Due to long-term burial in the ground or water, the plant cell walls of some wooden cultural relics were degraded by microorganisms, the structure of cell wall was loose and filled with water. The moisture content of these wooden cultural relics is much higher than that of normal wood. We collectively refer to these wooden cultural relics as Waterlogged Archaeological Wood (WAW).
1. Introduction
The Nanhai No. 1 shipwreck was a wooden merchant ship of the Southern Song Dynasty (1127 to 1279 AD), which sank in the South China Sea in Guangdong province, China. After the Nanhai No. 1 shipwreck was salvaged as a whole out of the sea, it was placed in the Maritime Silk Road Museum [1]. Many precious cultural relics had been unearthed from the Nanhai No. 1 shipwreck. Among all the cultural relics from the Nanhai No. 1 shipwreck, the wooden hull cultural relic was the most precious and the most difficult to protect, not only because the Nanhai No. 1 shipwreck is huge in volume, but also because the corrosion degree of the hull was uneven. The excavation of the wooden hull cultural relics ran through the excavation of the Nanhai No. 1 shipwreck since 2013. Maritime wooden cultural relics had been immersed in seawater for a long time, and their preservation environment was closed, at a constant temperature, constant pressure, high salt, and oxygen deficient environment. When they were excavated, the preservation environment changed into an open environment where the temperature, humidity and air circulation were not easy to control. Moreover, the control of oxidation, corrosion, microorganism biodegradation and other diseases would become extremely difficult. In order to maintain the stability of wooden cultural relics and control or delay the breeding and development of diseases, protection measures such as cleaning, moisturizing, desalination, reinforcement and anticorrosion and so on are usually taken. A series of scientific on-site protection measures laid the foundation for the transition from on-site protection of cultural relics to laboratory protection and restoration. During the excavation of the Nanhai No. 1 shipwreck, a large number of scattered individual pieces of wood were unearthed. After excavation, this hull wood was usually immersed in deionized water containing the metal chelating agent EDTA-2Na [2] and the antimicrobial agent isothiazolinone [3] for moisture stabilization, preliminary desalination, and microbial inhibition. At the same time, it is necessary to regularly monitor the water temperature, pH, concentration of main ions, conductivity, and microbial composition of the desalination buffer, and replace the desalination buffer regularly. The above treatment lays a solid foundation for the overall desalination, reinforcement, and protection of the hull in the later stage.
Wooden cultural relics are a carrier of ancient human civilization and represent a valuable material for the study of ancient history, art, science and technology, economy, and so on. Wooden cultural relics exist in many cultural sites, usually in the form of houses, tombs, hulls, decorations, and so on [4]. Wooden cultural relic can be divided into dry type and waterlogged type. In the original environment, the cellulose in the cell wall of waterlogged wooden cultural relics has been partially or completely degraded by bacteria or fungi. After the loss of degradation products, the structure of the cell wall becomes loose and even produces a large number of holes, and the original place of the cell wall is filled with water. This makes the moisture content of waterlogged archaeological wood (WAW) much higher than ordinary wood [5][6]. Generally, the moisture content of ordinary fresh wood is about 20%, while that of WAW can reach more than 500% [7][8]. The texture of WAW is fragile, so it is difficult to maintain a good condition after excavation. In general, WAW will be preserved in water after being excavated, and the temperature and humidity of the preservation environment will be as stable as possible. One is to maintain the moisture in the wood, so as to maintain the waterlogged state of the wooden cultural relic; the other is to cut off the air and avoid the further reproduction of aerobic microorganisms. The maritime wooden cultural relic is a kind of classic WAW. Due to long-term immersion in seawater, the intracellular electrolytes of maritime WAW have reached a full balance with seawater, and the wood contains a lot of salt. After the maritime WAW is excavated, many physical and chemical reactions will occur due to the change of environmental temperature and humidity, leading to the corrosion of wood. A high concentration of salts can degrade the fibers in WAW. When the environmental temperature and humidity change, some salts may crystallize and dissolve repeatedly, which will lead to fiber degradation and fracture. In addition, there are insoluble salts such as sulfur iron compounds in maritime WAW that easily oxidize into sulfuric acid during long-term exposure, leading to wood acidification and corrosion [2][9]. For example, in the protection of the Swedish warship Vasa, the problem of the acidity of wood and iron compounds in wood was highly apparent [10][11][12]. Research on the impact of biological pathways of iron and sulfur oxidization on the protection of the Mary Rose was also being studied [13]. At the same time, due to the large volume of maritime WAW, the desalination process is very slow. Therefore, it is necessary to carry out a long-term desalination treatment for maritime WAW to reduce its salt content.
