Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lily Guo and Version 1 by Remedios Castro Mejías.
The production of fruit vinegars as a way of making use of fruit by-products is an option widely used by the food industry, since surplus or second quality fruit can be used without compromising the quality of the final product. The acetic nature of vinegars and its subsequent impact on the organoleptic properties of the final product allows almost any type of fruit to be used for its elaboration.
- Fruit Vinegar Production
- organoleptic properties
- acetification process
1. Introduction
Vinegar has been part of the human diet since ancient times and has been widely used as a preservative, condiment, aromatizer, and even as a healthy drink. Moreover, it has also been traditionally used in ancient medicine because of its medicinal properties [1]. Vinegar can be made from any carbohydrate source, amylaceous, or sugary substrate through two successive fermentations: alcoholic fermentation, which is carried out by means of yeasts, and acetic fermentation, with acetic bacteria as the protagonist.
Every year, large amounts of fruit are produced and wasted since the excess cannot be consumed or because the fruits are considered of a second or third quality category. According to the FAO [2], 21.6% of the fruit produced in the world is wasted, starting from the post-harvest stage until its distribution. Very often, fruit is rejected simply because of its “imperfect” appearance or inadequate size, even if the fruit is perfectly edible. It is true that although there are alternatives such as the production of fruit purees, juices, or even fruit jams, large quantities are still wasted as the fruit is left in the fields until it decomposes or is immediately disposed of as waste. These actions lead to both ecological and economic problems; therefore, environmental pollution and rising prices can be the consequence of fruit overproduction. Hence, alternatives that can use this surplus and thus reduce the impact generated on the fruit industry are extremely valuable. Some possible options, related to the vinegar industry, could be the maceration of fruits with vinegar, the enrichment of vinegars with fruit fiber, or the employment of fruits for vinegar production.
As it has been mentioned, one of the possible uses of fruit industry residues is the elaboration of macerated vinegars using different parts of fruit. The peels of citrus fruits such as orange, lemon, lime, grapefruit, or the entire strawberry have been employed several times for the maceration with vinegar [3,4,5][3][4][5]. Some examples of vinegar maceration with other fruits such as banana, passion fruit, or apple have also been found in the literature [6].
Maceration is not the only way to make use of fruit waste. The dietary fiber extracted from these wastes can be used to enrich other foods. The dietetic fiber derived from fruits is increasingly introduced in the market these days because of its higher nutritional quality compared to the dietetic fiber derived from cereals. Several authors have studied dietary fiber from orange, lemon, lime, grapefruit, or apple peels [7,8,9,10][7][8][9][10]. Other authors [11] studied the enrichment of vinegar with dietary fibers from orange and lemon, and it was observed that with that enrichment, the volatile and polyphenolic compounds contained in the starting vinegar were enhanced, among which the orange fiber was the one that provided the highest content in volatiles and the lemon fiber was the one that provided the highest content in polyphenols.
Another option to exploit this surplus fruit would be the production of vinegars directly from them. Although the most commonly consumed vinegar in the world is wine vinegar (from grapes), there are many types of vinegars according to the raw material used for their production. Some of the most important examples are rice or sake vinegar, malt vinegar, cider vinegar, or general fruit vinegars other than grapes. Nowadays, a really popular vinegar is apple vinegar, which seems to have considerable healthy benefits, such as weight loss, the lowering of the blood glucose levels in people with type 2 diabetes mellitus, or the lowering of the risk of heart diseases, among others [12].
The production of fruit vinegars as a way of making use of fruit by-products is widely employed by the food industry, since it allows them to exploit surplus and second-quality fruit without compromising the quality of the final product. The acetic nature of fruit vinegars and the high sensory impact that this acid produces on the organoleptic properties of the product allow almost any type of fruit to be used for its elaboration.
Although Asian countries were the first ones to become interested in this type of product, more and more scientific research is being carried out in other parts of the world on this matrix (Figure 1).
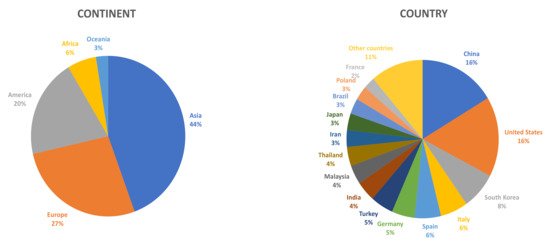
Figure 1.
Percent distribution of scientific articles on fruit vinegar published from 2015 to 2020, according to the origin of the research groups (continent/country) (Source: Scopus).
Figure 2 shows the distribution of the number of scientific articles on fruit vinegars published between 1990 and 2020. As can be seen, there has been an exponential growth in recent years, which would demonstrate the growing interest of the scientific community in this type of product.
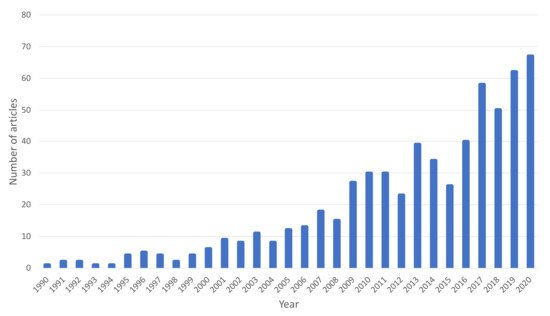
Figure 2. Number of scientific articles on fruit vinegar published per year (Source: Scopus).
With regard to the production process of fruit vinegars in particular, Figure 3 shows the different fruits for which two or more research studies have been found from 1990 to date for the production of fruit vinegars. It can be seen that many fruits have been explored for the elaboration of vinegars, the most common being apple, different berries, persimmon, strawberry, pineapple, cherry, orange, mango, or banana, among others. This figure does not include grapes as fruit, since this would require the inclusion of all the references to wine vinegars, which is not the object of this scientific review.
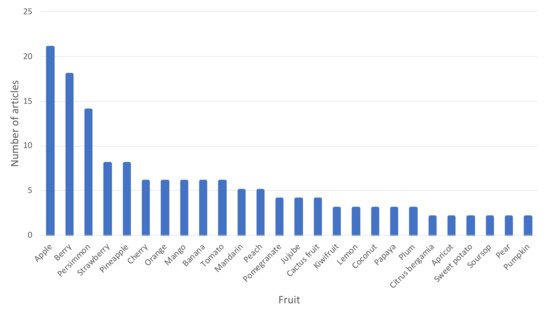
Figure 3. Different fruits (other than grapes) employed for the elaboration of fruit vinegars for which two or more scientific articles about the technological process have been found in the literature from 1990 to 2020.
This increasing tendency has enabled that some problems related to the authentication of fruit vinegars on the raw material, the elaboration process, or the geographical origin, have started [13]. The quality of fruit vinegar is related to the amount of some specific bioactive compounds. The addition of cheaper substitutes or the total substitution of these particular compounds, which define the quality of highly recognized vinegars, together with the possible use of false labeling, are usual authentication problems in the case of fruit vinegars. The different authentication methodologies used for the specific case of this type of product can be found in the bibliography. Unambiguous constituents [14[14][15],15], several molecular isotope ratios [16[16][17],17], spectroscopic techniques, such as infrared and fluorescence spectroscopy in combination with several chemometric techniques [13], and even electronic nose and electronic tongue [18] have been used to detect adulteration, mainly related to raw material and/or geographical origin.
