Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Natalia Manousi and Version 2 by Catherine Yang.
Bioanalysis is the scientific field of the quantitative determination of xenobiotics (e.g., drugs and their metabolites) and biotics (e.g., macromolecules) in biological matrices. The most common samples in bioanalysis include blood (i.e., serum, plasma and whole blood) and urine.
- bioanalysis
- graphene oxide
1. Introduction
Bioanalysis is the sub-discipline of analytical chemistry that deals with the quantitative determination of the exogenous and endogenous chemical compounds in biological matrices. Exogenous compounds are known as xenobiotics (e.g., drugs and their metabolites), while the endogenous ones are called biotics (e.g., macromolecules and biomarkers). Based on the analytical purpose, two distinct types of methodologies can be followed in bioanalysis: untargeted and targeted analysis. Targeted analysis is applied to determine a specific analyte or analytes, while untargeted analysis is mainly applied to obtain a maximum of information from the sample in order to isolate inter- or intra-individual variations and identify potential biomarkers [1][2][1,2]. Unequivocally, bioanalysis is a significant tool for many scientific fields, since it provides significant information regarding the absorption, distribution, metabolism and elimination of drugs [1].
A classical bioanalytical procedure is normally composed of two parts; the sample preparation and the detection/quantification of the target analyte [3][4][5][3,4,5]. Generally, the analysis of xenobiotics in biological matrices is a challenging procedure due to their low concentration, in combination with the complexity of the samples. Among the conventional samples that are examined for bioanalytical methods are blood serum, blood plasma, whole blood and urine while in the recent years, the analysis of alternative matrices (e.g., saliva, hair, nails, sweat and cerebrospinal fluid) has also gained attention [6]. Biological samples and pharmaceutical products are complex matrices that often contain macromolecules, inorganic salts and organic compounds that might exhibit similar chemical or physical properties with the target analytes. As a result, a clean-up and a preconcentration step are typically required [7][8][7,8].
2. Solid-Phase Microextraction
Solid-phase microextraction (SPME) was introduced in the early 1990s by Pawliszyn et al. [9][43] to address the need for rapid and solvent-free extraction techniques. In SPME, extraction takes place at the outer coating of a thin fused-silica fiber coated with a layer of adsorbent. For the extraction, the extracting phase is exposed to the sample matrix either directly (direct immersion SPME) or at the headspace above the sample (headspace SPME) for a certain time span to reach equilibrium. Subsequently, the fiber is removed and desorption of the adsorbed analytes is performed by the addition of an appropriate solvent or thermally in the injection port of a gas chromatograph (GC) [10][44]. In SPME, the fiber coating plays a vital role in the extraction process, and the development of new coatings with high extraction efficiency is a significant research direction in this field [11][45]. This sample preparation technique exhibits a plethora of benefits including high sensitivity, small sample volume, rapidity and simplicity, while it can also be solvent-free [12][46].
Hajebi et al. [11][45] developed electrospun polyamide/GO/polypyrrole composite nanofibers for the headspace SPME of methamphetamine from urine samples prior to its determination by GC-MS. The composite fibers combined high surface-to-volume ratio and spun ability around a thin rod of the polyamide nanofibers with the great mechanical, thermal, and chemical stability and multifunctionality of GO/polypyrrole. Under optimum conditions, the LOD and the limits of quantification (LOQ) for the target analyte were 0.9 and 3.0 μg·L−1, respectively.
GO and functionalized GO derivatives have been also evaluated as stationary phases for in-tube SPME. In-tube SPME is an automated variation of conventional SPME that utilizes an open tubular capillary column as an extraction device [13][47]. This sample preparation technique offer various advantages in terms of automation, preconcentration and miniaturization [14][48]. Various GO composite materials have been evaluated as adsorbents for in-tube SPME in bioanalysis. Shamsayei et al. [15][49] developed a polythiophene/GO nanostructured electrodeposited coating for on-line electrochemically controlled in-tube SPME of amitriptyline and doxepin as antidepressant drugs prior to their determination by HPLC-UV. The composite coating was prepared on the inner surface of a stainless-steel tube by a facile in-situ electro-deposition method. In the composite coating, the GO acted as an anion dopant, as well as sorbent. Compared to polythiophene and GO coatings, the composite coating, exhibited long lifetime, good mechanical stability, as well as large specific surface area. A poly-ethylenedioxythiophene-GO electrodeposited coating fabricated on the inner surface of stainless steel tube has been also reported for the in-tube SPME of letrozole from plasma samples [16][50]. Also in this case, the GO acted both as anion dopant and as sorbent. Compared to pure poly-ethylenedioxythiophene coating, the combination of poly-ethylenedioxythiophene and GO created a more efficient sorbent the target analyte. Chen et al. [17][51] developed a GO/poly(3,4 ethylenedioxythiophene)/polypyrrole composite and used it as adsorbent for the in-tube SPME of 8-hydroxy-2′-deoxyguanosine, 3-hydroxyphenanthrene and 1-hydroxypyrene. The composite coating was electrodeposited on the internal surface of a stainless-steel tube and it exhibited good chemical and mechanical stability, high extraction efficiency, good resistance to matrix interference, as well as long lifespan.