During the preservation of maritime WAW, a variety of disease problems may occur. Therefore, it is necessary to regularly monitor the properties of wood, the nature of the desalination buffer, and microbial biodegradation, and replace the desalination buffer regularly. Once maritime WAW is excavated, it will come into contact with the surrounding environment, which may cause biodegradation problems. Biodegradation is mainly caused by microbial activities, mainly including bacteria and fungi, whose rapid growth and secretion of secondary metabolites may lead to damage to cultural relics [14]. Wood is an excellent organic substrate for the growth of microorganisms [15]. There are many bacteria and fungi that can produce cellulolytic enzymes and ligninolytic enzymes [16][17][18]. Some researchers analyzed the bacterial community in 108 samples of WAW from different ages and identified a variety of bacteria [19]. The main disease microorganisms of the “Xiaobaijiao No. 1” shipwreck were erosion bacteria (EB) and tunneling bacteria (TB) [15]. EB and soft rot fungi were found to be active in WAW [20].
1.简介
南海一号沉船是一艘南宋(1127年至1279年)的木制商船,沉船于中国广东省的南中国海。将南海一号沉船从海中整体捞出后,将其放置在海上丝绸之路博物馆[ 1 ]。南海一号沉船事故中发现了许多珍贵的文物。在南海一号沉船的所有文物,木制船体文物是最珍贵和最难保护的,这不仅是因为南海一号沉船的体积巨大,而且还因为南海一号沉船的腐蚀程度高。船体不平坦。木壳文物的挖掘工作始于2013年以来的南海一号沉船的挖掘。海上木文物已长时间浸泡在海水中,并且它们的保存环境处于恒温恒压的状态下处于封闭状态,高盐和缺氧的环境。当他们被挖掘时,保存环境变成了一个开放的环境,在那里温度,湿度和空气流通不易控制。而且,控制氧化,腐蚀,微生物生物降解和其他疾病将变得极为困难。为了保持木制文物的稳定性和控制或延缓疾病的繁殖和发展,通常采取诸如清洁,保湿,淡化,增强和防腐等保护措施。一系列科学的现场保护措施为从文物的现场保护到实验室的保护和恢复重叠了基础。在发掘南海一号沉船的过程中,发掘扩展散落的木头碎片。开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[而且,控制氧化,腐蚀,微生物生物降解和其他疾病将变得极为困难。为了保持木制文物的稳定性和控制或延缓疾病的繁殖和发展,通常采取一种清洁,保湿,淡化,增强和防腐等保护措施。各种科学的现场保护措施为从文物的现场保护到实验室的保护和恢复替代了基础。在发掘南海一号沉船的过程中,发掘收缩散落的木头碎片。开挖后,通常将这种船体木材浸入含金属螯合剂EDTA-2Na [而且,控制氧化,腐蚀,微生物生物降解和其他疾病将变得极为困难。疾病的繁殖和发展,通常采取采取清洁,保湿,淡化,增强和防腐等保护措施。各种科学的现场保护措施为从文物的现场保护到实验室的保护和恢复破坏了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片。开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[微生物的生物降解和其他疾病将变得极为困难。为了保持木制文物的稳定性和控制或延缓疾病的繁殖和发展,通常采取诸如清洁,保湿,淡化,增强和防腐等保护措施。各种科学的现场保护措施为从文物的现场保护到实验室的保护和恢复。奠定了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片。开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[微生物的生物降解和其他疾病将变得极为困难。为了保持木制文物的稳定性和控制或延缓疾病的繁殖和发展,通常采取诸如清洁,保湿,淡化,增强和防腐等保护措施。一系列科学的现场保护措施为从文物的现场保护到实验室的保护和恢复奠定了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片。