2. Fermentation Processes
Vinegar is produced through a two-stage fermentation process, the first being the conversion of fermentable sugars into ethanol by yeasts, generally Saccharomyces species, and the second being the oxidation of ethanol by bacteria, generally Acetobacter species. Fermentation is a key process in the production of fruit vinegars, during which many volatile compounds, polyphenols, and organic acids, among others, are modified through chemical and microbial actions.
2.1. Alcoholic Fermentation
After the raw material preparation, the alcoholic fermentation plays a crucial role in vinegar production. Fermentation is an ancestral technique for the preservation of food and is considered a simple, natural, and valuable biotechnological process. The advantage of this technology lies with the maintenance and/or improvement of the safety, nutritional, sensory, and shelf-life properties of food products from plants [79][19].
°Brix grades are closely related to alcoholic fermentation, as they reflect the content of sugars, which will determine the alcoholic grade that can be obtained, and this depends on the raw material used as well as on the culture of the microorganisms used for the fermentation process. Table 1 lists the fruit used as raw material and the °Brix degrees that fruit juices are expected to have [80][20].
Table 1. Minimum °Brix expected from the different fruit juices.
Minimum Brix expected from the different fruit juices.
| Common Name | Botanic Name | °Brix |
|---|---|---|
| Apple | Malus domestica | 11.2 |
| Apricot | Prunus armeniaca | 11.2 |
| Banana | Musa x paradisiaca | 21.0 |
| Blackcurrant | Ribes nigrum | 11.0 |
| Grape | Vitis vinifera | 15.9 |
| Grapefruit | Citrus x paradisi | 10.0 |
| Guava | Psidium guajava | 8.5 |
| Lemon | Citrus limon | 8.0 |
| Mango | Mangifera indica | 13.5 |
| Orange | Citrus sinensis | 11.2 |
| Passion Fruit | Passiflora edulis | 12.0 |
| Peach | Prunus persica | 10.0 |
| Pear | Pyrus communis | 11.9 |
| Pineapple | Ananas comosus | 12.8 |
| Raspberry | Rubus idaeus | 7.0 |
| Cherry | Prunus cerasus | 13.5 |
| Strawberry | Fragaria x ananassa | 7.0 |
| Tomato | Lycopersicon esculentum | 5.0 |
| Tangerine | Citrus reticulata | 11.2 |
Fermentation time is also a variable parameter in this process. Different fermentation times have been described in the scientific literature for musts from different raw materials. For example, in the case of cranberry as a starting fruit, Da Silva Fonseca et al. [65][21] set the alcoholic fermentation time at 125 h and used the commercial strain Saccharomyces cerevisiae bayanus; and Yan et al. [81][22] used S. cerevisiae AS2.316 yeast for 192h to ferment premier Rabbiteye cranberry juice. However, longer times have been described, such as in the case described by other authors [82][23] who produced wine from Brigitta blueberry using a fermentation period of 35 days at 13 °C and the strain Saccharomyces cerevisiae. bayanus. Generally, the fermentation time depends on the fruit used, its sugar level, and the physical state in which it is presented (juice, crushed, chopped, etc.). It could also be affected by the concentration of microorganisms, the sugar content, or the fermentation temperature. Similarly, the alcoholic fermentation can be carried out by spontaneous fermentation or using a starter culture, which also affects the duration of the process and the properties of the final product [30][24].
There are other parameters that could also affect the alcoholic fermentation, such as the fermentation temperature, the composition of the substrate, the tolerance to alcohol by the yeast used, the pH, or the sugar concentration [83][25]. Usually, by increasing the fermentation temperature, the fermentation rate increases. However, a much higher temperature could inhibit the growth of the microorganisms and, therefore, affect the fermentation rate. Torija et al. [84][26] found that the maximum speed for alcoholic fermentation was 35 °C. However, temperature tolerance is also dependent on sugar concentration. Other authors found that the fermentation of molasses at 35 °C was possible at 20% of sugar concentration but not at 22% [85][27]. The pH of the medium is also an important parameter that affects cell growth and fermentation efficiency. Usual values of pH are between 3.5 and 5.5, depending of the fruit employed. Moreover, not all yeasts have the same tolerance to ethanol, and this fact could also affect the alcoholic fermentation process [86][28]. On the other hand, increasing sugar concentration will increase the osmotic pressure and viscosity of the medium and would inhibit yeast growth and ethanol production [83][25]. Usual values of sugar content in the medium for a good fermentation rate should not exceed 20%. To increase the alcoholic content, avoiding the inhibition of the fermentation by substrate, a second addition of sucrose could be done after the initial level of sucrose has been consumed by microorganisms [80,87][20][29]. By this procedure, a higher content of acetic acid could be achieved in the final vinegar.
It should also be noted that the alcoholic fermentation of the sugars generates a number of by-products including glycerol, which after ethanol is the alcohol that is most widely used by acetic acid bacteria. However, the excess of glycerol may reduce the ethanol yield during wine production. Factors such as temperature, aeration, sugar concentration, and osmotic stress could influence the production of glycerol during alcoholic fermentation [88][30]. Some authors have found differences in the ethanol/glycerol ratio produced under static and dynamic (agitation) alcoholic fermentation conditions, with a maximum ratio of 29 after 27 h in the agitated fermentation toward 47 after 45 h in the static one. Glycerol is a non-aromatic compound. Nevertheless, it can significantly contribute to wine’s quality, providing sweetness and fullness [89][31]. Glycerol acts as a carbon source for Acetobacter species and protects them from hard conditions such as high pH situations. In this way, acetic acid bacteria can survive and energetically grow for a long time in a glycerol-containing medium. Acetic acid bacteria can employ glycerol as a carbon source and transform it into dihydroxyacetone (DHA) [90][32]. Therefore, the ethanol/glycerol ratio is a key parameter for the vinegar quality. In particular, a high ratio should be adequate for an optimal acetification step. Some of the scientific research studies that include the ethanol/glycerol ratio were performed by Lea [91][33] where 0.23–0.56% glycerol content is present in apple vinegar, while in other study [75][34] on cherry vinegars, glycerol content levels were lower.
In relation to the differences found for glycerol/ethanol ratio according to an agitated or static alcoholic fermentation, wines from agitated/static process presented 5.73 and 6.81% (v/v) of alcohol content, and the values of total acidity were 3.9 and 4.4 g/L, respectively, with volatile acidity of 1.1 and 1.2 g of acetic acid/L, for the agitation and static processes. A pH value of 4.0 for both fermentation processes was found. The agitated fermentation showed a higher ash content and total dry extract, 11.25 g/L toward 8.63 g/L in the static process. Regarding volatile compounds, in general, the agitated process produced a wine with a lower volatile content: ethyl acetate, 18.1 mg/L for the agitation and 23.2 mg/L for the static process; acetaldehyde, with values of 22.1 and 89.9 mg/L for agitation and static fermentation, respectively; furfural; some alcohols such as methanol and isoamyl alcohol; etc. In summary, the productivity in the agitation process was higher than in the static, and shorter times were required [92][35].
