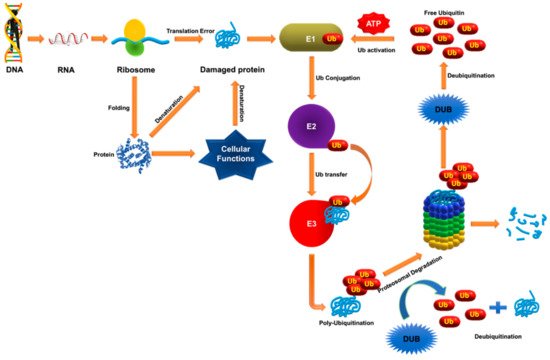
USP7 was initially identified as ICP0 (herpes simplex virus protein)-stabilizing protein
[112]. Several studies have reported that USP7 mediates stabilization of ICP0 enzyme–inducing proteasome-dependent degradation of a number of proteins, including p53 and promyelocytic leukemia protein by protecting it from auto-ubiquitination
[113]. MDM2, the murine double-minute oncogene (HDM2-human orthologue), is a substrate for USP7 which negatively regulates the tumor-suppressor protein p53. Under normal conditions, USP7 stabilizes intracellular MDM2 concentrations, which in turn drive steady ubiquitination of p53, which targets it for proteasome-mediated degradation
[114]. In MM, reduction in p53 expression occurs at the later stages of cancer, along with overexpression of USP7. Studies have shown that inhibition of USP7 causes auto-ubiquitination and degradation of MDM2, resulting in p53 stabilization inducing apoptosis via G1 phase arrest
[115]. The UPS appears to play an essential role in tumorigenesis, and preclinical and clinical studies have helped develop proteasome inhibitor bortezomib as a target against MM. However, recent studies have demonstrated possible off-target toxicity, development of resistance toward bortezomib, and its limited application in p53-deficient MM cells
[116][117][116,117]. Given the link between upregulation of USP7 and tumor aggressiveness in MM, an alternate therapeutic approach of using USP7 inhibitors would represent a major advance. Downregulation of USP7 by P5091 (a USP7 inhibitor) in MM cell lines, an MM xenograft model, and patient-derived tumor cells can create a potent and specific inhibitor that enhances degradation of HDM2, as well as upregulation of p53 and p21 expression, resulting in cell cytotoxicity
[118]. USP7 regulates p53 activity by deubiquitinating and stabilizing it, and overexpression of USP7 induces p53-dependent apoptosis. The N-terminal domain of USP7 is involved in p53-USP7 interactions, and also contains a TRAF domain and an EBNA1 binding domain. Human TRAF regulates lymphocyte survival, while EBNA1 is a viral onco-protein responsible for the immortalization of cells and development of B-cells lymphomas
[119]. Chronic lymphocytic leukemia (CLL) is underscored by genetic aberrations at a particular locus in ATM and p53 genes, which are associated with DDR. These genes play critical roles in DDR-mediated tumor suppression. Defects in these genes facilitate accumulation and persistence of genetic mutations, increasing genomic instability, which ultimately results in tumorigenesis as well as therapeutic drug resistance.
Recent studies have reported that USP7 modulates the stability of RAD18, a DNA damage-responsive E3 ubiquitin ligase, which in turn regulates p53 expression in CLL. In CLL models and patient-derived tumor samples, ATM-p53 mediated DDR is inactivated, while expression of USP7 was upregulated, a relationship which correlates the findings of a study of an MM model showing similar overexpression of USP7. Inhibition of USP7 by the chemotherapeutic inhibitor HBX19818 and siRNA-mediated downregulation of USP7 resulted in significant increases in tumor-cell apoptosis and disruption in homologous recombination repair due to genotoxicity
[120]. Preclinical studies have shown that USP7 small-molecule inhibitors such as P22077, P5091, GNE-6640, GNE-6776, and HBX19818 are well tolerated, induce efficient tumor-cell cytotoxicity, and selectively inhibit target molecules in in vitro and in vivo conditions
[118][121][118,121]. The effect of USP7 on the turnover of p53 and DDR-associated proteins, along with the efficacy of USP7 inhibitors on hematological malignancies, provides a proof of concept for the evaluation of USP7 as a potential pharmacological target in hematological tumors
[120].
