1. Clinical Importance of Interferon-Based Biopharmaceuticals and Market Overview
The lack of effective therapies for the treatment of a variety of human diseases has caused numerous health issues
[1], representing the major driving force of Research and Development (R&D) activities toward the development of innovative medicines. In this regard, the emergence of biopharmaceuticals has allowed tremendous improvements in life quality
[2], being at the cornerstone of the progress achieved in the last decades on the prevention and treatment of a wide range of diseases (e.g., cancer, infectious diseases, neurodegenerative diseases, among others). Biopharmaceuticals, also called biotherapeutics or biologicals, are products of biological origin such as proteins, nucleic acids, blood-derived products, somatic cells, or derivatives that are produced or extracted from living sources (e.g., microorganisms, cells, plants, or animals)
[3][4][5][3,4,5]. Nowadays, recombinant therapeutic proteins and antibodies are considered the most abundant types of biopharmaceutical products in the market
[3]. The success of biopharmaceutical-based therapies is linked to the development of recombinant DNA technology in late 1970s, which has allowed the large-scale production of human proteins and strongly stimulated systematic clinical investigations using new therapeutic approaches
[6]. Following the approval of the first biopharmaceutical-insulin-in 1982, this market has been rapidly growing. According to the literature, from 2015 to 2018, approximately 112 biopharmaceuticals were approved in the United States of America (USA) and in the European Union (EU), essentially doubling the typical five-yearly historical approval pace and thus demonstrating the high demand for such products
[3]. The overall growth of the biopharmaceutical market occurs due to two factors: the first one is related to the increment in the use of this type of product, and the second is closely related to the appearance of biosimilars
[3][7][8][3,7,8]. Biosimilars are biological products similar to already existing medicines whose patents have expired
[7][8][7,8], entering into the market with lower costs while exhibiting the same effects (quality, safety, and effectiveness) as the original biopharmaceutical
[7]. Moreover, the global sales of therapeutic proteins have been increasing, being forecasted to increase on the approval of this type of therapeutic biomolecule in the coming years
[3][7][3,7].
Among therapeutic proteins, the role of interferons (IFN) should be underlined, as they have been marketed for over 30 years with a considerable impact on the global therapeutic proteins market
[3]. However, as recently highlighted by Timmerman
[9] on the history of interferon’s trajectory, from the viral interference to the Hoffmann-La Roche product (Roferon A
®, Hoffmann-La Roche, Basel, Switzerland), a series of obstacles had to be overcome-namely, restrictions to working with recombinant DNA, -to be in line with the interests of commercial partners and their demands for patent protection while addressing the desire by academic researchers working in the field for scientific outputs. IFN sales peaked between the 1980s and 2000s, as they were abundantly marketed and classified as “multiple drugs”, with an increasing range of therapeutic effects.
In a period of just six years, from 1986 to 1992, the world IFN market increased by approximately $740 million
[10]. More recently, the global IFN market was valued at $6.9 billion in 2019, and it was estimated that it could grow to about $7.5 billion by 2020 due to an increasing demand for the use of IFNs along with antiretrovirals and antimalarial drugs in the treatment of SARS-CoV-2 disease (COVID-19) patients
[11]. Furthermore, these projections are supported by the increasing incidence of chronic diseases, such as hepatitis B, hepatitis C, and multiple sclerosis, coupled with the use of IFNs in combinatorial therapies, the increasing adoption of IFN biosimilars with possible prophylactic or therapeutic effectiveness against virus pandemics, the advent of novel drug delivery systems, and continuous R&D activities involving IFNs
[11]. Due to their relevance, several IFN products are currently in different stages of clinical trials. By January 2021, 172 active clinical trials involving the application of therapeutic IFN-based products were at different stages of development: 2 are in early phase 1, 50 in phase 1, 70 in phase 2, 28 in phase 3, and 6 in phase 4 of clinical trials
[12].
The different clinical applications of IFNs and their corresponding marketed biological medicines are summarized in
[3][13][14][3,13,14]. Several IFN subtypes are well established in the market for the treatment of several pathologies, mainly in oncological treatment, as well as multiple sclerosis and chronic hepatitis C. To date, 21 formulations for the administration of IFN have received approval from EU and USA regulatory agencies, of which five have been withdrawn from the market—Infergen
® (Three Rivers Pharmaceuticals, Warrendale, PA, USA) in 2006 (EU), Roferon A
® (Hoffmann-La Roche, Basel, Switzerland) in 2007, Viraferon
® (Schering-Plough Corporation, Brussels, Belgium) in 2008 (EU), Albinterferon
®/Albuferon
® in 2010 (Novartis, Basel, Switzerland; Human Genome Sciences, Rockville, MD, USA), and ViraferonPeg
® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) in 2021 (EU). Rather than safety and efficacy issues, these products have been generally withdrawn from market due to requests of marketing authorization holders and the availability of similar products in market.
