Mycotoxins are secondary metabolites of filamentous fungi that contaminate food products such as fruits, vegetables, cereals, beverages, and other agricultural commodities.
- contamination
- aflatoxins
- ochratoxin A
- patulin
- toxicity
- detoxification
1. Introduction
Mycotoxins are naturally occurring, poisonous compounds produced from filamentous fungi or molds that can be found in foods. Mycotoxins have a huge set of chemical compounds generated by diverse mycotoxigenic fungi species
[1]
. Over 400 toxic metabolites are produced by more than 100 fungi species
[2]
. Humans are exposed to mycotoxins through the consumption of contaminated foods
[3]
. They can pose negative health effects, ranging from acute toxicity to chronic symptoms, such as kidney damage, liver damage, immune deficiency, and cancer
.
Cereal grains and fruits can be infected by molds at various stages of production, for example, during cultivation, harvesting, and storage
[6]
. The contamination of mycotoxins is a worldwide problem, but it is more serious in humid and warm environmental conditions that favor the growth of fungi and production of mycotoxins. As secondary metabolites, mycotoxins are very durable chemical components that can be transmitted from raw materials to processed products such as beverages, which can pose a serious health risk to consumers (Figure 1).
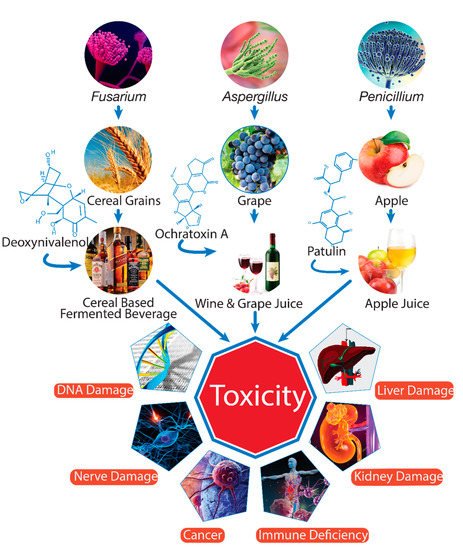
Figure 1.
Mycotoxin contamination of beverages and adverse effects on health (drawn using Adobe Illustrator CC software).
Over the last few years, distinguishable progress in society has driven reforms in the world beverage market. Consumers are becoming more conscious about the effect of diet on their health. Beverages are not only responsible for providing energy and hydration but also for strengthening health and preventing nutrition-related defeciencies
[7]
. The application of effective measures to protect consumers from the toxic effects of mycotoxins and, subsequently, to defend against public health is very significant and crucial.
2. Major Mycotoxins in Beverages
2.1. Aflatoxins
Aflatoxins (AFs) are mainly produced by Aspergillus spp. In most of the cases, contamination with AFs takes place after harvesting and during storage. Inappropriate management during transportation and storage including exposure to conditions such as high humidity (˃65%) and temperatures rapidly increases the AF concentration in food.
2.2. Ochratoxin A
Ochratoxins (OTs) are group of mycotoxins that are mostly generated by Aspergillus and Penicillium species. The occurrence of OTA-producing fungi and the level of OTA may vary with the climatic conditions
[8]
. OTA is generally found in subtropical areas and in high-temperate climate regions and can be available in various food products in these areas, for example, beer, wine, and grape products
[9]
. Table 1 summarizes the major mycotoxins responsible for the contamination of beverages.
Major mycotoxins involved in the contamination of beverages.