In relation to the influence of a possible agitation during alcoholic fermentation, scarce literature can be found in which both methodologies, static and dynamic conditions, were compared for fruit wines. Coelho et al. carried out a study in which the production of four fruit wines, orange, mango, cherry, and banana, were optimized. All the fermentation studies were carried out under agitation conditions. The alcoholic fermentation did not affect the fruits’ antioxidant activity, and the orange and cherry vinegars showed the highest antioxidant activities in concordance with the values found for this parameter in the raw materials. In this case, cherry vinegars were the most acceptable ones from a sensory point of view [93][36].
Microbiological Aspects of Alcoholic Fermentation on Fruit Vinegars
-
Spontaneous Alcoholic Fermentation
Numerous studies have been carried out on natural or spontaneous alcoholic fermentation processes [29,31,52,53,74,94][37][38][39][40][41][42]. During natural fermentation, the changing environmental conditions favor the proliferation of the most suitable native microbiota for the processing of the raw material. The stricter the growth conditions, the greater the selective pressure exerted on the native microorganisms. Song et al. [74][41] produced black raspberry vinegar using strains of native yeasts for the alcoholic fermentation. These native yeast strains showed improved growth and an increased ethanol production rate in comparison with other commercial yeasts. In addition, some differences in terms of physical–chemical properties of the final vinegars produced could be observed depending on the type of yeast used for the alcoholic fermentation, as well as an increased antioxidant capacity when using native yeasts.
The use of native yeasts and spontaneous alcoholic fermentation also have some drawbacks, such as the higher risk of contamination with other undesirable microorganisms, the uncertainty about the properties of the obtained product, the usual longer periods employed for the beginning of the fermentation, or the possibility of having a lower population of yeasts, which could interfere with the fermentation process. However, Hidalgo et al. [53][40] obtained the alcoholic substrate for the elaboration of persimmon vinegar both by natural fermentation and through inoculation of S. cerevisiae, and in both fermentations, the same yeast population was reached: 108 cells/mL. Similar values of yeast population were found in the natural fermentation of other fruits such as gabiroba [95][43], apple [96][44], strawberry [97][45], or pineapple [94][42] when used for the production of alcoholic beverages.
In most spontaneous fermentations, a microbial succession takes place, and quite often, lactic acid bacteria and yeasts dominate at the beginning of the process. Generally, it is yeasts other than Saccharomyces that start the spontaneous alcoholic fermentation, until finally, S. cerevisiae is the one to dominate the process [53][40]. These consume sugars and produce ethanol, which inhibits the growth of many bacteria species, which results in a longer shelf life of the products. This phenomenon has been described for gabiroba wine [95][43], although in fermentations that yield a low final alcohol content, Saccharomyces may not always appear [94][42]. In the spontaneous fermentation of persimmon, P. guilliermondii, H. uvarum, Z. florentinus and Cryptococcus sp. were isolated during the entire fermentation process [53][40]. Non-Saccharomyces yeasts usually present a higher diversity when spontaneous fermentations are carried out, since inoculation with selected yeasts usually reduces the growth of native yeasts [98][46]. The presence of non-Saccharomyces yeasts is related to the sugar concentration or the presence of organic acids in raw material, and these yeasts may alter the volatile profile of the wine produced, compared to that produced mainly with Saccharomyces strains [53][40]. Although non-Saccharomyces yeasts are usually employed in alcoholic fermentation to reduce alcohol content of wine [99][47], and they can affect the quality parameters during wine fermentation [100][48], scarce literature exists regarding the effect of these species on the quality of fruit vinegars. These kinds of yeasts are usually common when subtracts with high sugar concentration (30–50%) and low values of pH (<3) are employed, such as in the case of traditional balsamic vinegar [101][49]. Some authors found that some non-Saccharomyces yeast strains such as Candida ethanolica, Pichia membranifaciens, and Saccharomycodes ludwigii were present in conventional and organic apple cider vinegars, presenting a high acetic acid resistant, and the differences in the composition of microbiota could influence the chemical composition and sensorial quality of vinegars [102][50]. Other studies have shown that non-Saccharomyces yeast species such as Candida and Saccharomycodes appeared during the initial and middle stages of acetification for wine vinegar or kombucha vinegar, often showing more beneficial effects with positive metabolic activities [103][51]. In addition, Kawa-Rygielska et al. demonstrated that the use of Saccharomyces non-cerevisiae strains such as Saccharomyces bayanus to carry out alcoholic fermentation significantly increased the content of biologically active compounds and antioxidant activity in cornelian cherry vinegars [75][34].
-
Alcoholic Fermentation using a Starter Culture
A starter culture is defined as a preparation containing a large number of cells of a particular microorganism that is added to the raw material to trigger and lead the fermentation process of a food product. This is a frequent practice to obtain the alcoholic medium for vinegar production, since it ensures the quality and reproducibility of the final product [104][52], and it also shortens the fermentation time and increases the safety of the product [29][37]. As an example, in a study on strawberry and persimmon vinegar production, the alcoholic fermentation took place more rapidly when yeast inoculation was used, since the lag phase was shorter [52,53][39][40]. Numerous studies are available in the literature in which the most commonly used yeast for alcoholic fermentation is Saccharomyces cerevisiae [28[53][54][55][56][57][58][59][60][21][61][62][63][64],37,45,47,48,49,56,63,65,105,106,107,108], but others can also be found where mixed cultures are used, such as the mixture of Saccharomyces cerevisiae with Lactobacillus plantarum for the production of citrus vinegar [38][65]. In this study, the contents of sweet and umami free amino acids were higher when the mixture was employed, and flavor groups such as esters, alcohols, and aldehydes also significantly improved. From an organoleptic point of view, citrus vinegar produced with the mixture showed higher intensity for sweet and umami attributes, as well as flowery and fruity ones. Moreover, the utilization of mixed culture in alcoholic fermentation significantly improved the antioxidant activity in citrus vinegar [38][65].