Table 1. Therapeutic interferons approved in the United States of America (USA) and European Union (EU).
|
| Interferon (IFN) Type/Subtype |
|
| Clinical Indication |
|
| Commercial Name |
|
| Active Pharmaceutical Ingredient |
|
| Approval Date |
|
|
| IFNα (I) |
|
| IFNα-2a |
|
| Hairy cell leukemia; AIDS-related Kaposi’s sarcoma; Chronic myelogenous leukemia; Cutaneous T-cell lymphoma; Chronic hepatitis B and C; Follicular lymphoma; Malignant melanoma |
|
| Roferon A® Hoffmann–La Roche (Basel, Switzerland) |
|
| IFNα-2a (E. coli) |
|
| 1986 (EU) |
| 1986 (USA) |
|
|
| Chronic hepatitis B; Chronic myelogenous leukemia; Melanoma |
|
| Pegasys® Hoffmann–La Roche (Basel, Switzerland) |
|
| PEGylated IFNα-2a (E. coli) |
|
| 2002 |
| (USA and EU) |
|
|
| IFNα-2b |
|
| Multiple myeloma; Chronic myelogenous leukemia; Chronic hepatitis B and C; Carcinoid tumor; Hairy cell leukemia; Follicular lymphoma; Malignant melanoma; Condylomata acuminate; Kaposi’s sarcoma |
|
| Intron A®, Alfatronol® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) |
|
| IFNα-2b (E. coli) |
|
| 1986 (USA) |
| 1986 (EU) |
|
|
| Chronic hepatitis B and C |
|
| Viraferon® (Schering-Plough Corporation, Brussels, Belgium) |
|
| IFNα-2b (E. coli) |
|
| 2000 (EU) |
|
|
| Chronic hepatitis C |
|
| Rebetron® (Schering-Plough Corporation, Brussels, Belgium) |
|
| ribavirin/IFNα-2b (E. coli) |
|
| 1999 (USA) |
|
|
| Chronic hepatitis C |
|
| ViraferonPeg® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) |
|
| PEGylated IFNα-2b (E. coli) |
|
| 2000 (EU) |
|
|
| Chronic hepatitis C |
|
| PegIntron® (Schering-Plough Corporation, Brussels, Belgium) |
|
| PEGylated IFNα-2b (E. coli) |
|
| 2001 (USA) |
| 2000 (EU) |
|
|
| Chronic hepatitis C |
|
| Albinterferon®/Albuferon® (Novartis—Basel, Switzerland; Human Genome Sciences, Rockville, MD, USA) |
|
| Fusion protein of albumin and IFNα-2b (E. coli) |
|
| 2010 (USA) |
|
|
| Melanoma |
|
| Sylatron™ (Merck & Co., Inc, Kenilworth, NJ, USA) |
|
| PEGylated IFNα-2b (E. coli) |
|
| 2011 (USA) |
|
|
| IFNα-2c |
|
| Chronic viral hepatitis; HIV infection |
|
| Berofor® (Boehringer Ingelheim, Lda, Ingelheim am Rhein, Germany) |
|
| IFNα-2c (E. coli) |
|
| 1989 (USA) |
|
|
| IFNα (I) |
|
| IFNα-n3 |
|
| Condyloma acuminate |
|
| Alferon N® AIM ImmunoTech (Philadelphia, PA, USA) |
|
| IFNα-n3 (human leukocytes) |
|
| 1987 (USA) |
|
|
| IFNα-n1 |
| (lymphoblastoid) |
|
| Chronic hepatitis B and C; Hairy cell leukemia; HPV infection |
|
| Wellferon®Glaxo Wellcome (London, United Kingdom) |
|
| IFNα-n1 (human lymphoblastoid cells) |
|
| 1997 (USA) |
|
|
| IFNα-con-1 |
|
| Chronic hepatitis C |
|
| Infergen® (Three Rivers Pharmaceuticals, Warrendale, USA) |
|
| IFNα (E. coli) |
| IFNα + Ribavirin ( E. coli) |
|
| 2001(USA) |
|
|
| IFNβ (I) |
|
| INFβ-1a |
|
| Multiple sclerosis |
|
| Avonex® (Biogen Idec, Maidenhead, United Kingdom) |
|
| IFNβ-1a (CHO cells) |
|
| 1996 (USA) |
| 1997 (EU) |
|
|
| Rebif® (EMD Serono, London, United Kingdom) |
|
| Glycosylated IFNβ-1a (CHO cells) |
|
| 2002 (USA) |
| 1998 (EU) |
|
|
| Plegridy® (Biogen Idec, Maidenhead, United Kingdom) |
|
| PEGylated IFNβ-1a (CHO) |
|
| 2014 (EU and US) |
|
|
| INFβ-1b |
|
| Multiple sclerosis |
|
| Betaseron® (Chiron—Emeryville, USA; Berlex Laboratories, Richmond, VA, USA) |
|
| IFNβ-1b (differs from human protein in that Cysteine-17 is replaced by Serine) (E. coli) |
|
| 1993 (USA) |
|
|
| Betaferon® (Bayer Pharma, Leverkusen, Germany) |
|
| 1995 (EU) |
|
|
| Extavia® (Novartis Europharm, Camberley, United Kingdom; Novartis Pharmaceuticals, East Hanover, NJ, USA) |
|
| IFNβ-1b (E. coli) |
