Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Lutfun Nahar.
Dihydrofuranocoumarin, chalepin (1) and furanocoumarin, chalepensin (2) are 3-prenylated bioactive coumarins, first isolated from the well-known medicinal plant Ruta chalepensis L. (Fam: Rutaceae) but also distributed in various species of the genera Boenminghausenia, Clausena and Ruta.
- Ruta chalepensis
- Rutaceae
- chalepin
- chalepensin
1. Introduction
Chalepin (1; mol formula: C19H22O4; mol weight 314) and chalepensin (2; mol formula: C16H14O3; mol weight 254) (Figure 1) are, respectively, a dihydrofuranocoumarin and a furanocoumarin, with a prenylation at C-3 of the coumarin core structure. These coumarins, as the names imply, were first isolated from Ruta chalepensis L. (Fam: Rutaceae), but are also found in other Ruta species, e.g., R. angustifolia and a few other plants of the genus Clausena (Fam: Rutaceae), e.g., Clausena anisata (Willd.) Hook. F. ex Benth. [1,2,3,4][1][2][3][4]. While chalepin (1), also known as heliettin, is optically active, chalepensin (2), also known as xylotenin, does not possess any optical activity. Although these coumarins are rather rare in the sense that there are not many 3-prenylated naturally occurring furanocoumarins reported to date, there are quite a good number of bioactivity studies carried out on these compounds. The present review critically appraises publications on bioactivity of these 3-prenylated furanocoumarins in the light of their feasibility as novel therapeutic agents and covers their natural distribution in the plant kingdom, as well as a plausible biosynthetic route.
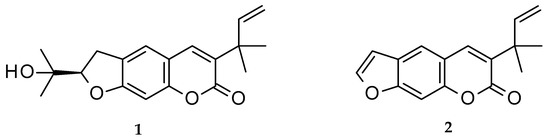
Figure 1.
Structures of chalepin (
1
) and chalepensin (
2
).
2. Distribution
First isolated from Ruta chalepensis more than half a century ago, chalepin (1) and chalepensin (2) have been further reported mainly from various species of the genera Clausena and Ruta of the family Rutaceae [4,5][4][5]. It appears that these compounds exclusively occur in the family Rutaceae [1,2,3[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20],4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20], and predominantly within these two genera. However, Boenminghausenia albiflora var. japonica (Hook.) Rchb. Ex Meisn and B. sessilicarpa H. Lev. also produce chalepensin (2) [6,20][6][20] and this genus is phylogenetically close to the genus Ruta [21]. Chalepensin (2) was further found in the leaves of Esenbeckia alata (Karst and Triana) Tr. and Pl. [9], while E. grandiflora Mart. was reported to produce chalepin (1) [10]. Interestingly, the genus Esenbeckia Kunth. is a part of a small group of phylogenetically distant Rutaceae including the genera Clausena and Ruta, where 3-prenylated coumarins like 1 and 2 are generally produced [9]. Thus, co-occurrence of these 3-prenylated furanocoumarins in these genera might have some chemotaxonomic implications, at least at the family level, within the family Rutaceae. The distribution of these two coumarins (1 and 2) is summarized in Table 1. Within the source plants these compounds are well distributed almost in all parts, leaves, stem, flowers and fruits. Although not chalepensin (2) itself, a series of 5-O-prenylated chalepensin derivatives were reported from Dorstenia foetida Schweinf., a medicinal plant from the family Moraceae, distributed in various countries in the Middle-East Asia [22].
Table 1.
Distribution of chalepin (
1
) and chalepensin (
2
) in the plant kingdom.