During alcoholic fermentation, besides the production of ethanol, a large number of chemical compounds are normally modified. For example, Su and Silva [67][66] made rabbiteye cranberry vinegar using S. cerevisiae for the alcoholic fermentation, and this fermentation reduced the total anthocyanin and polyphenol content of the by-products, but it did not affect the antioxidative activity. In contrast, Kong et al. [64][67] found no significant differences in polyphenolic content and antioxidative activity when alcoholic fermentation was carried out with added to dry yeast for the production of papaya vinegars. This modification of substances might be influenced by the type of fermentation used (spontaneous or with starter culture). Úbeda et al. [30][24] when producing strawberry vinegars showed that the wines produced using starter culture presented half the anthocyanin content, in comparison to those obtained by spontaneous fermentation. Regarding the modification of the volatile composition during alcoholic fermentation, Úbeda, Callejón, et al. [56][59] found that the yeast strain used influenced the production of acetaldehyde and higher alcohols during the alcoholic fermentation of strawberry or persimmon for the production of vinegars. In another study on the production of gabiroba wine [95][43], the inoculated yeasts produced larger amounts of ethanol and higher alcohols compared to those obtained using native yeasts. In relation to organic acids, several authors have found important variations of these compounds during the alcoholic fermentation when a starter culture for the production of fruit vinegars was used [64,109][67][68]. Concretely, the concentration of lactic acid increased and was accumulated during alcoholic fermentation, whereas other acids, such as malic or citric acid decreased significantly in the production of wine from peach [109][68]. Ascorbic acid content can also increase during alcoholic fermentation because yeast could produce precursor antioxidant molecules such as D-erythroascorbic acid [110][69]. Lorenzini et al. [111][70], in the fermentation of apple juices with Saccharomyces and non-Saccharomyces strains, observed that the malic acid content was similar in all ciders. The content in acetic acid was low in cider produced by the two Saccharomyces strains, T. debrueckii TD291 and Z. bailii ZB3, while S. bacillaris YR21 was the largest producer for this organic acid. Succinic acid is the main acid produced by yeasts during alcoholic fermentation. A high amount of this acid could influence negatively on the final quality of fruit wines. Duarte et al. [112][71] found similar concentrations of ethanol, glycerol, and malic acid for three raspberry wines obtained with three yeast strains (CAT-1, UFLA FW 15, and S. bayanus CBS 1505), whereas the wine fermented by UFLA FW 15 showed the highest amount of succinic acid (7.9 g/L).
Other authors [64][67] for the elaboration of papaya vinegar carried out a pasteurization process before and after the alcoholic fermentation in order to eliminate any possible microorganisms and prevent any undesired modifications of the sample’s content. In this case, they used active dry S. cerevisiae (ADS) yeast in powder form, which under anaerobic conditions and at an incubation temperature of 30 °C for 7 days allowed the production of papaya wine.
Alternatively, the starter culture can be achieved by cell immobilization. Encapsulation is the most often used immobilization method. This method consists of confining the intact active cells within a specific region. Some of its advantages are the following: stimulation of the production, prolongation and excretion of secondary metabolites (e.g., aromatic compounds), continuous cell recovery and reuse, and protection against unfavorable environments, among others [113,114][72][73].
Another important advantage that this technology provides consists of the reduction of processing costs and the possibility of customizing the properties of the product of interest, such as improving its organoleptic characteristics and safety, or generating specific functional properties such as the increase of antioxidant capacity derived from the polyphenolic content, melatonin production released by yeasts, or probiotic and immunomodulatory properties. Immobilization mimics the cellular aggregation phenomenon that normally occurs when microorganisms grow in natural environments. Several substances have been investigated to be used as an aid for immobilization. Leonés et al. [80][20] used two types of commercial yeasts: Saccharomyces cerevisiae AWRI 796 and Saccharomyces cerevisiae var. bayanus for the alcoholic fermentation that was required for the production of lemon vinegar. For each strain, they carried out both submerged and immobilized culture in alginate spheres. The best conditions for the alcoholic fermentation were obtained when Saccharomyces cerevisiae var. bayanus was used in a submerged culture, since a higher alcoholic degree was reached. This could be probably explained by the fact that when yeasts move freely in the medium, a larger amount of nutrients is at their disposal than when they are immobilized and, therefore, this improves the performance of the process.
2.2. Acetic Fermentation
Once the sugar has been transformed into ethanol, the next fermentation that takes place in the process to elaborate fruit vinegars is the acetic fermentation, which consists of the oxidation of the alcohol into acetic acid. This is an oxygen-dependent reaction, and, therefore, as the amount of oxygen decreases with the alcoholic fermentation, once the sugar is depleted, the oxygen concentration must be increased again for the acetic fermentation to take place.
The dynamic changes in the microbial community during acetic fermentation are different from those taking place during other stages of fermentation [115][74]. The high concentration of ethanol at the initial stages and the high acidic conditions of the middle and final stages suggest that most of the bacteria present are acetic acid bacteria. Therefore, the biotransformation of ethanol into acetic acid is usually performed by that type of bacteria. When bacteria use acetic acid as a carbon source, a peroxidation of the acetate can occur, which in turn leads to over-oxidation and to the formation of carbon dioxide and water [60][75]. If there are no losses due to evaporation or over-oxidation, the total concentration—the sum of the ethanol concentration (% v/v) plus the total acidity (% w/v)—should remain constant over the acetification process.
As already mentioned, the metabolism of acetic acid bacteria is aerobic; however, they can survive under anaerobic conditions or with very low oxygen concentrations since they have the possibility to use quinones instead of oxygen as the final electron acceptor [116,117][76][77].
On the other hand, it is also known that the concentration of ethanol could exert an inhibitory effect on acetic acid bacteria when it is above 50 g/L (approximately 6% v/v), this being more pronounced in discontinuous processing [118][78]. For this reason, there are studies, such as the one by Davies et al. [119][79] on the production of orange vinegar, where orange wine, which had an alcoholic content of 13–14%, was diluted in order to facilitate the action by acetic acid bacteria. The dilution caused a variation in the concentration of nutrients, and a solution with minerals and a source of nitrogen had to be added.
The optimal growth temperature for these bacteria is between 25 and 30 °C, while the maximum temperature that they can tolerate may reach 40 °C [120,121][80][81]. Since the oxidation of ethanol into acetic acid is an exothermic reaction, excessive temperatures could destroy acetic bacteria and increase the evaporation of volatile compounds, such as ethanol or acetic acid. If this is the case, the resulting vinegar quality and yield would be affected. In order to prevent these negative effects, the fermenter should be equipped with heat dissipation systems, such as cooling coils [122][82]. It has been proven that by slightly increasing the fermentation temperature, the productivity of the process can be enhanced, even though it could favor the oxidation processes and the loss of aromatic components. However, the use of temperature gradients during the acetification process is proposed as a suitable solution, which would slow down the process and at the same time would prevent these previously mentioned inconveniences. Fregapane et al. [123][83] observed that a variation of only two centigrade degrees at the beginning of the acetic fermentation (32 °C) and subsequently decreasing the temperature to 30 °C produced a 15% increment in acetic acid production and a shortening of the processing time from 29 hours to 24.5 hours in comparison with an isothermal fermentation at 30 °C.
Acetic acid bacteria are a wide and well-distributed group that can be found in fruits, flowers, honey, soil, juices, and fermented beverages, among others [124][84]. In terms of taxonomy, there are currently 19 genera classified under acetic acid bacteria [125][85]. The exploitation of those bacteria has a long history in fermentation processes, and nowadays, they represent an emerging field in biotechnological applications such as the biosynthesis of chemical products or food science. Their most recognized application at present is the production of vinegar, and the species of the genera Acetobacter, Gluconobacter, and Gluconacetobacter are the most commonly used for this purpose. Generally, Acetobacter aceti is the most widely used bacterium in the vinegar industry, since it is the one that usually starts the acetic fermentation [43][86], while Gluconobacter can provide a slightly different taste to vinegar due to the production of gluconate [126][87]. However, the production of D-gluconic acid has also been detected in acetic acid bacteria such as Acetobacter syzygii [127][88]. Hidalgo et al. [53][40], during the production of persimmon vinegar, identified bacteria such as Acetobacter malorum, Gluconacetobacter saccharivorans, Acetobacter pasteurianus, Acetobacter syzygii, Gluconacetobacter intermedius, or Gluconacetobacter europaeus, among others. In another study carried out using the acetic acid bacteria isolated from blueberries, several different genera of these were identified through biochemical tests (Acetobacter, Gluconobacter, Asaia, Gluconacetobacter and Swaminathania) dependent on the different varieties of blueberries used for the experiments [31][38].