|
| 2008 (US) |
|
|
| IFNγ (II) |
|
| INFγ-1b |
|
| Chronic granulomatous disease; Osteopetrosis |
|
| Actimmune® (Vidara Therapeutics, Dublin, Ireland) |
|
| IFNγ-1b (E. coli) |
|
| 1990 (US) |
|
|
| Imukin® (Boehringer Ingelheim, Lda, Ingelheim am Rhein, Germany) |
|
| 1996 (US) |
|
Abbreviations: CHO–Chinese hamster ovary; E. coli–Escherichia coli. Note: Data taken from [3][13][14][3,13,14].
The commercialized formulations are produced mainly using Escherichia coli (E. coli) as host, except for Plegridy® (Biogen Idec, Maidenhead, UK), Rebif® (EMD Serono, London, UK), and Avonex® (Biogen Idec, Maidenhead, UK), which are produced using Chinese hamster ovary (CHO) cells, and Alferon N® (AIM ImmunoTech, Philadelphia, PA, USA) and Wellferon® (Glaxo Wellcome, London, UK)), respectively, from human leukocytes and human lymphoblastoid cells. Moreover, some of the final products are available as PEGylated versions of IFNs, such as PegIntron®/Rebetol® (Schering-Plough Corporation, Kenilworth, NJ, USA) combo pack, PEG-Intron® (Merck Sharp & Dohme, Kenilworth, NJ, USA), ViraferonPeg® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA), Intron A® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA), and Plegridy® (Biogen Idec, Maidenhead, UK), envisaging to enhance their stability and blood circulation half-life.
Considering the relevance of IFNs for the treatment of several pathologies and their projected role in novel therapeutic regimens, as well as their essential role in improving patient health, this review article provides a comprehensive overview of the manufacturing of IFN-based biopharmaceuticals. The first section addresses the description of interferon characteristics, classification, and signaling pathways. The history and evolution of the manufacturing of IFNs are overviewed in the second section, subcategorized into the upstream stage, downstream stage, and formulation and delivery, in which representative works are outlined. An outlook is presented at the end of this work, complemented with foreseeable prospects for underdeveloped aspects of biopharmaceutical research and therapeutics involving IFNs.
2. Interferons Classification and Mechanisms of Action
In 1957, Isaacs and Lindenmann first saw a viral interference effect caused by bioactive material isolated from infected cells
[15], thus assigning the term “
interferon” to this interfering agent. Later, in 1978, due to improved molecular biology tools and developments on the upstream stage allowed researchers to obtain sufficient amounts of IFN with which to perform a reduced physical and chemical characterization of this biomolecule
[16]. IFNs are natural cell-signaling glycoproteins produced by eukaryotic cells in response to viral infections, tumors, and other biological inducers, and thus represent part of the first line of defense of vertebrates against infectious agents
[13][17][13,17].
IFNs cannot be classified as a single protein
[16]; instead, they require use of different letters-α, β, and γ-to refer to the main classes of IFNs, which are, respectively, produced by leukocytes, fibroblasts, and lymphocytes (T cells and natural killer cells)
[18]. In 1985, a new class (ω) was introduced in humans
[19], and class τ
[20] was further discovered in ovine cells. Furthermore, depending on their properties and their ability to bind to cell receptors, IFNs can also be classified into three different types (I to III), with each type displaying the ability to bind to a specific receptor and to trigger different signal transduction pathways and immunological responses, as shown in .