| Mycotoxins | Products Contaminated | Producing Microorganisms | References |
|---|
| Aflatoxins B1, B2, G1, G2 |
A. parasiticus, A. repens, A. ruber, A. tamarii, and | ||
| Aflatoxins | |||
| Orange, apple juice, grape juice, grapefruit peel | |||
B1, B2, G1, G2 | |||
| Aspergillus chevallieri | , | A. flavus, A. niger, A. oryzae, | |
| Orange, apple juice, grape juice, grapefruit peel | Aspergillus chevallieri, A. A. wentii | ||
| flavus | , | A. niger, A. oryzae, A. parasiticus, A. repens, A. ruber, A. tamarii, and A. wentii | |
| [ | 10 | ] | [11] |
| Ochratoxin A (OTA) | Grape juice, coffee, beer, and wine | A. ochraceus, A. carbonarius, A. niger, A. tubingensis, and | |
| Ochratoxin A (OTA) | Grape juice, coffee, beer, and wine | A. ochraceus, A. Penicillium expansum | |
| carbonarius | , | A. niger, A. tubingensis, and Penicillium expansum | |
| [ | 10 | ] | [12] |
| Patulin (PAT) | Fruit juices | Penicillium expansum, P. patulum, Aspergillus clavatus, Byssochlamys fulva, and B. nivea |
|
| [ | 13 | ] | [14] |
| Fumonisins (FBs) | Beer | Fusariumproliferatum, | |
| Fumonisins (FBs) | |||
| F. verticillioides | , | and | |
| Beer | Fusariumproliferatum, F. F. nygamai | ||
| verticillioides | , and F. nygamai | ||
| [ | 15 | ] | [16][17] |
| Trichothecenes (type B: Deoxynivalenol (DON)) |
Plant-based beverages, beer | F. graminearum, F. cerealis, and | |
| Trichothecenes (type B: Deoxynivalenol (DON)) |
Plant-based beverages, beer | F. graminearum, F. cerealis, and F. F. culmorum | |
| culmorum | |||
| [ | 16 | ] | [18][19][20] |
| Trichothecenes (type A: HT-2) |
Functional vegetable milks, beer | F. sporotrichioides,and F. langsethiae | |
| [ | 20 | ] | [21] |
| Trichothecenes (type A: T-2 toxin) |
Plant-based milks, beer | F. sporotrichioides, and | |
| Trichothecenes (type A: T-2 toxin) |
Plant-based milks, beer | F. sporotrichioides, and F. F. langsethiae | |
| langsethiae | |||
| [ | 19 | ] | [21] |
| Zearalenone (ZEN) | Beer, wine | F. graminearum, F. culmorum, F. equiseti, F. cerealis, F. verticillioides, and | |
| Zearalenone (ZEN) | Beer, wine | F. graminearum, F. F. incarnatum | |
| culmorum | , | F. equiseti, F. cerealis, F. verticillioides, and F. incarnatum | |
| [ | 16 | ] | [22] |
| Alternaria toxins (TeA, AOH, AME) | Fruit juices, wine, beer | Alternaria alternate, A. tenuissima, and A. arborescens | |
| [ | 23 | ] | [24] |
2.3. Patulin
Patulin (PAT) is predominantly generated from various Penicillium, Aspergillus, and Byssochlamys species and possesses various hazardous features such as toxicity, carcinogenicity, and mutagenicity. P. expansum, B. fulva, and B. nivea
Patulin (PAT) is predominantly generated from various Penicillium, Aspergillus, and Byssochlamys species and possesses various hazardous features such as toxicity, carcinogenicity, and mutagenicity. P. expansum, B. fulva, and B.
are significant PAT-producing microorganisms. Patulin has been identified in many foods, particularly in fruits and beverages
nivea are significant PAT-producing microorganisms. Patulin has been identified in many foods, particularly in fruits and beverages
[25]
.
2.4. Fumonisin
Fumonisin (FB) mycotoxins are secondary metabolites of Fusarium spp, mostly Fusarium verticillioides and
F. proliferatum
. It was found to be a contaminant of wheat, corn, and barley.
2.5. Trichothecenes
Trichothecenes (TCs) belong to a large group of structurally related toxins, mainly produced by fungal species of the Fusarium genus
[26]
. T-2 and HT-2 toxins have been detected in barley, oat, maize, wheat, rice, beer, and plant-based milks, especially in oat- and soy-based milks and beverages
[19][26][27][28]. Deoxynivalenol (DON) is synthesized by different species of the Fusarium genus, mainly by Fusarium culmorum and Fusarium graminearum
. Deoxynivalenol (DON) is synthesized by different species of the Fusarium genus, mainly by Fusarium culmorum and Fusarium
in cereals
graminearum in cereals
[29]
. It also contaminates cereal-based food products, for instance, pasta, bread, and beer.
2.6. Zearalenone
Zearalenone (ZEN) is produced by various species of Fusarium, mainly F. graminearum,
F. culmorum
, and F. sporotrichioides. It infects corn, wheat, barley, oat, and rye, mainly in areas with temperate climates
[30]
.