开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[为了保持木制文物的稳定性和控制或延缓疾病的繁殖和发展,通常采取诸如清洁,保湿,淡化,增强和防腐等保护措施。一系列科学的现场保护措施为从文物的现场保护到实验室的保护和恢复奠定了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片,开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[为了保持木制文物的稳定性和控制或延缓疾病的繁殖和发展,通常采取诸如清洁,保湿,淡化,增强和防腐等保护措施。一系列科学的现场保护措施为从文物的现场保护到实验室的保护和恢复奠定了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片,开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na [通常采用补强和防腐等措施。一系列科学的现场保护措施为从文物的现场保护到实验室的保护和恢复突破了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片。开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[通常采取补强和防腐等措施。一系列科学的现场保护措施为从文物的现场保护到实验室的保护和恢复奠定了基础。在发掘南海一号沉船的过程中,发掘了大量散落的木头碎片。开挖后,通常将这种船体木材浸入含有金属螯合剂EDTA-2Na盐[ 2 ]和抗微生物剂异噻唑啉酮[ 3 ]用于稳定水分,初步淡化和抑制微生物。同时,有必要定期监测水温,pH值,主离子浓度,电导率和脱盐缓冲液的微生物组成,并定期更换脱盐缓冲液。上述处理为后期的船体整体淡化,加固和保护损坏了替换的基础。
木制文物是古代人类文明的载体,是研究古代历史,艺术,科学技术,经济等方面的宝贵材料。木制文物存在于许多文化遗址中,通常以房屋,陵墓,船体,装饰品等形式存在[ 4. ]。木制文物可分为干式和淹水式。在原始环境中,浸水的木制文物的细胞壁中的纤维素已被细菌或真菌部分或完全降解。在损失降解产物之后,细胞壁的结构变得松散,甚至产生大量的孔,并且细胞壁的原始位置充满了水。这使得淹水考古木(WAW)比普通木[高得多的水分含量5,6 ]。一般来说,普通新鲜木材的水分含量为约20%,而WAW的可以达到超过500%[ 7,8 ]。由于WAW的质地易碎,因此开挖后难以保持良好状态。通常,WAW挖掘后将被保存在水中,并且保存环境的温度和湿度将尽可能稳定。一种是保持木材中的水分,以保持木质文物的浸水状态。另一种是切断空气,避免需氧微生物的进一步繁殖。海上木制文物是一种经典的WAW。由于长期浸泡在海水中,海上WAW的细胞内电解质已与海水充分平衡,木材中含有大量盐分,挖掘海上WAW后,由于环境温度和湿度的变化,将发生许多物理和化学反应,导致木材腐蚀。高浓度的盐浓度WAW中的纤维降解。结果,海上WAW中还存在不溶性盐,例如硫铁化合物,在长期暴露过程中容易氧化成硫酸,从而导致木材酸化和腐蚀[ 2,9 ]。例如,在瑞典军舰瓦萨的保护,在木材木材和铁化合物,酸性的问题是高度明显的[ 10,11,12 ]。还研究了铁和硫氧化的生物途径对玛丽玫瑰保护的影响[ 13 ]。同时,由于海上WAW数量巨大,海水淡化过程非常缓慢。因此,有必要对海洋WAW进行长期的脱盐处理以降低其盐含量。
在海上WAW的保存过程中,可能会发生多种疾病问题。因此,有必要定期监测木材的性质,脱盐缓冲液的性质和微生物的生物降解,并定期更换脱盐缓冲液。海上WAW挖掘后,将与周围环境接触,这可能会导致生物降解问题。生物降解主要是由微生物活动引起的,主要包括细菌和真菌,它们的快速生长和次生代谢的分泌可能导致文物的破坏[ 14 ]。木材是微生物生长的极好的有机基质[ 15 ]。有许多细菌和真菌可产生纤维素分解酶和木质素分解酶[ 16,17,18 ]。一些研究人员分析了108个不同年龄的WAW样品中的细菌菌群,并鉴定出各种细菌[ 19 ]。“小白礁1号”沉船的主要病害微生物是侵入细菌(EB)和隧道细菌(TB)[ 15 ]。发现WAW中有EB和软腐精细活性[ 20 ]。因此,有必要综合考虑木材的性质和微生物问题,以更好地解决WAW的各种疾病。
2. Analysis of Chemical Components in the Wood2.木材中化学成分分析
The contents of lignin, holocellulose and ash are shown in Table 1. According to literature reports, in fresh Pinus wood, the lignin content is generally about 25%, the holocellulose content is generally about 75%, and the ash content is generally less than 1% [21][22]. Through comparison, it can be found that the holocellulose content was much lower than that of fresh wood, while the ash content was higher than that of fresh wood. Since most of the cellulose had been degraded and lignin is difficult to