5.4. USP9X
USP9X is a substrate-specific DUB with a highly conserved sequence in
Drosophila and humans. Overexpression of USP9X is associated with poor prognosis in various cases of hematological malignancies, such as CML, B-cell malignancies, and MM. Studies have shown that MM patients overexpressing USP9X are at higher risk of death and are associated with a poor prognosis of cancer
[122]. Induced MCL1, an essential apoptotic regulator protein for the survival of stem and progenitor cells of multiple lineages, is expressed at abnormally high levels in B- and mantle-cell lymphomas, CML, and MM. While the mechanism of overexpression of MCL1 in cancer is not completely understood, USP9X is thought to stabilize MCL1 by removing degradative Lys-48–linked polyubiquitin chains. Increased expression of USP9X is highly correlated with increased MCL1 in diffuse B-cell lymphomas and MM. Knockdown of USP9X results in downregulation of MCL1, which enhances cell apoptosis in human follicular lymphomas and B-cell lymphomas
[123]. Increased MCL1 and USP9X protein expression has been detected during relapses of AML, acute lymphocytic leukemia (ALL)
[124] and MM
[125], and is associated with increased tumor survival. Inhibition of USP9X by WP1130 downregulates MCL1 protein, inducing apoptosis in CML cell lines
[103]. Selective silencing of USP9X in CML cell lines resulted in downregulation of MCL1 and increased sensitivity toward drug and apoptotic stimuli
[126]. Preclinical trials with the USP9X inhibitors ABT-737 and ABT-263 demonstrated that they could increase proteasomal degradation of MCL1 through USP9X inhibition
[123].
CML is associated with an abnormality in chromosomes, resulting in unregulated expression of Bcr-Abl, and causing aberrant tyrosine kinase activity. Bcr-Abl kinase inhibitors such as imatinib showed high efficacy in CML patients. However, long-term exposure to this drug results in acquired drug resistance and disease progression at later stages. In-depth analysis also showed that resistance to imatinib is correlated with an increase in expression of USP9X. Treatment with WP1130, an anti-leukemia drug, results in downregulation of Bcr-Abl and USP9X-mediated apoptosis in CML
[126]. Another novel small molecule, EOAI3402143 (with properties similar to WP1130), selectively inhibits USP9X and USP24, induces apoptosis in malignant B-cell lines, and also blocks or regresses myeloma tumors in mice
[127]. Inhibition or knockdown of USP9X may therefore be a therapeutic target in various hematological malignancies with abnormal USP9X activity.
USP9X also exhibits mitotic activity due to its role in the regulation of chromosome alignment and segregation by spindle assembly checkpoint (SAC) targeting survivin and Aurora B and other inhibitors of apoptosis proteins. SAC-induced mitotic arrest coupled with knockdown of USP9X are important targets for anti-neoplastic therapies
[128]. USP9X binds specifically to numerous substrates in different types of cells. It also plays an important role in T-cell proliferation, T-helper-cell differentiation, and cytokine production
[119][129][119,129]. Recent studies have demonstrated that USP9X deubiquitinates the X-linked inhibitor of apoptotic protein (XIAP) to promote mitotic survival in aggressive B-cell lymphomas through RNAi-mediated knockdown of USP9X. Overexpression of USP9X is also correlated with increased expression of XIAP, which has been identified as a predictive biomarker for chemotherapy resistance in diffuse B-cell lymphomas
[129]. Indeed, USP9X is involved in the regulation of various mitotic and apoptotic proteins and its overexpression is associated with various hematological malignancies, making USP9X a potential theurapeutic target. Deeper insights into the mechanisms involved in signaling pathways associated with USP9X would help develop more effective drugs. In addition, unbiased determination of USP9X targets and its regulation may yield a more comprehensive assessment of DUB activity in cancer cells. Additional studies to determine key components in the apoptotic pathway and a role for USP9X in this process may help develop more effective cancer therapies.
5.5. USP14
USP14 is a DUB associated with the 19S proteasome, which dynamically regulates the magnitude and nature of its activity, but its role in disease development is unclear
[130]. Various studies have revealed that USP14 is associated with numerous types of cancer. Specifically, it was reported that upregulated expression of USP14 is associated with leukemia and may be implicated in apoptosis
[131]. Various proteasome inhibitors, such as bortezomib
[132], carfilzomib
[133], and MLN9708
[134], have contributed significantly toward treatment and survival in MM and B-cell-related malignancies in patients. However, increasing development of resistance of cancer cells against chemotherapy has been a main roadblock in development of treatment for malignancies. Studies have shown that MM cells can be protected from chemotherapy-induced apoptosis by the phenomenon called cell adhesion mediated drug resistance (CAM-DR)
[135]. USP14 reportedly contributes to CAM-DR by upregulating the anti-apoptotic protein Bcl-xl and Wnt 3 signaling pathways. Studies have shown that USP14 is significantly overexpressed in CAM-DR models and downregulated in apoptotic models of MM. Moreover, upregulation of USP14 in MM models could enhance anti-apoptotic cell-adhesion abilities, thus promoting drug resistance in MM
[136].