Table 2. Classification of interferons based on the type of receptor through which signaling takes place. Adapted from Diamond and collaborators
[21].
|
| IFN Type |
|
| Class |
|
| Discovery Year |
|
| Receptor Binding |
|
|
| I |
|
| α |
|
| 1957 |
|
| High binding affinity to IFNAR2, which then recruits low-affinity IFNAR1 to form the signaling competent ternary complex |
|
|
| β |
|
| 1957 |
|
|
| ω |
|
| 1985 |
|
|
| τ |
|
| 1996 |
|
|
| II |
|
| γ |
|
| Early 1970s |
|
| Affinity for IFNGR (IFNGR1 and IFNGR2) |
|
|
| III |
|
| λ1 |
|
| 2003 |
|
| High binding affinity to IFNLR1, which then recruits low-affinity IL-10Rβ to form signaling competent ternary complex |
|
|
| λ2 |
|
|
| λ3 |
|
|
| λ4 |
|
2009 (EU) |
|
|
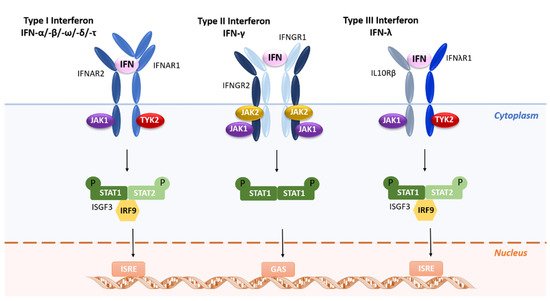
Briefly, type I IFNs bind to a heterodimeric receptor composed of two chains, IFNAR1 and IFNAR2, leading to the activation of the receptor-associated Janus-activated kinases (JAKs) TYK2 and JAK1, respectively ()
[22][23][24][25][22,23,24,25]. The next step in this signal transduction pathway is tyrosine phosphorylation of signal transducers and activators of transcription—STAT1 and STAT2—and the subsequent assembling of the heterotrimeric IFN-stimulated gene factor 3 (ISGF3) transcription factor complex. Distinctly, type II IFNs bind to a different cell-surface receptor consisting of IFNGR1 and IFNGR2 subunits, which in turn associate with JAK1 and JAK2, respectively, leading to phosphorylation of STAT1 ()
[26]. Finally, type III IFNs bind to a heterodimeric cytokine receptor composed of an IL-28R-binding chain and IL-10R2 that is shared with the IL-10 family of cytokines ()
[27]. The signaling cascade is like that of type I IFNs, in which the ISGF3 transcription factor complex binds to ISRE (IFN-stimulated response element) elements in gene promoters to induce transcription of IFN-inducible genes (ISGs). However, coordination and cooperation of multiple distinct signaling cascades, including the mitogen-activated protein kinase p38 cascade and the phosphatidylinositol-3-kinase cascade, are required for the generation of responses to IFNs
[13].
Figure 1.
Receptor activation or ligand-receptor complex assembled by type I, type II, or type III interferons.
Since their discovery by Isaacs and Lindenmann, IFNs have been known for their antiviral and antitumoral activities. These proteins own a broad spectrum of activity that impacts cellular metabolism and differentiation, and thus the antitumor effects appear to be due to a combination of direct antiproliferative effects, as well as indirect immune-mediated effects
[16][17][28][29][16,17,28,29]. Accordingly, IFNs have been used in clinical practice to promote immune responses against infections and to treat autoimmune disorders and cancer, among others
[16][17][16,17]. Furthermore, they can have synergistic or additive effects between them and with other biological response modifiers. The antiproliferative activity of IFN can be classified as direct or indirect
[17][29][30][17,29,30], depending on if they inhibit the growth of cancer cells by stopping the cell cycle, apoptosis, or differentiation
[17][30][17,30], or if they activate immune cells, such as T cells and natural killer (NK) cells, stimulating the immune system against oncogenesis and controlling tumor development
[29][30][29,30]. The antiviral mechanism of IFN, like the antiproliferative mechanism, is based on the control of gene expression
[17]. The antiviral response strongly depends on the virus, the host cell, and the type of IFN. The infection of a cell by a virus induces the production of IFN, which can then exert an autocrine or paracrine action on the surrounding cells. This phenomenon triggers the expression of proteins regulated by this IFN, which collectively constitute, in a very generalized way, the antiviral response responsible for inhibiting virus multiplication
[17][28][31][17,28,31]. Schreiber and coworkers
[32] determined the binding affinities (to isolated IFN receptor chains 1 and 2) and biological activity (antiproliferative and antiviral models) of IFNα subtypes. The authors found that the binding affinity and antiproliferative activity correlated with each other, but that for antiviral potency, there were several cases where the relationship appeared to be more complex than simple binding
[32]. According to the authors, the concordance of the binding with the activity for most of the subtypes suggests that receptor binding events play a major role in the activity profiles of these molecules
[32].