However, the use of starter cultures is far from being applied at a large scale, mainly for two reasons: firstly, because it would not be economically profitable, since vinegar is a generally inexpensive product and elaborating it with a starter culture would increase costs; and secondly, because the nutritional requirements by acetic bacteria present considerable difficulties for their cultivation and conservation in laboratories [1]. Nevertheless, there have been studies that have used bacterial inocula such as Acetobacter malorum for the production of strawberry vinegar [52][39]. However, these authors observed that this strain of bacteria was displaced by Gluconacetobacteria when the acetic fermentation took place in wooden barrels. Another example would be the study carried out by Boonsupa [58][89], who spent 15 days experimenting with the acetic fermentation of blackberry, blueberry, and raspberry wines inoculated with Acetobacter pasteurianus. The same authors carried out the fermentation of banana vinegar using the same bacterial strain [60][75].
Although it is still a field in which scarce scientific literature is found, the use of acetic acid bacteria as starter of the fermentation would present several advantages, compared to spontaneous acetic fermentation. According to Hidalgo et al. [52][39], the use of starter cultures induced a fast beginning of the acetification and provided the appropriate conditions for the correct development of the process, avoiding stuck acetification. Moreover, in the inoculated processes, the final acidity of vinegars seems to be higher. Concretely, in this study, the samples inoculated with Acetobacter cerevisiae reached higher acidity values (from 6.6% to 6.9% (w/v)) in shorter times than those with spontaneous fermentation (5.5% w/v after 28 days).
Úbeda et al. [56][59] reported differences of 2 acetic degrees between spontaneous and inoculated fermentation of strawberry vinegars. In addition, the study of the aromatic fraction demonstrated that inoculated acetification carried out in wood barrels yielded vinegars with a better aroma profile. The same authors described significant differences in ethyl acetate content, which increased from wine to vinegar when starter cultures were employed, with values from 83 to 682 mg/L, whereas for spontaneous processes, it diminished, due to hydrolysis phenomena, showing values from 45 to 483 mg/L [56][59]. This could affect the final organoleptic properties of the vinegars, because ethyl acetate presents a strong “glue” odor, so this character would be more intense when inoculated fermentations are carried out.
Higher alcohols and methanol also showed differences between spontaneous fermentations and fermentations performed with selected acetic acid bacteria. Regarding the former ones, their consumption was higher in those vinegars fermented with starters, whereas methanol showed a larger decrease in the spontaneous fermentation. Finally, it is worth mentioning that there are some studies in which high contents of polyphenols and antioxidant activities have been reported in fruit vinegars produced with A. pasteurianus [58,60][89][75]. Therefore, it seems that by the careful selection of the bacteria strain employed in the acetic fermentation, some bioactive components could also be promoted to the final product.
As can be seen, the composition of fruit vinegars depends on the acetic acid bacteria strain that carries out the fermentation. In addition, faster fermentations are usually obtained when starters are employed. Therefore, a comprehensive control can be obtained if starters are employed in the production of fruit vinegars. In this way, products with the expected composition and organoleptic properties by producers and consumers can be obtained. The type of microorganism used both, in alcoholic and acetic fermentation, affects the final characteristics of the vinegar produced. However, scarce information about different acetic acid strains and the consequences of their use in the production of fruit vinegars is available. That could be a future research subject in order to obtain fruit vinegars even from the same raw material but with different sensory properties, increasing, in this way, the variety of acetic products that can be commercialized.
Acetification Systems
There are basically two acetification methods: surface and submerged cultivation systems.
In the surface culture method, acetic acid bacteria grow abundantly on the surface of the medium, at the liquid–gas interface, where the highest concentration of oxygen is present. This is considered a static method, where the presence of bacteria at the interface is limited for physical reasons. There are numerous investigations where this method of acetification has been applied to the elaboration of fruit vinegars [28,32,37,44,46,49,58,66,67,73,106,128,129][53][90][54][91][92][58][89][93][66][94][62][95][96]. The acetic acid values obtained using this method of fermentation on surface cultures are usually not too high. For instance, Özen et al. [44][91] used this surface cultivation method for the elaboration of cherry vinegars, both from fresh juice and from concentrate, achieving acidity values above 4.6%. Cejudo-Bastante et al. [37][54], during the elaboration of orange vinegars by means of surface cultivation, obtained similar values of acidity (around 4%), and the final vinegars presented good organoleptic characteristics. On the other hand, Fatima and Mishra [32][90] obtained acidity values between 5% and 6% in coconut water vinegars and slightly less in banana skin vinegars. In addition, the times employed for the fermentation by means of surface culture are usually relatively long. Fermentation times as long as 144 days have been reported for the acetic fermentation of black raspberry vinegars [73][94], although shorter periods have also been reported, such as 45 days for plum vinegars [46][92], 30 days for cherry vinegars [44][91], or 15 days for berry vinegars [58][89]. A reference has been found in which the Schützenbach method was used for the elaboration of blueberry vinegars [31][38]. This method, which allows an acceleration of the process, uses wood shavings to support the bacteria, and the liquid is pumped over the shavings to increase the oxygen supply. Using this system, the process of acetification was accelerated to 9–24 days compared to the traditional method of surface cultivation that required more than 30 days.
On the other hand, the methods of fermentation using submerged culture are based on the presence of a culture of bacteria freely submerged within the liquid to be fermented. Air is constantly supplied (either on its own or enriched with oxygen), and no additional support is provided to the bacteria [130][97]. For these methods, acetifiers are used, which are usually automated and provide a high flow of oxygen (Figure 4). Therefore, these methods usually present higher yields than those obtained through surface culture fermentation [131][98].
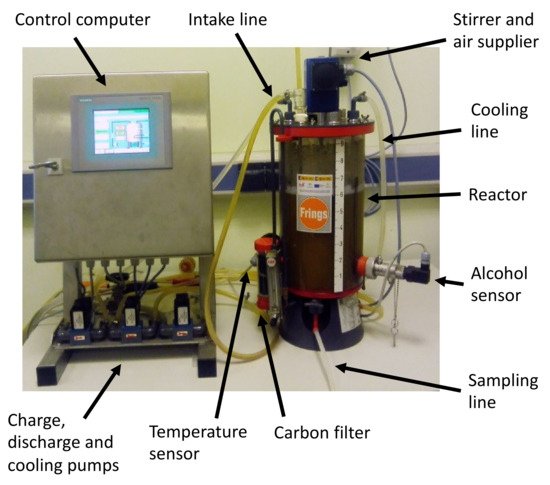
Figure 4.
