the recruitment and activation of monocytes and macrophages. Nevertheless, they also have a role in the ending of the inflammatory response through the secretion of anti-inflammatory molecules [42][52]. With those mediators, activated fibroblasts can influence macrophages’ polarization toward M1/pro-inflammatory or M2/anti-inflammatory phenotypes [43][53]. Immunosuppression further relies on negative feedbacks utilizing the production of soluble mediators, such as inducible nitric oxide synthase (iNOS), by mesenchymal cells in response to a cocktail of pro-inflammatory cytokines IFNγ, TNFα, IL-1α, or IL-1β as demonstrated in mice [44][54].
Ending of wound healing is also mediated by apoptosis and deactivation of the activated fibroblasts by epigenetic changes [45][55]. While those regulatory mechanisms are extensively studied in organs such as skin, liver, or lung, little is known considering the gut. Nevertheless, one can suppose that the mechanisms involved remain quite similar [46][56]. For example, the combination of cytokines, such as IFNγ, TNFα, and IL-1β, induces a caspase-dependent apoptosis of human intestinal myofibroblasts [47][57]. IL-1β, or factors released during the inflammatory process such as HGF or bFGF, can trigger apoptosis of myofibroblasts in lung fibroses [48][58] and skin wounds [49][59], respectively. It has also been proposed that apoptosis of lung fibroblasts occurs after the withdrawal of the pro-survival growth factors TGFβ and PDGF during the later stage of wound healing [50][51][60,61]. The shift from tissue repair to pathogenic inflammation through myofibroblast apoptosis evasion has been recently reviewed [52][62]. To summarize, it seems that myofibroblasts are poised to self-destruct when tissue repair ends because of the absence of pro-survival signals. Nevertheless, some biomechanical and biochemical feedback loops can maintain those positive feeds; thereby, tipping the balance toward persistence. Another mechanism of cell death is speculated and relies on programmed necrosis through high production of COX2 when human dermal fibroblasts are clustered and cultivated in three-dimension spheroids [53][63]. However, another team did not observe such processes, thereby, keeping the question open [54][64].
3.2. Wounds That Do Not Heal: Persistent Fibroblast Activation in Chronic Inflammation
The inflammatory bowel diseases, two main subtypes of which are Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, relapsing inflammatory disorders of the gastrointestinal tract. IBD afflicts several millions of persons worldwide. Their incidence is high among developed countries and increases steadily. These diseases are characterized not only by an excessive immune response to gut microbiota dysbiosis but also by an important impairment of the epithelial renewal process, leading to an abnormal mucosal repair/healing. Indeed, a major complication of UC is its evolution into cancer.
An issue in chronic inflammation is the persistence of an activated stroma. The phenotypic stability of activated fibroblasts in IBD and CRC is supported by the establishment of an autocrine signaling favorable to the maintenance of their phenotype. Leukemia inhibitory factor (LIF)-induced constitutive activation of the JAK1/STAT3 signaling pathway has been suggested to induce epigenetic changes both via DNMT3b DNA methyltransferase activation and continuous pro-inflammatory cytokine secretion [55][69]. Indeed, during chronic inflammation, IL-6, TNFα, and IL-1β maintain fibroblasts in an activated state [56][57][70,71]. Although transient fibroblast activation is mandatory to support the intestinal epithelium’s regeneration, a persistent activation promotes fibrosis, contributes to the persistence of the inflammation, as found in IBD, and later on, favors cancer initiation and progression. Indeed, cytokine signaling and immune response alteration are known to be associated with IBD and CRC [55][58][59][69,72,73].
4. From Sustained Inflammation to Tumorigenesis
4.1. How It All Starts
For years, CRC development dogma implicated the emergence of successive genetic alterations leading to tumorigenesis, described as the “adenoma–carcinoma sequence”. The well-characterized mutagenic sequence in CRC begins with an inactivating mutation in the APC gene, a key downregulator of the Wnt/β-catenin proliferative pathway, and continues with the acquisition of subsequent mutations of oncogenes, such as KRAS, PI3KCA, SMAD4, and TP53, forming a malignant tumor [60][86]. A number of studies proposed ISC as cells of origin for CRC since activating mutations of the Wnt/β-catenin pathway in LGR5+, Bm1+, or Prom1+ cells lead to adenoma development in mice [5][6][7][5,6,7]. However, such mutations in differentiated cells form stalling microadenomas, suggesting that alternative factors are necessary for tumor initiation. Vermeulen et al. proposed a model based on a competition between mutants and wild-type (WT) ISC for the occupation of the crypt [61][87]. In this model, mutant cells have a prominent, but not total probability, to conquer the crypt depending on multiple parameters including bottom crypt position. Interestingly, they calculated that a dominant negative mutated TP53 clone has a competitive advantage versus WT only in the context of colitis. Here, mutated ISC selection depends on physiological ISC niche spatial occupancy and dominance. Moreover, as previously said, several clinical investigations demonstrated an increased risk for CRC apparition in patients subject to IBD [62][63][64][65][88,89,90,91], although some studie contested this affirmation [66][92]. As a matter of interest, CRC risk appears to be dependent on the type, the duration, and the severity of the pathology. It strongly suggests that the microenvironment of the crypt is involved. The colorectal crypt environment during chronic inflammation is highly disturbed. Persistent activated fibroblasts and immune cells are concentrated near the epithelium, where they intensively secrete growth factors, pro-survival molecules, pro-inflammatory cytokines, and ECM components to stimulate tissue repair and fibrosis. In this way, they also create the breeding ground for tumorigenesis. Tumor initiation occurring in a physiological context (stochasticity, i.e., sporadic CRC) or in an inflammatory or regenerative context (i.e., colitis-associated cancer (CAC)) is wildly different and could explain differences between these two types of CRC development. However, in this manuscript we will not develop the occurrence of sporadic colorectal cancer and the role of CAF in this tumorigenesis since it has been recently very nicely reviewed [11].
4.2. Cancer-Associated Fibroblasts: Colorectal Cancer’s Band Leader
The incidence of CAFs in tumor progression is better understood; however, it becomes more complex as the knowledge progresses [67][68][69][112,113,114]. Indeed, CAFs promote cancer development through tumor environment remodeling, cancer stemness enhancing, cancer cell feeding, and immune response suppression, but evidence shows that CAFs can also turn into tumor inhibitors. This suggests, and it was confirmed by recent studies, that CAF populations are heterogeneous [70][71][98,99]. A recent study demonstrated the importance of two CAF subpopulations involved in BMP signaling regulation that ensure the balance between stemness enrichment and tumor cell differentiation in the mouse and human CRC organoid model [72][115]. A first population, associated with a poor prognosis in human CRC, is characterized by the increased secretion of GREM1, the BMP antagonist known to play a predominant role in the physiological ISC niche. By analogy with fibroblasts’ role in the physiological regulation of the stem cell population, in CRC, the CAF population secreting GREM1 is associated with tumor stemness. Conversely, the CAF population described to promote differentiation and to block stemness is characterized by enrichment in immunoglobulin superfamily containing leucine-rich repeat (ISLR) protein causing BMP pathway stimulation and is, thus, associated with a good prognosis in CRC. The role of CAF in CRC progression and aggressiveness has become prominent thanks to transcriptomic analysis demonstrating that poor prognosis and resistance to radiotherapy correlate with a gene signature linked to stromal cells rather than epithelial tumor cells [73][74][116,117].