Co-inhibition of USP14 and UCHL5 by novel DUB inhibitor VLX1570 revealed potent tumor-specific apoptotic activity in drug-resistant tumor cells of Waldenstrom macroglobulinemia (WM), an incurable non-Hodgkin lymphoma
[115][137][115,137]. Recently it was also established that targeted inhibition of USP14 and UCHL5 with the novel small-molecule proteasome inhibitor b-AP15 induced proteotoxic stress and apoptosis in tumor cells of WM, without affecting proteolytic activity of the 20S proteasome
[138]. The turnover of many cell-cycle regulatory proteins such cyclin-dependent kinase (CDK) 1A and 1B as well as p53 protein is controlled by b-AP15. Accumulation of cell-cycle inhibitors and regulatory proteins results in cell-cycle arrest, along with increased DNA damage markers
[130], suggesting b-AP15 exhibits genotoxic properties. In addition, b-AP15–mediated inhibition of proteasome deubiquitinating activity suppressed tumor progression and organ infiltration in different in vivo solid tumor models of an AML
[139]. b-AP15 mediated inhibition along with siRNA knockdown of USP14 and UCHL5 induces synergistic apoptotic activity in MM tumor cells and overcomes resistance to bortezomib
[140]. A recent study involving selective inhibition of USP14 by IU1 treatment accelerated the degradation of proteins under proteotoxic stress in MM
[141], but inhibition of both 19S proteasome-associated DUBs resulted in the accumulation of polyubiquitinated proteins
[130]. Taken together, these results suggest a redundancy between USP14 and UCHL5, with either one required for proteasomal function.
We propose that deubiquitinating activity of the 19S regulatory subunit of proteasome can be a potential pharmacological target for cancer treatment. Use of broad-spectrum DUB inhibitors along with proteasome inhibitors such as bortezomib can be effective in the treatment of hematological malignancies. Further investigation of the role of USP14 may provide a preliminary theoretical basis for its application in clinical research.
5.6. USP24
USP24 is a 2620-amino-acid protein containing a catalytic ubiquitin C-terminal hydrolase domain and an ubiquitin-associated domain. Although the function of USP24 is poorly understood, a recent study demonstrated overexpression of USP24 protein in certain cancer types during the later stages of disease progression. Studies have also demonstrated that USP24 promotes cancer malignancy by inducing IL-6 transcription into tumor-infiltrating leukocytes, vascular endothelial cells, and cancer-associated fibroblasts
[142]. In another study, selective inhibition of USP9X by the inhibitor WP1130 reduced MCL1 levels and induced apoptosis in MM cells. However, significant upregulation of USP24 was observed when USP9X was inhibited, emphasizing its role in MM cell survival
[143]. USP24 was also found to regulate survival of MM cells in absence of USP9X. Direct USP24 knockdown resulted in apoptosis of myeloma cells associated with a reduction in MCL1 levels. Dose-dependent inhibition of USP9X and USP24 activity by a modified compound of WP1130, EOAI3402143, increased cell apoptosis and completely regressed myeloma tumors in mice models
[127]. Even though certain reports suggest an indirect role of USP24 in certain hematological malignancies, the function of USP24 in disease prognosis remains unclear. Further studies should help identify the role of USP24-mediated post-translational modification in the interaction of tumor cells and tumor associated microenvironment.
5.7. CYLD
NF-κB transcriptional factors and associated signaling pathways play a central role in activation of the innate and adaptive immune responses, and are involved in cancer development, tumor angiogenesis, and progression
[144]. CYLD is a negative regulator of NF-κB signaling and its loss inhibits apoptosis by activation and expression of NF-kB-dependent cells’ pro-survival genes
[145]. The CYLD gene was first identified in association with suppression of multiple skin tumors in cases of familial cylindromatosis
[146]. CYLD is an essential mediator of RIPK1- and RIPK3-dependent necroptosis
[145].