| Plant Names | Family | Chalepin (1) | Chalepensin (2) | References |
|---|
| Boenminghausenia albiflora | var. | japonica | (Hook.) Rchb. Ex Meisn. | Rutaceae | − | + | [6] |
| Boenminghausenia sessilicarpa | H. Lev. | Rutaceae | − | + | [20] | ||
| Clausena anisata | (Willd.) Hook. F. ex Benth. | Rutaceae | + | − | [4,7] | [4][7] | |
| Clausena emarginata | C. C. Huang | Rutaceae | + | − | [4,7] | [4][7] | |
| Clausena indica | (Dalz.) Oliver | Rutaceae | + | + | [1] | ||
| Clausena lansium | (Lour.) Skeels | Rutaceae | + | + | [8] | ||
| Esenbeckia alata | (Karst & Triana) Tr. & Pl. | Rutaceae | − | + | [9] | ||
| Esenbeckia grandiflora | Mart. | Rutaceae | + | − | [10] | ||
| Ruta angustifolia | L. Pers | Rutaceae | + | − | [3,11,12] | [3][11][12] | |
4. Drugability’ of Chalepin (1) and Chalepensin (2)
“Drugability” can simply be defined as the ability of a compound to be used as a pharmaceutical drug. In order for a molecule to be developed as a drug, it must have certain physicochemical characteristics, which can be measured or predicted by various experimental or mathematical models. The Lipinski rule of five, formulated in 1997 by Christopher A. Lipniski, can be used, albeit not conclusively, to predict whether a compound could be an ideal candidate as a drug molecule, i.e., whether a compound possesses “druglikeness” or not [57][27]. This rule states that an orally active drug does not have more than one violation of the following criteria: a molecular mass less than 500 Daltons, no more than five hydrogen donors, no more than 10 hydrogen bonds and an octanol-water partition coefficient (log P) that does not exceed five. Sometimes an additional criterion, “molar refractivity should be between 40–130” is also added to the above rule. If we consider these criteria in relation to chalepin (1) and chalepensin (2), both compounds tend to follow Lipinski rule of five, and there is no violation of this rule whatsoever (Table 3), which suggests that these compounds possess “druglikeness” or “drugability” and have the potential for further development as commercial drugs. However, it must be noted that this rule of five was originally presented to aid the development of orally bioavailable drugs and was not intended for guiding the medicinal chemistry in the development of all small-molecule drugs. Moreover, there is hardly any reliable experimental bioavailability data available on these coumarins (1 and 2) to make any connections between bioavailability and the predicted values for the criteria shown in Table 3.
Table 3. “Druglikeness” of chalepin (1) and chalepensin (2) *.
| Criteria | Chalepin (1) | Chalepensin (2) | ||||
|---|---|---|---|---|---|---|
| Molar mass | 314 | 254 | ||||
| Hydrogen bond donor | 1 | 0 | ||||
| Hydrogen bond acceptors | 4 | 3 | ||||
| Log | P | 3.72 | 4.32 | |||
| Molar refractivity | 86.6 cm | 3 | 72.5 cm | 3 | ||
| Lipinski rule of 5 violation | 0 | 0 | ||||
| Ruta chalepensis | ||||||
| L. | ||||||
| Rutaceae | + | + | [ | 5,13,14,15] | [5][13][14][15] | |
| Ruta graveolens | L. | Rutaceae | + | + | [16,17] | [16][17] |
| Ruta montana | L. | Rutaceae | − | + | [18] | |
| Stauranthus perforatus | Liebm. | Rutaceae | + | + | [19] |
+ = Found; − = Not found.
3. Biosynthesis
Like all other coumarins, the biosynthesis of chalepin (1) and chalepensin (2) begins from the simple coumarin umbelliferone, which is formed from the amino acid L-phenylalanine through the formation of trans-cinnamic acid, p-coumaric acid, 2-hydroxy-p-coumaric acid, 2-glucosyloxy-p-coumaric acid and 2-glucosyloxy-p-cis-coumaric acid aided by different enzymes, e.g., cinnamate 4-hydroxylase and 4-coumarate-CoA ligase, 4-coumaroyl 2′-hydroxylase (Figure 2) [23,24][23][24]. Sharma et al. [25] studied the biosynthesis of chalepin (1) in Ruta graveolens. They suggested that 3-(1,1-dimethylallyl)-umbelliferone could be the key intermediate for the biosynthesis of chalepin (1), and the dihydrofuran moiety in chalepin (1) is formed via prenylation, aided by dimethylallyldiphosphate, at C-6 of the core coumarin skeleton followed by oxidative cyclization with neighboring hydroxyl function at C-7. Generally, prenyltransferases (6-prenyltransferase was identified in R. graveolens as a plastidic enzyme) are considered the enzymes involved in the biosynthesis of furano-/dihydrofuranocoumarins through umbelliferone prenylation. Further oxidation of chalepin (1) could lead to the formation of the furanocoumarin chalepensin (2) in a similar fashion as observed in the conversion of marmesin to psoralen [26]. In fact, biosynthesis of chalepin (1) resembles that of 3-prenylated furanocoumarin, rutamarin (acetyl-chalepin) [26]. At this moment, it is not clear from the literature if the prenylation at C-3 takes precedence over that on C-6. In fact, the published information on the biosynthesis of these coumarins 1 and 2 is rather extremely limited, and much work, especially using radioisotopes is much needed to explore other possible routes to the biosynthesis of these compounds.