2.7. Alternaria Toxins
The main Alternaria mycotoxins are Tenuazonic acid (TeA), Alternariol (AOH), and alternariol monomethyl ether (AME). Alternaria
The main Alternaria mycotoxins are Tenuazonic acid (TeA), Alternariol (AOH), and alternariol monomethyl ether (AME). Alternaria spp., mainly Alternaria alternata, A.
spp., mainly Alternaria alternata, A. tenuissima, and
tenuissima, and
A. arborescens
produce Alternariols and are found in a large range of foods including berries, prune nectar, carrot juice, apple juice concentrate, grape juice, raspberry juice, cranberry juice, beer, and red wine
.
3. Detection and Quantification of Mycotoxins in Beverages
In most cases, mycotoxin levels in contaminated food and beverages can be very low, and this necessitates the development of a suitable, rapid, and sensitive detection method. Various analytical testing procedures have been developed for mycotoxin detection and quantification due to their diverse forms
[33]
. Normal chromatographic procedures are usually time consuming and cost intensive; therefore, a range of methods, mostly based on immunological principles, have been developed and commercialized for quick determination
[34]. Some common mycotoxin detection methods in beverages as well as beverage-producing crops are summarized in Table 3.
. Some common mycotoxin detection methods in beverages as well as beverage-producing crops are summarized in Table 2.
Overview of common detection methods for mycotoxins in beverages as well as beverage-producing crops.
| Analytical Methods | Detection Method | Toxin | Applicability | LOD | References | Advantages | Disadvantages |
|---|
| TLC | CCD | Patulin | Apple Juice | 14 µg/L | [35] | Time saving, specific fluorescence spot on UV light | Limited plate length and environmental effects on measurement |
| HPLC | FD | OTA | Wheat | 23 pg | [36] | Fast, high resolution data, accurate and easily reproducible. Less training required | Expensive and method development could be challenging |
| MS/MS | Wine | 0.005 ng/ml | [37] | ||||
| FD | 0.09 µg/L | [38] | |||||
| AFs | Food items | 1.6-5.2 mg/kg | [39] | ||||
| UV and FD | Milk | 0.13–0.16 mg/L | [40] | ||||
| LC | FD | OTA | Wine | 0.07 ng/ml | [41] | Several mycotoxin detections, high sensitivity, provides confirmation | Expensive, required expertise In case of MS, sensitivity depends on ionization |
| ZEN | Barley, Maize, Wheat | 100 µg/Kg | [42] | ||||
| AFB1 | Corn | 2–5 ng/g | [43] | ||||
| MS/MS | Trichothecenes | Wheat and maize | 0.2–3.3 µg/Kg | [44] | |||
| Automated microarray chip reader | Chemiluminescence | OTA | Coffee | 0.3 µg/L | [45] | High throughput, multiplexed, parallel processing method | Not so common to their variability and reproducibility, require intensive labor |
| Electro-polymerization onto surface | SPR | ZEN | Corn | 0.3 ng/ml | [46] | Suitable for cereals sample, sim- plicity, portability, and ease to use, can be used in field |
Optimization and validation not reported for this method |
| Immunochromatographic strip | Highly Luminescent Quantum Dot Beads | AFB1 | Maize | 0.42 pg/ml | [47] | A simple method for rapid screening, superior performance | Required expertise |
| Direct, competitive magneto-immunoassay | SPR | OTA | Beverages | 0.042 µg/L | [48] | Rapid, cost effective, and sensitive | |
| Electrochemical | FB | Maize | 0.33 µg/L | [49][50] | |||
| Lateral flow immunoassay | Colorimetric | 199 µg/Kg | [51] | Fast, one-step assay, no washing step, low cost and simple | Qualitative or semi quantitative results, sample volume governs precision | ||
| Photonics immobilization technique | Quartz-crystal microbalance (QCM) | Patulin | Apple puree | 56 ng/ml | [52] | Specific, higher sensitivity, generality, response (only requires a few minutes), flexibility, and portability |
The decrease of the signal in the presence of high analyte concentrations, in situ analysis |
| Surface-enhanced Raman scattering (SERS)-based immunoassay | Silica-encapsulated hollow gold nanoparticles | AFB1 | Food | 0.1 ng/ml | [53] | Enhanced ELISA method | Hard to synthesize and expensive |
| ELISA | UV absorbance | AFM1 | Milk | 4–25 ng/L | [54] | Fast, simple, economical, high sensitivity, simultaneous analysis of multiple samples, easy to screen | Lack of precision at low concentrations, matrice interference problems, possible false-positive/negative results |
| ZEN | Maize | 0.02 µg/L | [55] | ||||
| AFB1 and AFM1 | Food items | 0.13-0.16 mg/L | [40] | ||||
| Electrochemical | FB | Corn | 5 µg/L | [56] |
4. Mitigation Policies of Mycotoxin Contamination in Beverages
Nearly all mycotox
Thins are thermally resistant and cannot be simply degraded by normal heat treatment methods during food processing or household cooking methods [17]. Nolayer chromatography = TLC, High performance liquid chrmally, mycotoxin contamination in beverages can be controlled by preventing the contamination of agricultural raw materials used for the production of beverages [57][58].