Typical system for submerged culture fermentation.
However, surface cultivation methods have traditionally been considered as being suitable for the production of quality vinegars. Molelekoa et al. [129][96] used surface and submerged culture for the production of marula vinegar (a fruit from South Africa). When surface culture was used for the fermentation process, the final product had a higher antioxidant and anti-radicals power.
The investigations that have used the method of elaboration of fruit vinegars by means of submerged culture are also very numerous, of which orange has been the most commonly used fruit [28[53][99][79],42,119], followed by pomegranate [45,71][55][100]. Nevertheless, other research studies have been found on strawberries [62][101], persimmon [48][57], peach [109][68], tomato [132][102], lemon [80][20], apricot [76][103], and marula [129][96].
Fermentation times when using submerged culture are usually much shorter than those used in surface culture. Cejudo-Bastante et al. [28][53] compared the two systems of acetification for the elaboration of orange vinegar, and 6 weeks was used for the fermentation in surface culture, as opposed to 22 hours used for the fermentation in submerged culture. The constant supply of oxygen during the whole process is vital, since these species are strictly aerobic, and an interruption of the air supply may result in the death of the culture [133][104]. At the beginning of the process, the level of the air flow must be maintained at low levels, around 1 L of O2/h L of substrate, to favor the reproduction of the bacteria. This should be increased to values around 7.5 L of O2/h L of substrate after the acetic fermentation has begun [28,80,132][53][20][102].
Many compounds are normally degraded during the acetic fermentation process in vinegar production, and this fact is more pronounced when the submerged culture method is used. This is explained by the increased yield of the process. In an experiment in which strawberry vinegars were developed, 91% of the anthocyanins were lost during the acetic fermentation, compared to just 19% losses during the alcoholic fermentation [62][101]. In another investigation with pomegranate vinegars, it was observed that volatile and polyphenolic compounds increased during the alcoholic fermentation but decreased during the acetic one [45][55]. Another study reported reductions around 60% of the polyphenolic content in pomegranate vinegars compared to the starting juice [71][100]. Although it is difficult to avoid these losses of bioactive compounds during the acetification process due to the biochemical nature of the process, it could be attenuated when surface culture is employed. When operating with submerged culture, a high amount of oxygen is supplied in order to accelerate the oxidation reaction of alcohol into acetic acid. For an industrial tank holding 25,000 L and operating at an acetification rate of 0.2% acetic acid · h−1, it will require about 20,000 L of oxygen (at 20 °C, 1 atm) per hour (i.e., 26.7 kg O2·h−1) [131][98]. Taking into account that air is used to supply the oxygen and that only 60–90% of the oxygen supplied is employed to oxidize ethanol, the amount of air required at 20 °C and 1 atm would be 100,000–150,000 L·h–1. This high amount of air would also provoke the acceleration of other oxidative reactions of bioactive compounds and therefore would favor some degradation processes when submerged culture is employed. As it has been commented previously, the selection of specific strains of acetic acid bacteria that favored the production of bioactive components could help diminish these losses. For example, the production of D-gluconic acid, which has been demonstrated to be an interesting compound with healthy properties [134][105], could be favored by the specific strains of acetic acid bacteria employed, such as Gluconobacter japonicus, or Gluconobacter oxydans [135][106]. Usually, Gluconobacter strains are generally more ketogenic than Acetobacter strains. Thus, Gluconobacter strains oxidize a broader range of substrates compared to Acetobacter, such as alcohols, sugars, sugar acids, or sugar alcohols, and therefore, the corresponding oxidation products are accumulated in the medium [136][107]. For the preservation of the volatile fraction, De Ory et al. [137][108] proposed an acetic acid fermentation reactor equipped with a closed gas recycling system that prevents any loss of volatile compounds due to evaporation. With this system, the evaporative losses were reduced to 0% during the acetic acid fermentation process.
Three modes of operating the fermenter are available when working in submerged culture: discontinuous, semi-continuous, or continuous [131][98]. Virtually all the research related to the development of fruit vinegars uses the semi-continuous mode, in which the whole fermenter is not discharged when the process of acetification has been completed. Instead, only part of it is discharged, while another part is used as the starter for the next fermentation cycle, which speeds up the process [138][109]. The discharge volume may vary, but it is usually between one-half and two-thirds of the fermenting volume. For example, Hornedo-Ortega et al. [62][101] developed strawberry vinegar by operating in a semi-continuous mode and discharged about 73% of the fermenting volume. On the other hand, Cejudo-Bastante et al. [132][102] used this method for the production of tomato vinegars, performing a 66% discharge, the same value of Leonés et al. [80][20] for the production of lemon vinegars.
Regarding the effect of acetification system on sensory properties of fruit vinegars other than grapes, only one reference has been found in the literature, in which both systems were employed for the production of orange vinegar and sensory evaluation was carried out [28][53]. The submerged culture produced more pungent vinegars, with higher scores of the descriptors “fruity”, “floral”, and “glue”, and with better values of “general impression”, compared to vinegars obtained by means of surface culture. In another work concerning Turkish grape vinegar, the authors obtained higher values of acidity and contents of volatile compounds with the surface culture method [139][110]. However, regarding sensory characteristics, these authors reported significant differences only for ethyl acetate odor and aromatic intensity (higher for surface culture); the rest of descriptors were significantly similar for both acetification systems.
References
- Solieri, L.; Giudici, P. Vinegars of the World; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 1–16.
- Food and Agriculture Organization. The State of Food and Agriculture 2019: Moving Fordward on Food Loss and Waste Reduction; Food and Agriculture Organization: Rome, Italy, 2019.
- Bastante, M.J.C.; Guerrero, E.D.; Mejías, R.C.; Marín, R.N.; Dodero, M.C.R.; Barroso, C.G. Study of the Polyphenolic Composition and Antioxidant Activity of New Sherry Vinegar-Derived Products by Maceration with Fruits. J. Agric. Food Chem. 2010, 58, 11814–11820.
- Cejudo-Bastante, M.J.; Durán, E.; Castro, R.; Rodríguez-Dodero, M.C.; Natera, R.; García-Barroso, C. Study of the volatile composition and sensory characteristics of new Sherry vinegar-derived products by maceration with fruits. LWT 2013, 50, 469–479.
- Bruna-Maynou, F.J.; Castro, R.; Rodríguez-Dodero, M.C.; Barroso, C.G.; Durán-Guerrero, E. Flavored Sherry vinegar with citric notes: Characterization and effect of ultrasound in the maceration of orange peels. Food Res. Int. 2020, 133, 109165.
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Establishment of the Volatile Signature of Wine-Based Aromatic Vinegars Subjected to Maceration. Molecules 2018, 23, 499.
- Vegas, C.; González, Á.; Mateo, E.; Mas, A.; Poblet, M.; Torija, M.J. Evaluation of representativity of the acetic acid bacteria species identified by culture-dependent method during a traditional wine vinegar production. Food Res. Int. 2013, 51, 404–411.
- Larrauri, J.; Rupérez, P.; Bravo, L.; Saura-Calixto, F. High dietary fibre powders from orange and lime peels: Associated polyphenols and antioxidant capacity. Food Res. Int. 1996, 29, 757–762.