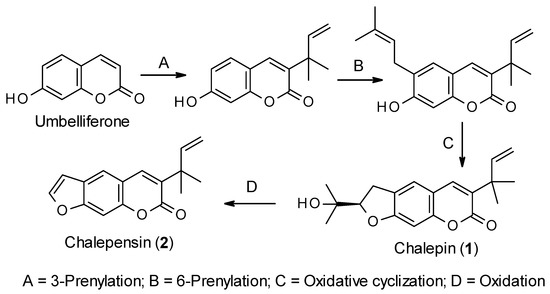
Figure 2.
Putative biosynthetic route for the formation of chalepin (
1
) and chalepensin (
2
).
* Data obtained from ChemSpider (www.chemspider.com, (accessed on 24 February 2021)) and DrugBank (https://go.drugbank.com/drugs/DB02205, (accessed on 24 February 2021)).
References
- Joshi, B.S.; Gawad, D.H. Isolation of some furanocoumarins from Clausena indica and identity of chalepensin with xylotenin. Phytochemistry 1971, 10, 480–481.
- Mohr, N.; Budzikiewicz, H.; El-Tawil, B.A.H.; El-Beih, F.K.A. Further furoquinoline alkaloids from Ruta chalepensis. Phytochemistry 1982, 21, 1838–1839.
- Richardson, J.S.M.; Sethi, G.; Lee, G.S.; Malek, S.N.A. Chalepin: Isolated from Ruta angustifolia L. Pers induces mitochondrial mediated apoptosis in lung carcinoma cells. BMC Complementary Altern. Med. 2016, 16, 389.
- Tamene, D.; Endale, M. Antibacterial activity of coumarins and carbazole alkaloids from roots of Clausena anisata. Adv. Pharmacol. Sci. 2019, 2019, 5419854.
- Quintanilla-Licea, R.; Mata-Cardenas, B.D.; Vargas-Villarreal, J.; Bazaldua-Rodriguez, A.F.; Angeles-Hernandez, I.K.; Garza-Gonzalez, J.N.; Hernandez-Garcia, M.E. Antiprotozoal activity against Entamoeba histolytica of plants used in northeast Mexican traditional medicine. Bioactive compounds from Lippia graveolens and Ruta chalepensis. Molecules 2014, 19, 21044–21065.
- Yamahara, J.; Miki, S.; Murakami, H.; Matsuda, H.; Fujimura, H. Screening test for calcium antagonists in natural products. The active principles of Boebminghausenia albiflora var. japonica. Yakuhaku Zasshi 1987, 107, 823–826.
- Hu, S.; Guo, J.-M.; Zhang, W.-H.; Zhang, M.-M.; Liu, Y.-P.; Fu, Y.-H. Chemical constituents from stems and leaves of Clausena emarginata. China J. Chin. Mater. Med. 2019, 44, 2096–2101.
- Adebajo, A.C.; Iwalewa, E.O.; Obuotor, E.M.; Ibikunle, G.F.; Omisore, N.O.; Adewunmi, C.O.; Obaparusi, O.O.; Klaes, M.; Adetogun, G.E.; Schmidt, T.J.; et al. Pharmacological properties of the extract and some isolated compounds of Clausena lansium sten bark: Anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 2009, 122, 10–19.