Imatography = HPLC, Liquid chromatography = LC, Enzyme-linked implementation of good manufacturing practices will help to ensure safe beverage production without mycotoxin residues. Good manufacturing practices (GMPs) include the use of proper sorting, processing, drying, cooling, and storage conditions for agricultural raw materials. Complete reduction in the number of mycotoxins, or at least a number not higher than the maximum allowable limits, can be achieved by different pre- and postharvest treatmentsunosorbent assay = ELISA, FD = Fluorescence detection, Ultraviolet = UV, Charge-coupled device = CCD, Surface plasmon resonance = SPR, Mass spectrometry = MS.
4. Mitigation Policies of Mycotoxin Contamination in Beverages
Nearly all mycotoxins are thermally resistant and cannot be simply degraded by normal heat treatment methods during food processing or household cooking methods
[17]
. Normally, mycotoxin contamination in beverages can be controlled by preventing the contamination of agricultural raw materials used for the production of beverages [57][58].
Implementation of good manufacturing practices will help to ensure safe beverage production without mycotoxin residues. Good manufacturing practices (GMPs) include the use of proper sorting, processing, drying, cooling, and storage conditions for agricultural raw materials. Complete reduction in the number of mycotoxins, or at least a number not higher than the maximum allowable limits, can be achieved by different pre- and postharvest treatments [59]
.
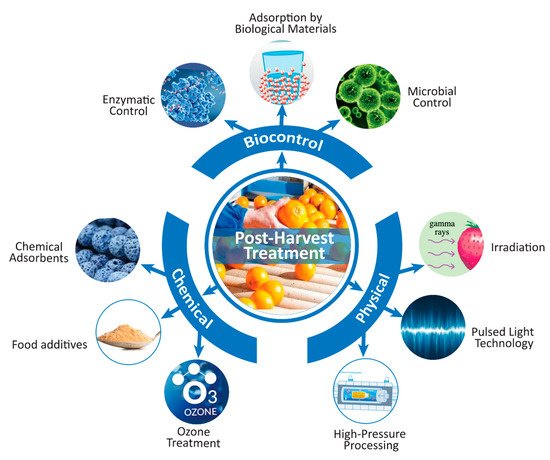
Figure 2.
Scheme for reducing the mycotoxin concentration in beverages using postharvest treatments (drawn using Adobe Illustrator CC software).
5. Critical Challenges of Mycotoxins in Beverages
Mycotoxins possess very stable chemical structures that remain unchanged after pasteurization treatment. It has been reported that proper selection, adequate cleaning and washing, and careful sorting of fruits are very crucial factors for the mitigation of mycotoxin contamination during the manufacturing of beverages
[60]
. As children drink more juices than wine as compared to adults, therefore, the incidence of mycotoxins in fruit juices is a matter of serious concern
.