degrade, the percentage of lignin in the wood has increased to 63%. Therefore, the wood degradation degree of WAW from the Nanhai No. 1 shipwreck was high, and the ash content of the WAW was high. In addition, the changes of lignin and holocellulose contents in April and July 2019 were not obvious, indicating that the degradation of the WAW became slow during the desalination. The ash content in July 2019 was significantly lower than that in April 2019, indicating that some inorganic salt ions were slowly removed after immersion in desalination buffer.木质素,全纤维素和灰分的含量示于表1中。据文献报道,在新鲜的松木材,木质素含量通常为约25%,综纤维素含量通常为约75%,以及灰分含量通常小于1%[ 26,27 ]。通过比较,可以发现全纤维素含量远低于新鲜木材,而灰分含量则高于新鲜木材。由于大多数纤维素已经降解,木质素难以降解,因此木质素在木材中的百分比已增加到63%。因此,南海一号沉船中WAW的木材降解度很高,且WAW的灰分含量很高。此外,2019年4月和2019年7月木质素和全纤维素含量的变化不明显,表明在海水淡化过程中WAW的降解变得缓慢。2019年7月的灰分含量明显低于2019年4月的灰分含量,这表明某些无机盐离子在浸入脱盐缓冲液后被慢慢去除。
Table 1. Lignin, holocellulose, and ash content of WAW in NH.W2 in April and July 2019.
表1。2019年4月和7月NH.W2中WAW的木质素,全纤维素和灰分
| Lignin Content (%) |
Holocellulose Content (%) |
Ash Content (%) |
| 20 April 2019 |
表2。2019年8月和9月NH.W2脱盐缓冲液中的铁元素含量。
| Iron Element Content (g/L) |
| 61.32 |
4.72 |
13.02 |
| 1 August 2019 |
127.23 |
。在原来的脱盐缓冲液,氯-和SO 4 2-。存在并不原始脱盐缓冲液中的钠+主要存在于EDTA-2Na盐中,原始的Na +的含量为460毫克/升我们发现,在脱盐缓冲浸渍后,钠的含量+,氯-和SO 4 2-。的中脱盐缓冲均较原始脱盐缓冲显关系着更高此外,钠的含量+,氯-和SO 4 2-经过经过的脱盐处理后趋于稳定。结果表明,浸泡在脱盐缓冲液中后,WAW中的可溶性盐逐渐被除去。
Table 3. Anion and cation content of the desalination buffer in NH.W2 in May, June and October 2019.
表3。2019年5月,6月和10月NH.W2中脱盐缓冲液的阴离子和阳离子含量。
| Na+ Content (mg/L) |
Cl− Content (mg/L) |
SO42− Content (mg/L) |
| 29 May 2019 |
786.30 |
386.12 |
162.23 |
12 July 2019 |
64.96 |
4.43 |
3.86 |
| 28 June 2019 |
Mean Value |
| 10 September 2019 |
63.14 |
4.575 |
8.44 |
| 611.47 |
| 745.33 |
512.67 |
219.22 |
| 22 October 2019 |
786.30 |
396.81 |
170.95 |
| 木质素含量(%) |
纤维素含量(%) |
灰分(%) |
| 铁元素含量(g / L) |
| 2019年4月20日 |
61.32 |
4.72 |
13.02 |
| 2019年8月1日 |
127.23 |
2019年7月12日 |
64.96 |
4.43 |
3.86 |
| 绝对 |
63.14 |
4.575 |
8.44 |
3. Analysis of Iron Element Content in the Buffer3.缓冲液中铁元素含量的分析
The iron contents of the desalination buffer are shown in Table 2. We found that the iron content in the desalination buffer increased significantly after one month of the desalination treatment. The results showed that some insoluble salts mainly composed of sulfur iron compounds in WAW could be removed slowly after soaking in desalination treatment.