- Ubandorivera, J. Mexican lime peel: Comparative study on contents of dietary fibre and associated antioxidant activity. Food Chem. 2005, 89, 57–61.
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401.
- Marrufo-Curtido, A.; Cejudo-Bastante, M.J.; Rodríguez-Dodero, M.C.; Natera-Marín, R.; Castro-Mejías, R.; García-Barroso, C.; Durán-Guerrero, E. Novel vinegar-derived product enriched with dietary fiber: Effect on polyphenolic profile, volatile composition and sensory analysis. J. Food Sci. Technol. 2015, 52, 7608–7624.
- Launholt, T.L.; Kristiansen, C.B.; Hjorth, P. Safety and side effects of apple vinegar intake and its effect on metabolic parameters and body weight: A systematic review. Eur. J. Nutr. 2020, 59, 2273–2289.
- Cavdaroglu, C.; Ozen, B. Authentication of Vinegars with Targeted and Non-targeted Methods. Food Rev. Int. 2021, 1–18.
- Laurent, D. Food Traceability and Authenticity Based on Volatile Compound Analysis. In Food Traceability and Authenticity; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 216–231.
- Luo, M.; Zheng, Y.; Xiong, C.; Li, B.; Chen, S.; Bai, W.; Zeng, Y.; Li, Y.; Zhang, X. A Geographical Discrimination of Shanxi Extra Aged Vinegars Using Polyalcohols as the Discriminators. J. AOAC Int. 2013, 96, 1048–1053.
- Cagliani, L.R.; Scano, P.; Consonni, R. NMR-Based Metabolomics: Quality and Authenticity of Plant-Based Foods. In Modern Magnetic Resonance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1709–1727.
- Jamin, E.; Thomas, F. SNIF-NMR Applications in an Economic Context: Fraud Detection in Food Products. In Modern Magnetic Resonance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1405–1416.
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Advances in Electronic Noses and Tongues for Food Authenticity Testing. In Advances in Food Authenticity Testing; Woodhead Publishing: Kidlington, UK, 2016; pp. 201–225.
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106.
- Leonés, A.; Durán-Guerrero, E.; Carbú, M.; Cantoral, J.M.; Barroso, C.G.; Castro, R. Development of vinegar obtained from lemon juice: Optimization and chemical characterization of the process. LWT 2019, 100, 314–321.
- Fonseca, M.D.S.; Santos, V.A.Q.; Calegari, G.C.; Dekker, R.F.H.; Barbosa-Dekker, A.D.M.; Da Cunha, M.A.A. Blueberry and honey vinegar: Successive batch production, antioxidant potential and antimicrobial ability. Braz. J. Food Technol. 2018, 21.
- Yan, H.G.; Zhan, W.H.; Chen, J.H.; Ding, Z.E. Optimization of the alcoholic fermentation of blueberry juice by AS 2.316 Saccharomyces cerevisiae wine yeast. Afr. J. Biotechnol. 2012, 11, 3623–3630.
- López, N.E.L.; Leiva, V.U.; Carrasco, C.A. Development of a distilled-like alcoholic drink from blueberry (Vaccinium corymbosum) cv. Brigitta, and sensory analysis. Acta Agron. 2015, 65, 1–8.
- Ubeda, C.; Callejón, R.; Hidalgo, C.; Torija, M.; Troncoso, A.; Morales, M. Employment of different processes for the production of strawberry vinegars: Effects on antioxidant activity, total phenols and monomeric anthocyanins. LWT 2013, 52, 139–145.
- Zohri, A.A.; Ramadan, A.M.; El-Tabakh, M.M.; Al-Tantawy, K. Key factors affecting the efficiency of ethanol fermentation using beet molasses. Egypt. Sugar J. 2015, 8, 27–52.
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int. J. Food Microbiol. 2003, 85, 127–136.
- Morimura, S.; Ling, Z.Y.; Kida, K. Ethanol production by repeated-batch fermentation at high temperature in a molasses medium containing a high concentration of total sugar by a thermotolerant flocculating yeast with improved salt-tolerance. J. Ferment. Bioeng. 1997, 83, 271–274.
- Vamvakas, S.-S.; Kapolos, J. Factors affecting yeast ethanol tolerance and fermentation efficiency. World J. Microbiol. Biotechnol. 2020, 36, 1–8.
- Silva, M.E.; Neto, A.B.T.; Silva, W.B.; Silva, F.L.H.; Swarnakar, R. Cashew wine vinegar production: Alcoholic and acetic fermentation. Braz. J. Chem. Eng. 2007, 24, 163–169.
- Siqueira, P.F.; Karp, S.G.; Carvalho, J.C.; Sturm, W.; Rodríguez-León, J.A.; Tholozan, J.-L.; Singhania, R.R.; Pandey, A.; Soccol, C.R. Production of bio-ethanol from soybean molasses by Saccharomyces cerevisiae at laboratory, pilot and industrial scales. Bioresour. Technol. 2008, 99, 8156–8163.
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The Microbiology of Wine and Vinifications. In Handbook of Enology; Wiley: Hoboken, NJ, USA, 2006.
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384.
- Lea, A.G. Cider Vinegar. In Processed Apple Products; Van Nostrand Reinhold: New York, NY, USA, 1989.
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piórecki, N. Bioactive Compounds in Cornelian Cherry Vinegars. Molecules 2018, 23, 379.
- Tanaka, C.A.; Ruzon, F.I.; Caldeirão Rodrigues Miranda, L.; Galvan, D.; Spinosa, W.A.; Castro-Gómez Hernan, R.J. Modeling and Kinetics of Bioconversion and Chemical Properties (Wine and Vinegar) from Banana Pulp By-Products. Preprints 2016.
- Coelho, E.; Vilanova, M.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT 2015, 62, 1043–1052.
- Mas, A. Technological process for production of persimmon and strawberry vinegars. Int. J. Wine Res. 2010, 2, 55.
- Hidalgo, C.; García, D.; Romero, J.; Mas, A.; Torija, M.J.; Mateo, E. Acetobacter strains isolated during the acetification of blueberry (Vaccinium corymbosum L.) wine. Lett. Appl. Microbiol. 2013, 57, 227–232.
- Hidalgo, C.; Torija, M.; Mas, A.; Mateo, E. Effect of inoculation on strawberry fermentation and acetification processes using native strains of yeast and acetic acid bacteria. Food Microbiol. 2013, 34, 88–94.
- Hidalgo, C.; Mateo, E.; Mas, A.; Torija, M. Identification of yeast and acetic acid bacteria isolated from the fermentation and acetification of persimmon (Diospyros kaki). Food Microbiol. 2012, 30, 98–104.
- Song, N.-E.; Jeong, D.-Y.; Baik, S.-H. Application of indigenous Saccharomyces cerevisiae to improve the black raspberry (Rubus coreanus Miquel) vinegar fermentation process and its microbiological and physicochemical analysis. Food Sci. Biotechnol. 2019, 28, 481–489.