- Garcia-Beltran, O.; Areche, C.; Cassels, B.K.; Suarez, L.E.C. Coumarins isolated from Esenbeckia alata (Rutaceae). Biochem. Syst. Ecol. 2014, 52, 39–40.
- Oliveira, F.M.; Santana, A.E.G.; Conserva, L.M.; Maia, J.G.S.; Guilhon, G.M.P. Alkaloids and coumarins from Esenbeckia species. Phytochemistry 1996, 41, 647–649.
- Richardson, J.S.M.; Aminudin, N.; Malek, A.; Nurestri, S. A Compound from Ruta angustifolia L. Pers exhibits cell cycle arrest at s phase, suppresses nuclear factor-kappa B (NF-kappa B) pathway, signal transducer and activation of transcription 3 (STAT3) phosphorylation and extrinsic apoptotic pathway in non-small cell lung cancer carcinoma (A549). Pharmacogn. Mag. 2017, 13, S489–S498.
- Fakai, M.I.; Malek, S.N.A.; Karsani, S.A. Induction of apoptosis by chalepin through phosphatidylserine externalisations and DNA fragmentation in breast cancer cells (MCF7). Life Sci. 2019, 220, 186–193.
- Ulubelen, A.; Ertugrul, L.; Birman, H.; Yigit, R.; Erseven, G.; Olgac, V. Antifertility effects of some coumarins isolated from Ruta chalepensis and R. chalepensis var. latifolia in rodents. Phytother. Res. 1994, 8, 233–236.
- Elbeih, F.K.; Eltawil, B.A.H.; Baghlaf, A.O. Constituents of local plants.12. Coumarin and chalepensin, a further constituent of Ruta chalepensis L. J. Chin. Chem. Soc. 1981, 28, 237–238.
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70.
- Orlita, A.; Sidwa-Gorycka, M.; Paszkiewicz, M.; Malinski, E.; Mumirska, J.; Siedlecka, E.M.; Lojkowska, E.; Stepnowski, P. Application of chitin and chitosan as elicitors of coumarins and furoquinoline alkaloids in Ruta graveolens l. (Common rue). Biotechnol. Appl. Biochem. 2008, 51, 91–96.
- Wu, T.S.; Shi, L.S.; Wang, J.J.; Iou, S.C.; Chang, H.C.; Chen, Y.P.; Kuo, Y.H.; Chang, Y.L.; Teng, C.M. Cytotoxic and antiplatelet aggregation principles of Ruta graveolens. J. Chin. Chem. Soc. 2003, 50, 171–178.
- Ulubelen, A.; Doganca, S. Constituents of the aerial parts of Ruta montana. Fitoterapia 1991, 62, 279.
- Anaya, A.L.; Macias-Rubalcava, M.; Cruz-Ortega, R.; Garcia-Santana, C.; Sanchez-Monterrubio, P.N.; Hernandez-Bautista, B.E.; Mata, R. Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico. Phytochemistry 2004, 66, 487–494.
- Yang, Q.-Y.; Tian, X.-Y.; Fang, W.-S. Bioactive coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007, 9, 59–65.
- Wei, L.; Xiang, X.-G.; Wang, Y.-Z.; Li, Z.-Y. Phylogenetic relationships and evolution of the Androecia in Ruteae (Rutaceae). PLoS ONE 2015, 10, e0137190.
- Heinke, R.; Franke, K.; Porzel, A.; Wessjohann, L.A.; Ali, N.A.A.; Schmidt, J. Furanocoumarins from Dorstenia foetida. Phytochemistry 2011, 72, 929–934.
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. Prog. Chem. Org. Nat. Prod. 2017, 106, 241–304.
- Nahar, L.; Sarker, S.D. Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry, 2nd ed.; Wiley and Sons: Chichester, UK, 2019.
- Sharma, R.B.; Raj, K.; Kapil, R.S. Biosynthesis of chalepin in Ruta graveolens. Indian J. Chem. Sect. B—Org. Chem. Incl. Med. Chem. 1998, 37, 247–251.
- Grundon, M.F. Biosynthesis of aromatic hemiterpenes. Tetrahedron 1978, 34, 143–161.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26.
More