Physical methods can be applied at large and small scales for a wider range of food, but some physical methods including irradiation have negative effects on the nutritional, antioxidant, and sensorial properties of food. Chemical methods are easy to use and comparatively cheap, but their main limitation is the toxicity of residues and secondary products. Additionally, the toxicity of the mycotoxin-degraded products needs to be measured. Although the adsorption of mycotoxins by chemical adsorbents is one of the most inexpensive detoxification methods, the safety of absorbent materials and the removal of the adsorbent–mycotoxin complex from foods is still challenging. In addition, the overall sensorial quality and final quality parameters (color, clarity, brix, titratable acidity, pH, and TSS) can be adversely affected by chemical treatments. Biological control methods are healthy and environmental friendly. However, microbial approaches may deteriorate the food quality by absorbing nutrients and releasing metabolites into the food matrices. Additionally, biological control methods are more expensive than physical and chemical control measures. Another critical challenge is the commercialization of biological control methods by overcoming the limitations in translation from laboratory trials to commercial applications.
References
- Greeff-Laubscher, M.R.; Beukes, I.; Marais, G.J.; Jacobs, K. Mycotoxin production by three different toxigenic fungi genera on formulated abalone feed and the effect of an aquatic environment on fumonisins. Mycology 2020, 11, 105–117.
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609.
- Gacem, M.A.; Gacem, H.; Telli, A.; Khelil, A.O.E.H. Mycotoxins: Decontamination and nanocontrol methods. In Nanomycotoxicology; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 189–216.
- Amuzie, C.; Bandyopadhyay, R.; Bhat, R.; Black, R.; Burger, H.-M.; Cardwell, K.; Gelderblom, W.; Gong, Y.Y.; Groopman, J.; Kimanya, M.; et al. Effects of aflatoxins on aflatoxicosis and liver cancer. In Mycotoxin Control in Low- and Middle-Income Countries; International Agency for Research on Cancer: Lyon, France, 2015; Volume 9, pp. 13–16.
- WHO. Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2017.
- Ashiq, S. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Compr. Rev. Food Sci. Food Saf. 2015, 14, 159–175.
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302.
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869.
- Oteiza, J.M.; Khaneghah, A.M.; Campagnollo, F.B.; Granato, D.; Mahmoudi, M.R.; Sant’Ana, A.S.; Gianuzzi, L. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT Food Sci. Technol. 2017, 80, 200–207.
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 1–10.
- Probst, C.; Bandyopadhyay, R.; Cotty, P.J. Diversity of aflatoxin-producing fungi and their impact on food safety in sub-Saharan Africa. Int. J. Food Microbiol. 2014, 174, 113–122.
- Freire, L.; Guerreiro, T.M.; Pia, A.K.R.; Lima, E.O.; Oliveira, D.N.; Melo, C.F.O.R.; Catharino, R.R.; Sant’Ana, A.S. A quantitative study on growth variability and production of ochratoxin A and its derivatives by A. carbonarius and A. niger in grape-based medium. Sci. Rep. 2018, 8, 14573.
- Erdoğan, A.; Ghimire, D.; Gürses, M.; Çetin, B.; Baran, A. Patulin contamination in fruit juices and its control measures. Eur. J. Sci. Technol. 2018, 39–48.
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in apples and apple-based food products: The burdens and the mitigation strategies. Toxins 2018, 10, 475.
- Rheeder, J.P.; Marasas, W.F.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105.
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129.
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560.
- Kuzdraliński, A.; Solarska, E.; Muszyńska, M. Deoxynivalenol and zearalenone occurence in beers analysed by an enzyme-linked immunosorbent assay method. Food Control 2013, 29, 22–24.
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERs and liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 229, 336–372.
- Hamed, A.M.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Determination of Fusarium toxins in functional vegetable milks applying salting-out-assisted liquid–liquid extraction combined with ultra-high-performance liquid chromatography tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 2033–2041.
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 2020, 9, 137.
- Zhang, B.; Chen, X.; Han, S.Y.; Li, M.; Ma, T.Z.; Sheng, W.J.; Zhu, X. Simultaneous analysis of 20 mycotoxins in grapes and wines from Hexi corridor region (China): Based on a QuEChERS–UHPLC–MS/MS method. Molecules 2018, 23, 1926.
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777.
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017, 8, 1451.
- Ngea, G.L.N.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent trends in detecting, controlling, and detoxifying of patulin mycotoxin using biotechnology methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472.
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the mycotoxins incidence in Serbia in the period 2004–2016. Toxins 2018, 10, 279.