Table 2. Iron element content of the desalination buffer in NH.W2 in August and September 2019.
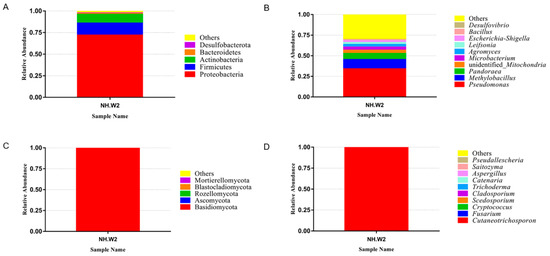
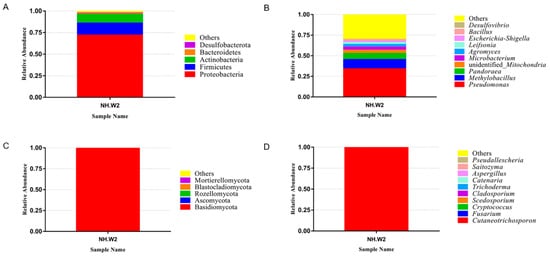
Leifsonia, and Escherichia-Shigella. Figure 1c,d show the composition and proportion of fungi in NH.W2 desalination buffer. Figure 1c represents the distribution of the fungi at the phylum level. The results show that at the phylum level, Basidiomycota accounts for the largest proportion, accounting for 99.63%, followed by Ascomycota, accounting for 0.25%. Figure 1d and Table 4 represent the distribution of the fungi at the genus level. We found that at the genus level, the most abundant fungi is Cutaneotrichosporon, accounting for 99.59%, followed by Fusarium and Cryptococcus, accounting for 0.18% and 0.03%, respectively.我们于2019年11月在NH.W2中检测了脱盐缓冲液的微生物组成。图2A ,B显示了NH.W2脱盐缓冲液中细菌的组成和比例。图2a中表示细菌在门水平的分布。结果表明,在门类中,变形杆菌占最大比例,占71.95%。紧随其后的是厚壁菌门和放线菌,分别占13.90%和10.04%。图2b中和表4表示细菌在属水平上的分布。我们发现,在属水平上,丰富最细菌的的英文假单胞菌,占34.06%。其次的英文甲基杆菌和潘多拉,分别占11.33%和7.25%。此外,不明还有身份的线粒体,微杆菌,Agromyces,Leifsonia状语从句:大肠杆菌,痢疾杆菌。图2c中,d显示了NH.W2脱盐缓冲液中真菌的组成和比例。图2c中表示真菌在门水平的分布。结果表明,在门类中,担子菌占最大比例,占99.63%,其次是子囊菌,占0.25%。图2d和表4代表面粉在属水平上的分布。我们发现,在属水平上,最丰富的粉末是角皮癣菌,占99.59%,其次是镰刀菌和隐球菌,分别占0.18%和0.03%。
图2.监测池8(NH.W2)中微生物群落的相对丰度。相对丰度以百分比显示。根据右侧的图例,为动物门和属着色。(甲)门水平细菌的相对丰度。(乙)属水平上细菌的相对丰度。(C ^)门水平真菌的相对丰度。(d)属水平上真菌的相对丰度。
表4.在NH.W2属中,脱盐缓冲液中优势细菌和优势真菌的相对丰度。
Figure 1. The relative abundance of microbial communities in monitoring tank 8 (NH. W2). The relative abundance is shown as a percentage. Phylum and genera are colored according to the legend on the right. (A) Relative abundance of bacteria at phylum level. (B) Relative abundance of bacteria at genera level. (C) Relative abundance of fungi at phylum level. (D) Relative abundance of fungi at genera level.