Proteolytic cleavage of CYLD through the para-caspase MALT-1 tissue results in NF-κB activation, which is a key step in the initiation of T-cell acute lymphoblastic leukemia (T-ALL)
[147][148][147,148]. Notch signaling regulates activation of the NF-κB signaling cascade and facilitates NF-κB nuclear retention during T-cell activation
[149]. Dysregulated Notch gene expression is a common feature of acute T-ALL
[150]. Recent evidence suggests that Notch-induced activation of NF-κB pathways plays a key role in T-cell leukemia, and the degree of downregulation of NF-κB is correlated with the severity of the disease
[151]. CYLD-mediated suppression of NF-κB signaling and Iκ-B kinase (IκK) expression and function weakens human T-ALL cells and also represses tumor growth in animal models
[152].
CYLD negatively regulates mitosis and cytokinesis
[153][154][153,154], and plays an important role in the regulation of microtubule dynamics and cell migration
[155][156][155,156] and apoptosis. CYLD can therefore be a novel target for the development of therapeutics against hematological malignancies. As is evident in previous studies, regulation of NF-κB signaling plays an important role in the initiation and pathogenesis of hematological malignancies. Along with CYLD, A20, and other DUBs, such as USP10
[157], USP11
[158], USP21
[159], USP15
[160], and OTULIN
[161], play important roles in the activation or inhibition of the NF-κB pathway. The studies make it clear that DUBs play vital roles in ensuring optimal signal transduction and homeostasis through tight regulation of cell death and NF-κB activation. The identification of novel DUBs and cross-talk between DUBs, which may reveal further mechanisms and functions of these DUBs in the pathogenesis of hematological malignancies. Screening of DUB-conditional knockout cell lines or animal models will likely help identify novel DUB targets and clarify their functions beyond inflammation, tumor suppression, and immunity. Although the data available regarding the role of DUBs in hematological malignancies continues to grow, the ultimate challenge will be to apply and translate this fundamental knowledge to the development of effective and novel therapies.
6. Deubiquitinases as Emerging Targets against Hematological Malignancies
Ubiquitination and deubiquitination play critical roles in various biological pathways closely associated with development of various cancers. Although knowledge about the precise role of DUBs in cancer pathology is limited, the list of DUBs that are altered genetically in human cancer cases has grown rapidly in recent years
[162]. Recent studies have presented DUBs as a genuine oncogene and tumor suppressor. DUBs that regulate cellular expression and turnover of oncogenic protein in various hematological malignancies have been identified. In recent years, inhibitors of the UPS have emerged as therapeutic targets for the treatment of various cancers. However, application of most of these inhibitors are hampered by low efficacy in hematological malignancies
[163]. The ability of DUBs to modulate the fate of a protein specifically and selectively gives them an advantage over targeting the UPS. For example, DUBs may increase or maintain levels of a tumor suppressor protein by decreasing its degradation by UPS or boost pathogenesis by reversing the fate of oncogenic proteins in the cell
[26]. Considering the advantages and ease of developing inhibitors over enzyme activators, research into the development of DUB inhibitors against hematological malignancies has been emphasized.
Recent approaches to targeting DUBs through various small-molecule inhibitors have produced promising results against various hematological malignancies. The novel regulatory particle b-AP15 together with lenalidomide, or dexamethasone, induces synergistic anti-MM activity
[140]. According to recent studies, b-AP15 and VLX1570 could also be a potential therapy for leukemia and WM by inhibiting 19S proteasome-associated DUBs such as USP14/UCHL5 and inducing tumor-cell apoptosis. VLX1570 along with dexamethasone was in clinical trial phase I/II but was terminated because of dose-limiting toxicity
[164]. Another potent DUB inhibitor WP1130, previously known as Degrasyn, targets deubiquitinases such as USP5, USP9X, USP14, USP24, and UCHL5. Recent studies concluded that WP1130-mediated inhibition of USP9X increases ubiquitination of an anti-apoptotic protein MCL1 which is highly expressed in drug-resistant MM tumors
[127][165][127,165]. The rapid degradation of MCL1 results in an increase in the sensitivity of these tumor cells to chemotherapy
[26][163][166][26,163,166]. USP24, which is closely related to USP9X, also plays a critical role in the survival of myeloma B cells by regulating MCL1 protein levels. Peterson and colleagues suggested that dual inhibition of USP9X and USP24 by WP1130 provides greater anti-myeloma activity. However, they developed an inhibitor that is three times as effective called EOAI3402143 (G9), which also had an improved therapeutic index. G9 inhibits USP5, increasing p53 accumulation and therefore is a promising approach to treating B-cell malignancies
[127][167][127,167]. At present, studies of USP7 and USP10 inhibitors (HBX19818 and P22077), which play key roles in many cellular processes, are under way. A lead-like inhibitor, HBX41108 and HBX19818, has been found to inhibit the catalytic activity of USP7 and induce p53-dependent apoptosis
[168][169][168,169].