- Arroyo-Manzanares, N.; Hamed, A.M.; García-Campaña, A.M.; Gámiz-Gracia, L. Plant-based milks: Unexplored source of emerging mycotoxins. A proposal for the control of enniatins and beauvericin using UHPLC-MS/MS. Food Addit. Contam. Part B 2019, 12, 296–302.
- Joint Food and Agriculture Organization; World Health Organization Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants: Fifty-Fifth Report of the JOINT/FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2001; p. 701.
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624.
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a Fusarium mycotoxin, zearalenone. Crit. Rev. Food. Sci. Nutr. 2020, 60, 2710–2729.
- Yvv, A.K.; Renuka, R.M.; Bodaiah, B.; Mangamu, U.K.; Vijayalakshmi, M.; Poda, S. Mycotoxin strategies: Impact on global health and wealth. Pharm. Anal.Acta 2016, 7, 1–11.
- Scott, P.M.; Lawrence, G.A.; Lau, B.P.Y. Analysis of wines, grape juices and cranberry juices for Alternaria toxins. Mycotoxin Res. 2006, 22, 142–147.
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204.
- Batrinou, A.; Houhoula, D.; Papageorgiou, E. Rapid detection of mycotoxins on foods and beverages with enzyme-linked immunosorbent assay. Qual. Assur. Saf. Crop. Foods 2020, 12, 40–49.
- Welke, J.E.; Hoeltz, M.; Dottori, H.A.; Noll, I.B. Quantitative analysis of patulin in apple juice by thin-layer chromatography using a charge coupled device detector. Food Addit. Contam. 2009, 26, 754–758.
- De Girolamo, A.; McKeague, M.; Miller, J.D.; DeRosa, M.C.; Visconti, A. Determination of ochratoxin A in wheat after clean-up through a DNA aptamer-based solid-phase extraction column. Food Chem. 2011, 127, 1378–1384.
- Campone, L.; Piccinelli, A.L.; Rastrelli, L. Dispersive liquid–liquid microextraction combined with high-performance liquid chromatography–tandem mass spectrometry for the identification and the accurate quantification by isotope dilution assay of Ochratoxin A in wine samples. Anal. Bioanal. Chem. 2011, 399, 1279–1286.
- Tessini, C.; Mardones, C.; von Baer, D.; Vega, M.; Herlitz, E.; Saelzer, R.; Silva, J.; Torres, O. Alternatives for sample pre-treatment and HPLC determination of ochratoxin A in red wine using fluorescence detection. Anal. Chim. Acta 2010, 660, 119–126.
- Zahn, M.; Jeong, M.L.; Wang, D.; Trinh, T.; Fay, B.; Ma, W. Product-specific sample clean-up and HPLC analysis of aflatoxins for a dietary product. Phytochem. Anal. 2009, 20, 335–337.
- Jiang, W.; Wang, Z.; Nölke, G.; Zhang, J.; Niu, L.; Shen, J. Simultaneous determination of aflatoxin B1 and aflatoxin M1 in food matrices by enzyme-linked immunosorbent assay. Food Anal. Methods 2013, 6, 767–774.
- Aresta, A.; Vatinno, R.; Palmisano, F.; Zambonin, C.G. Determination of Ochratoxin A in wine at sub ng/mL levels by solid-phase microextraction coupled to liquid chromatography with fluorescence detection. J. Chromatogr. A 2006, 1115, 196–201.
- MacDonald, S.J.; Anderson, S.; Brereton, P.; Wood, R.; Damant, A. Determination of zearalenone in barley, maize and wheat flour, polenta, and maize-based baby food by immunoaffinity column cleanup with liquid chromatography: Interlaboratory study. J. AOAC Int. 2005, 88, 1733–1740.
- Brera, C.; Debegnach, F.; Minardi, V.; Pannunzi, E.; Santis, B.D.; Miraglia, M. Immunoaffinity column cleanup with liquid chromatography for determination of aflatoxin B1 in corn samples: Interlaboratory study. J. AOAC Int. 2007, 90, 765–772.
- Santini, A.; Ferracane, R.; Somma, M.C.; Aragón, A.; Ritieni, A. Multitoxin extraction and detection of trichothecenes in cereals: An improved LC-MS/MS approach. J. Sci. Food Agric. 2009, 89, 1145–1153.