| |
| Pseudomonas |
34.06 |
Cutaneotrichosporon |
99.59 |
| Methylobacillus |
11.33 |
Fusarium |
0.18 |
| Pandoraea |
7.25 |
Cryptococcus |
0.03 |
| unidentified_Mitochondria |
3.99 |
Scedosporium |
0.01 |
| Microbacterium |
3.75 |
Cladosporium |
0.01 |
| Agromyces |
3.12 |
Trichoderma |
0.01 |
| Leifsonia |
2.53 |
Catenaria |
0.01 |
| Escherichia-Shigella |
2.09 |
Aspergillus |
0.01 |
| Bacillus |
1.18 |
Saitozyma |
0.01 |
| Desulfovibrio |
0.42 |
Pseudallescheria |
0.00 |
| Others |
30.28 |
Others |
0.16 |
4. Analysis of Anion and Cation Content in the Buffer4.缓冲液中熵和阳离子含量的分析
The anion and cation content of the desalination buffer are shown in Table 3. In the original desalination buffer, Cl− and SO42− did not exist. The Na+ in the original desalination buffer mainly existed in EDTA-2Na, and the content of original Na+ was 460 mg/L. We found that after immersion in the desalination buffer, the contents of Na+, Cl− and SO42− in the desalination buffer were significantly higher than those in the original desalination buffer. Moreover, the contents of Na+, Cl− and SO42− tended to be stable after a period of desalination treatment. The results showed that the soluble salt in WAW was gradually removed after immersion in the desalination buffer.
| 钠+含量(mg / L) |
氯-含量(毫克/升) |
SO 4 2−含量(mg / L) |
| 2019年5月29日 |
786.30 |
386.12 |
162.23 |
| 2019年6月28日 |
745.33 |
512.67 |
219.22 |
| 2019年10月22日 |
786.30 |
396.81 |
170.95 |
5. Microbial Diversity Analysis by High-Throughput Sequencing
5.通过高通量预测进行微生物多样性分析
We detected the microbial composition of the desalination buffer in NH.W2 in November 2019. Figure 1a,b show the composition and proportion of bacteria in NH.W2 desalination buffer. Figure 1a represents the distribution of the bacteria at the phylum level. The results show that at the phylum level, Proteobacteria accounts for the largest proportion, accounting for 71.95%. This is followed by Firmicutes and Actinobacteria, accounting for 13.90% and 10.04%, respectively. Figure 1b and Table 4 represent the distribution of the bacteria at the genus level. We found that at the genus level, the most abundant bacteria is Pseudomonas, accounting for 34.06%. Followed by Methylobacillus and Pandoraea, accounting for 11.33% and 7.25%, respectively. In addition, there are unidentified Mitochondria, Microbacterium, Agromyces, | 优势细菌属(%) |
|
优势面粉属(%) |
|
|---|
| 假单胞菌 |
34.06 |
角鲨 |
99.59 |
| 甲基杆菌 |
11.33 |
镰刀菌 |
0.18 |
| 潘多拉亚 |
7.25 |
隐球菌 |
0.03 |
| unidentified_线粒体 |
3.99 |
鞘孢菌 |
0.01 |
| 微细菌 |
3.75 |
枝孢菌 |
0.01 |
| 农杆菌属 |
3.12 |
木霉属 |
0.01 |
| 莱福索尼亚 |
2.53 |
卡特纳里亚 |
0.01 |
| 大肠埃希氏菌 |
2.09 |
曲霉 |
0.01 |
| 芽孢杆菌 |
1.18 |
斋藤 |
0.01 |
| 脱硫弧菌 |
0.42 |
假单胞菌 |
0.00 |
| 其他 |
30.28 |
其他 |
0.16 |
Table 4. Relative abundance of dominant bacteria and dominant fungi of the desalination buffer in the NH.W2 at the genus level.
| Dominant Bacteria Genus (%) |
|
Dominant Fungi Genus (%) |