Preclinical data demonstrated the anti-tumor efficacy of P5091 with lenalidomide, histone deacetylase inhibitor SAHA, or dexamethasone by inducing tumor-cell apoptosis in MM disease models
[118]. It also stabilizes p53 by inhibiting USP7 mediated-deubiquitination of MDM2, which degrades p53 tumor suppressors
[115][170][171][115,170,171], thus inhibiting cancer cell proliferation. HBX19818, P5091, as well as their analogs, including P045204, P22077, and HBX41108 have been shown to be potent inhibitors of USP7
[172]. P217564, a second-generation inhibitor, binds to the active site of USP7, inhibiting its activity
[173]. Along with USP7, P22077 and HBX19818 has also been reported to inhibit USP10, promoting degradation of FLT3-mutant AML cells
[174]. A small-molecule inhibitor, spautin-1 (for
specific and potent
autophagy
inhibitor-1), inhibits autophagy, and two DUBs, USP10 and USP13, which deubiquitinates two tumor suppressors, Beclin1, a subunit of Vps34 complexes, and p53
[175].
Despite the development of several inhibitors against DUBs, none of them have gone on to clinical trials, likely due to the complex structure of the catalytic domain of DUBs that share similarities with other DUB family members. Upon ubiquitin-binding, the active sites of DUBs also undergo conformational changes, posing a challenge when designing as well as binding the inhibitor to its specific DUB. The UPS consists of two regulatory enzymes, E3 ligase, and DUBs, which work as a complex, and understanding the dynamics of the complex, not just the DUBs, is essential to engineering a specific inhibitor. Moreover, it has been well documented that diverse DUBs play crucial roles in many cellular processes. Future research should be channeled toward developing small-molecule inhibitors that target the conserved catalytic cysteine of DUBs with stable and selective substrate-binding efficacy using new technologies. A high-throughput screening method should be made available with which researchers can determine the combination of DUBs and/or inhibitors that best modulate active pathways in cancer.
A better understanding of regulatory DUBs involved in inhibition or activation of hematopoietic processes and pathologies is expected to open new frontiers in the development of novel therapeutic drugs that target hematological malignancies and disorders. The goals are to enhance our understanding of dysregulated DUBs in hematopoiesis; design new therapeutic targets, and establish biomarkers that could be used in diagnosis and prognosis.
7. Conclusions
Since its discovery, the UPS has emerged as a key regulator of various proteins and factors involved in hematopoiesis, erythropoiesis, and angiogenesis. The roles of E1, E2, and E3 enzymes in governing the various pathways involved in hematopoietic regulation and pathologies have been studied extensively, but knowledge about the reversal of the activity of DUBs and their involvement in various hematological processes is limited. Several studies provide insight into dysregulated functioning of DUBs in various hematopoietic cells, which contribute to hematological pathologies. In this review, we described various DUBs that directly or indirectly regulate various hematopoietic processes.
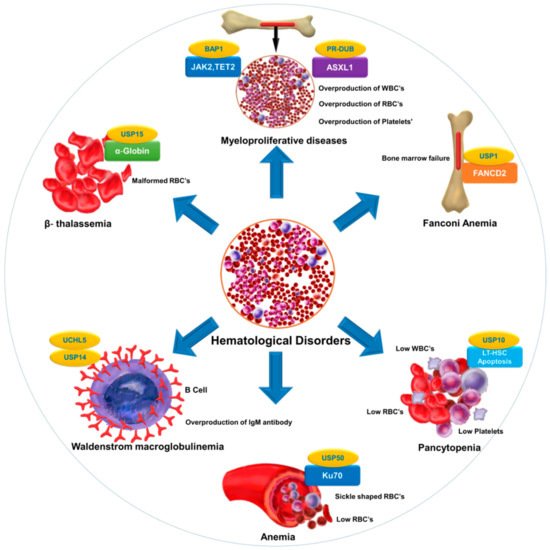
Selective inhibition or overexpression of DUBs has helped elucidate their roles in hematopoiesis, erythropoiesis, angiogenesis, and related abnormalities. Potent selective inhibitors of DUBs have shown promise for the treatment of hematological malignancies. Hematological disorders are the result of one or more malfunctioning components in the blood caused by intrinsic factors. DUBs are thought to play an important role in the etiology of various diseases and disorders and are therefore attractive drug targets. However, limited knowledge about substrate specificity and the molecular mechanisms of action of DUBs currently restricts their utility as novel therapeutic targets ().