Hollow-fiber SPME is another interesting alternative to conventional SPME, which eliminates the risk of cross-contamination and carry-over problems due to the utilization of disposable nature of the hollow fibers. Moreover, the hollow fibers can protect the sorbent, thus resulting in satisfactory stability and reliability of the extraction process [18][52]. Darvishnejad et al. [18][52] developed nanocubic cobalt oxide@GO nanocomposite reinforced hollow fibers for the hollow fiber-SPME of non-steroidal anti-inflammatory drugs from human urine prior to their determination by HPLC-UV. For this purpose, nanocubic Co3O4@GO was prepared through a hydrothermal method and dispersed in a solution in order to be immobilized in the wall pores of hollow fibers. Due to the synergistic effect between the Co3O4 nanocubes and the GO material, in combination with their unique three-dimensional interpenetrating porous network and high surface-to-volume ratio of GO, high extraction performance was reported.
Hyperbranched polyglycerol/graphene oxide nanocomposite has been employed for the reinforced hollow fiber solid/liquid phase microextraction of ibuprofen and naproxen from hair samples [19][53]. For this purpose, the surface of GO was modified with hyperbranched polyglycerol and the prepared nanocomposite was wetted by a few microliters of 1-octanol as organic solvent, followed by application for the extraction of the target analytes. The proposed method was found to be rapid, simple, sensitive and it required low solvent consumption.
3. Magnetic Solid-Phase Extraction
Magnetic solid-phase extraction (MSPE) is new approach of SPE with the usage of magnetic nanoparticles as adsorbents that was introduced by Safarikova et al. [20][54] in 1999. Since then, many scientists experimented with magnetic nanoparticles to develop MSPE protocols. Among a plethora of magnetic sorbents, magnetic graphene oxide (mGO) has attracted the interest of many researchers, due to its large surface area and its delocalized π-π electron interactions so as to extract metal ions or aromatic compounds from different samples [21][55]. MSPE exhibits various benefits including rapid phase separation, during which the sorbent can be retrieved from the sample with ease with the assistance of an external magnetic field [22][56]. The procedure consists of the following steps: (i) the addition of the magnetic nanoparticles in the sample containing the target analyte, (ii) the sorption of the analyte onto the adsorbent, (iii) the magnetic separation with the use of a strong magnet so as to pour the liquid sample containing possible interferences or other compounds that are not of interest, (iv) the addition of the elution solvent for the dispersion of the analyte, (v) the magnetic separation of the sorbent from the eluent, which is ready for analysis and (vi) the wash and regeneration of the sorbent for possible reusage. The regeneration step is required between different extraction/desorption cycles and it is typically performed by treating the sorbent with an appropriate organic solvent and aqueous solution to avoid incomplete elution of the target analytes that could limit the applications of the material [23][24][19,57]. The steps can also be seen in Figure 1.
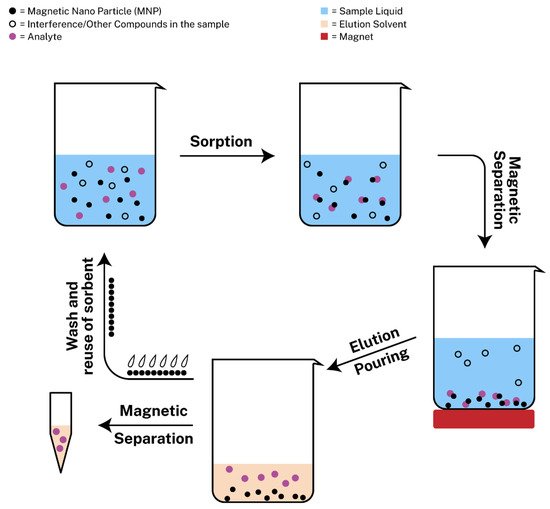

Figure 1. Schematic representation of magnetic solid phase extraction (MSPE).
Poly(2-aminobenzothiazole)-coated mGO nanocomposite was prepared by Asgharinezhad et al. for the determination of anti-inflammatory drugs and non-steroidal drugs in urine samples by HPLC-DAD [25][58]. This new material demonstrated high extraction efficiencies for diclofenac, naproxen and ibuprofen, with recoveries ranging from 85.5–90.5% for the three target analytes and low relative standard deviations.
Cephalosporins in spiked human urine were extracted with the usage of mixed hemimicelles of mGO and ionic liquid and determined by HPLC-UV. The ionic liquid used in this work was 1-hexadecyl-3-methylmidazoliumbromide, which was added along with a buffer solution of phosphate at pH 7 and an amount of mGO in a centrifuge tube, following ultrasonication in order to form mixed hemimicelles. A range of parameters that had an effect on extraction were studied and under optimal conditions, the LODs of the cephalosporins were 0.6–1.9 ng·mL−1, while the RSDs were low and the extraction recoveries were satisfactory [26][59]. Another team of scientists, prepared mGO coated with deep eutectic solvents (DES) to preconcentrate and extract methadone from urine and human blood plasma samples prior to its determination by GC-MS and GC-FID. The DES that showed the greatest results was choline chloride with 5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthalen-2-ol (TNO) at a molar ratio of 1:2 [27][60].