- Sauceda-Friebe, J.C.; Karsunke, X.Y.; Vazac, S.; Biselli, S.; Niessner, R.; Knopp, D. Regenerable immuno-biochip for screening ochratoxin A in green coffee extract using an automated microarray chip reader with chemiluminescence detection. Anal. Chim. Acta 2011, 689, 234–242.
- Chun, H.S.; Choi, E.H.; Chang, H.-J.; Choi, S.-W.; Eremin, S.A. A fluorescence polarization immunoassay for the detection of zearalenone in corn. Anal. Chim. Acta 2009, 639, 83–89.
- Ren, M.; Xu, H.; Huang, X.; Kuang, M.; Xiong, Y.; Xu, H.; Xu, Y.; Chen, H.; Wang, A. Immunochromatographic assay for ultrasensitive detection of aflatoxin B1 in maize by highly luminescent quantum dot beads. ACS Appl. Mater. Interfaces 2014, 6, 14215–14222.
- Yuan, J.; Deng, D.; Lauren, D.R.; Aguilar, M.-I.; Wu, Y. Surface plasmon resonance biosensor for the detection of ochratoxin A in cereals and beverages. Anal. Chim. Acta 2009, 656, 63–71.
- Wang, Y.-K.; Wang, Y.-C.; Wang, H.-A.; Ji, W.-H.; Sun, J.-H.; Yan, Y.-X. An immunomagnetic-bead-based enzyme-linked immunosorbent assay for sensitive quantification of fumonisin B1. Food Control 2014, 40, 41–45.
- Jodra, A.; López, M.Á.; Escarpa, A. Disposable and reliable electrochemical magnetoimmunosensor for Fumonisins simplified determination in maize-based foodstuffs. Biosens. Bioelectron. 2015, 64, 633–638.
- Molinelli, A.; Grossalber, K.; Krska, R. A rapid lateral flow test for the determination of total type B fumonisins in maize. Anal. Bioanal. Chem. 2009, 395, 1309–1316.
- Funari, R.; Della Ventura, B.; Carrieri, R.; Morra, L.; Lahoz, E.; Gesuele, F.; Altucci, C.; Velotta, R. Detection of parathion and patulin by quartz-crystal microbalance functionalized by the photonics immobilization technique. Biosens. Bioelectron. 2015, 67, 224–229.
- Ko, J.; Lee, C.; Choo, J. Highly sensitive SERS-based immunoassay of aflatoxin B1 using silica-encapsulated hollow gold nanoparticles. J. Hazard. Mater. 2015, 285, 11–17.
- Radoi, A.; Targa, M.; Prieto-Simon, B.; Marty, J.-L. Enzyme-Linked Immunosorbent Assay (ELISA) based on superparamagnetic nanoparticles for aflatoxin M1 detection. Talanta 2008, 77, 138–143.
- Tang, X.; Li, X.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Ding, X.; Lei, J.; Zhang, Z. Development and application of an immunoaffinity column enzyme immunoassay for mycotoxin zearalenone in complicated samples. PLoS ONE 2014, 9, e85606.
- Abdul Kadir, M.K.; Tothill, I.E. Development of an electrochemical immunosensor for fumonisins detection in foods. Toxins 2010, 2, 382–398.
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561.
- Adegoke, G.O.; Letuma, P. Strategies for the prevention and reduction of mycotoxins in developing countries. In Mycotoxin and Food Safety in Developing Countries; Intechopen: London, UK, 2013; pp. 123–136.
- Peng, W.X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153.
- Drusch, S.; Ragab, W. Mycotoxins in fruits, fruit juices, and dried fruits. J. Food Prot. 2003, 66, 1514–1527.
- Raiola, A.; Tenore, G.C.; Manyes, L.; Meca, G.; Ritieni, A. Risk analysis of main mycotoxins occurring in food for children: An overview. Food Chem. Toxicol. 2015, 84, 169–180.
- Ojuri, O.T.; Ezekiel, C.N.; Eskola, M.K.; Šarkanj, B.; Babalola, A.D.; Sulyok, M.; Hajšlová, J.; Elliott, C.T.; Krska, R. Mycotoxin co-exposures in infants and young children consuming household- and industrially-processed complementary foods in Nigeria and risk management advice. Food Control 2019, 98, 312–322.