In sum, both the antiviral and antiproliferative mechanisms are based on the regulation of gene expression
[28][30][28,30]. The proteins produced in response to the transcription and translation of these genes can have a direct or indirect action, leading in the latter case to the joint work of several aspects of the immune system
[17][30][17,30]. Structural studies
[33][34][33,34] have shown that type I IFNs consist of five α-helices (labeled A–E), which are linked by one overhand loop (AB loop) and three shorter segments (BC, CD, and DE loops)
[23]. The detailed analysis of the structure of this subclass of IFNs revealed similar α-helical cores but large structural differences in AB loops. These insights demonstrate that subtle sequence differences and specific structural rearrangements influence the IFN-receptor interaction and may hold the key for the observed differences in biological activity
[23]. Additional details on the structure of IFNs and their influence on IFN biological activities have been reviewed elsewhere
[17][23][35][36][37][17,23,35,36,37].
3. Therapeutic Cloned Interferons
Commercial IFN-based products were first derived from leukocytes and then from lymphoblastoid lines
[36]. However, as both protein extraction from natural producers and chemical synthesis undergoes inherent constraints that limit regular large-scale production, recombinant DNA technologies have rapidly become a choice for therapeutic protein production, including IFNs
[38]. The relatively small size (Mw ~20 kDa) and compactness of the IFN protein, combined with the lack of any functional glycosylation (at least in some cases, unglycosylated IFNs are predicted to be functionally identical to their glycosylated counterparts), has contributed to high yield and improved bioactivity
[36]. These therapeutic proteins are obtained ex vivo mostly in biological systems and must guarantee, in addition to full protein functionalities, a cost-effective industrial manufacturing in the absence of impurities (host cell proteins, DNA, aggregates, among others)
[38].
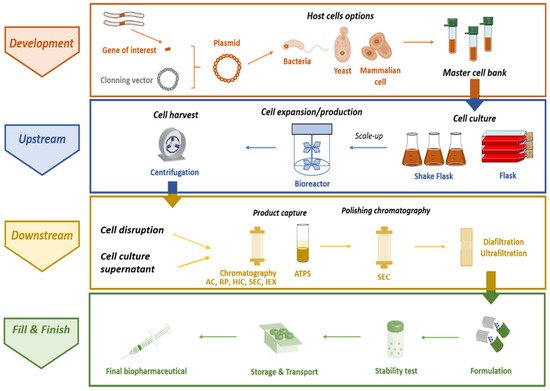
The complete manufacturing process to obtain recombinant therapeutic proteins comprises four main stages, summarized in : (i) the development stage, in which the gene of interest is isolated, cloned in a suitable plasmid, and then the recombinant plasmid is introduced in the selected host, allowing the master cell bank to be obtained; (ii) the production itself, or
upstream stage, which is associated with the choice of a particular expression system and respective culture conditions; (iii) the
downstream stage, including the recovery of the target protein, followed by its purification from a heterogeneous and highly complex matrix that generally encompasses chromatographic techniques (corresponding to the most expensive part of the process); and (iv) fill and finish, whereby the final product formulation is developed according to the method of administration, and the process must ensure that the stability and biological activity of the purified biopharmaceutical is maintained during storage and transport
[4][39][4,39]. Protein drugs must necessarily conform with quality constraints stricter than those expected in the production of enzymes for chemical industries, which consequently defines the choice of recombinant hosts, protocols, and production/purification strategies
[38]. Moreover, there is a generic consensus about the need to enable drugs for cell- or tissue-targeted delivery, aiming for a reduction in dosage, production costs, and side effects
[38]. To this end, therapeutic proteins are usually administered in formulations whose compositions are optimized to guarantee improved stability and delivery of target biopharmaceuticals. In general, the purity, activity, and safety of the finished products are ensured by critical aspects, including host cell development, cell culture, cell bank establishment, protein synthesis, purification process, and subsequent protein analysis, formulation, storage, and handling
[40].
Figure 2. Overview of the manufacturing of IFN-based biopharmaceuticals (ATPS–Aqueous two-phase system; AC–Affinity chromatography; IEX–Ion-exchange chromatography; HIC–Hydrophobic interaction chromatography; RP–Reverse phase chromatography; SEC–Size exclusion chromatography).