Figure 2. DUB as a novel target in hematological diseases. DUBs regulate the level and function of a protein by catalyzing removal of ubiquitin from the substrate protein. Dysregulation of DUBs contributes to the pathogenesis of various hematological disorders. The figure illustrates different hematological disorders and the associated DUBs that can provide novel targets for therapeutic interventions to treat these disorders.
provides a list of DUBs associated with blood disorders. Identification of other DUBs involved in the pathogenesis of other hematological disorders and more complete insight into the regulatory mechanisms of DUBs that govern disease progression will provide new perspectives in therapeutics.
Table 1. List of DUBs involved in hematological disorders.
| Disorder |
Associated Substrate |
Regulatory DUB |
Reference |
| Fanconi anemia |
FANCD2 |
USP1 |
[63][176] | [63,176] |
| Anemia |
Ku70 |
USP50 |
[8] |
| β-thalassemia |
α-globin |
USP15 |
[177] |
| Pancytopenia |
Reduction in LT-HSC |
USP10 |
[31] |
| Myeloproliferative diseases |
ASXL1, EZH2,JAK2,TET2 |
PR-DUB,BAP1 |
[178][179] | [178,179] |
| Waldenstrom macroglobulinemia (WM) |
Overexpression of USP14 and UCHL5 in drug-resistant WM-tumor cells |
USP14 and UCHL5 |
[137][180] | [137 | [181] | ,180,181] |
| Bone marrow failure |
B-cell factor 1 (Ebf1), paired box 5 (Pax5), and other B-lymphoid genes |
MYSM1 |
[45][182] | [45,182] |
| Malaria |
CD8+ T cells |
CYLD |
[162][183] | [162,183] |
Table 1. List of DUBs involved in hematological disorders.
Although DUBs may play important roles in the regulation of hematopoiesis, many questions about their involvement remain unexplored. The exact DUBs-mediated signaling pathways responsible for the progression of hematological disorders have yet to be elucidated. Much of this knowledge gap stems from the ability of a given DUB to regulate several hundreds of protein substrates. At the same time, a given protein substrate can be regulated by more than one DUB.
Despite progress toward the development of DUB inhibitors, a lack of specificity limits clinical application of a wide range of DUBs
[112]. This limitation may be overcome by improved understanding of self- versus trans-regulation of DUBs and applying this information to inhibitor synthesis prior to applying such DUB inhibitors to preclinical and clinical research. Furthermore, another possible mechanism of “dubbing” DUBs in cases where a particular DUB itself is regulated by another DUB should also be seriously considered
[13]. Expanding research on dubbing DUBs is expected to offer novel insights into understanding DUB regulatory networks and implementing innovative strategies to uncover and develop molecular therapies to treat hematopoietic diseases.
The DUBs discussed in this review raise the question of whether there is a master DUB regulator that can deubiquitinate multiple DUBs. However, conventional DUB-mediated therapeutic approaches involve targeting DUBs that contribute to hematological pathologies. Dubbing DUBs may allow for screening for a master DUB that regulates the key DUBs implicated in hematological pathologies. Once such master DUBs are identified, the task of generating selective and specific inhibitors of DUBs implicated in hematologic disorders may become easier. We hypothesize that mapping an exclusive inter-DUB regulatory network combined with wide proteomic-scale screening of crucial DUBs will increase our understanding of several unknown links that may be related to the DUB regulatory network in hematological malignancies.
The role of the DUBs in HSC maintenance and differentiation remains an active area of research. Further investigation into the localization and substrate specificity of DUBs, their interactions with other hematopoietic factors, and other data gaps will improve our knowledge about their role in hematopoiesis. Research in this direction will facilitate the development of specific inhibitors against the key DUBs implicated in hematopoiesis. Doing so, while minimizing undesirable side effects, should lead to exciting new opportunities in treating hematologic malignancies.