- Chanprasartsuk, O.-O.; Prakitchaiwattana, C.; Sanguandeekul, R.; Fleet, G.H. Autochthonous yeasts associated with mature pineapple fruits, freshly crushed juice and their ferments; and the chemical changes during natural fermentation. Bioresour. Technol. 2010, 101, 7500–7509.
- Duarte, W.F.; Dias, D.R.; Pereira, G.V.D.M.; Gervásio, I.M.; Schwan, R.F. Indigenous and inoculated yeast fermentation of gabiroba (Campomanesia pubescens) pulp for fruit wine production. J. Ind. Microbiol. Biotechnol. 2009, 36, 557–569.
- Morrissey, W.; Davenport, B.; Querol, A.; Dobson, A. The role of indigenous yeasts in traditional Irish cider fermentations. J. Appl. Microbiol. 2004, 97, 647–655.
- Cavaco, T.; Longuinho, C.; Quintas, C.; De Carvalho, I.S. Chemical and Microbial Changes during the Natural Fermentation of Strawberry Tree (Arbutus unedo L.) Fruits. J. Food Biochem. 2007, 31, 715–725.
- Beltran, G.; Torija, M.J.; Novo, M.; Ferrer, N.; Poblet, M.; Guillamón, J.M.; Rozès, N.; Mas, A. Analysis of yeast populations during alcoholic fermentation: A six year follow-up study. Syst. Appl. Microbiol. 2002, 25, 287–293.
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77.
- Benito, S.; Calderón, F. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54.
- Giudici, P.; Gullo, M.; Solieri, L. Traditional Balsamic Vinegar. In Vinegars of the World; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 157–177.
- Štornik, A.; Skok, B.; Trček, J. Comparison of Cultivable Acetic Acid Bacterial Microbiota in Organic and Conventional Apple Cider Vinegar. Food Technol. Biotechnol. 2016, 54, 113–119.
- Li, S.; Li, P.; Feng, F.; Luo, L.-X. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biotechnol. 2015, 99, 4997–5024.
- Degre, R. Selection and commercial cultivation of wine yeast and bacteria. In Wine Microbiology and Biotechnology; Fleet, G., Ed.; Taylor & Francis: London, UK, 1993; pp. 421–448.
- Cejudo-Bastante, C.; Durán-Guerrero, E.; García-Barroso, C.; Castro-Mejías, R. Comparative study of submerged and surface culture acetification process for orange vinegar. J. Sci. Food Agric. 2018, 98, 1052–1060.
- Cejudo-Bastante, C.; Castro-Mejías, R.; Natera-Marín, R.; García-Barroso, C.; Durán-Guerrero, E. Chemical and sensory characteristics of orange based vinegar. J. Food Sci. Technol. 2016, 53, 3147–3156.
- Kharchoufi, S.; Gomez, J.; Lasanta, C.; Castro, R.; Sainz, F.; Hamdi, M. Benchmarking laboratory-scale pomegranate vinegar against commercial wine vinegars: Antioxidant activity and chemical composition. J. Sci. Food Agric. 2018, 98, 4749–4758.
- Su, M.-S.; Chien, P.-J. Aroma impact components of rabbiteye blueberry (Vaccinium ashei) vinegars. Food Chem. 2010, 119, 923–928.
- Zou, B.; Wu, J.; Yu, Y.; Xiao, G.; Xu, Y. Evolution of the antioxidant capacity and phenolic contents of persimmon during fermentation. Food Sci. Biotechnol. 2017, 26, 563–571.
- Ubeda, C.; Callejon, R.M.; Troncoso, A.M.; Peña, F.; Morales, M.L.; Moreno-Rojas, J.M. Characterization of odour active compounds in strawberry vinegars. Flavour Fragr. J. 2012, 27, 313–321.
- Ubeda, C.; Callejón, R.; Hidalgo, C.; Torija, M.; Mas, A.; Troncoso, A.; Morales, M. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography—Mass spectrometry method. Food Res. Int. 2011, 44, 259–268.
- Koyama, M.; Ogasawara, Y.; Endou, K.; Akano, H.; Nakajima, T.; Aoyama, T.; Nakamura, K. Fermentation-induced changes in the concentrations of organic acids, amino acids, sugars, and minerals and superoxide dismutase-like activity in tomato vinegar. Int. J. Food Prop. 2016, 20, 888–898.
- Mohamad, N.E.; Yeap, S.K.; Beh, B.K.; Romli, M.F.; Yusof, H.M.; Kristeen-Teo, Y.W.; Sharifuddin, S.A.; Long, K.; Alitheen, N.B. Comparison of in vivo toxicity, antioxidant and immunomodulatory activities of coconut, nipah and pineapple juice vinegars. J. Sci. Food Agric. 2018, 98, 534–540.
- Budak, N.H. Bioactive components of Prunus avium L. black gold (red cherry) and Prunus avium L. stark gold (white cherry) juices, wines and vinegars. J. Food Sci. Technol. 2016, 54, 62–70.
- Seo, K.-I.; Lee, J.; Choi, R.-Y.; Lee, H.-I.; Lee, J.-H.; Jeong, Y.-K.; Kim, M.-J.; Lee, M.-K. Anti-obesity and anti-insulin resistance effects of tomato vinegar beverage in diet-induced obese mice. Food Funct. 2014, 5, 1579.
- Moon, Y.-J.; Choi, D.-S.; Oh, S.-H.; Song, Y.-S.; Cha, Y.-S. Effects of persimmon-vinegar on lipid and carnitine profiles in mice. Food Sci. Biotechnol. 2010, 19, 343–348.
- Chen, Y.; Huang, Y.; Bai, Y.; Fu, C.; Zhou, M.; Gao, B.; Wang, C.; Li, D.; Hu, Y.; Xu, N. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT 2017, 84, 753–763.
- Su, M.-S.; Silva, J.L. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2006, 97, 447–451.
- Ching, T.K.; Chin, W.H.; Ling, J.W.A.; Lazim, A.; Fazry, S.; Lim, S.J. Chemical Changes and Optimisation of Acetous Fermentation Time and Mother of Vinegar Concentration in the Production of Vinegar-like Fermented Papaya Beverage. Sains Malays. 2018, 47, 2017–2026.
- Xiang, J.L.; Du, L.; Liu, Z.J.; Li, X.L.; Luo, L.; Zhu, W.X. Changes in biochemical parameters and organic acid content during liquid fermentation of peach vinegar. Mod. Food Sci. Technol. 2015, 31, 193–198.
- Huh, W.-K.; Lee, B.-H.; Kim, S.-T.; Kim, Y.-R.; Rhie, G.-E.; Baek, Y.-W.; Hwang, C.-S.; Lee, J.-S.; Kang, S.-O. D-Erythroascorbic acid is an important antioxidant molecule in Saccharomyces cerevisiae. Mol. Microbiol. 1998, 30, 895–903.
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230.
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Vilanova, M.; Teixeira, J.A.; E Silva, J.B.A.; Schwan, R.F. Raspberry (Rubus idaeus L.) wine: Yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res. Int. 2010, 43, 2303–2314.
- De Backer, L.; Devleminck, S.; Willaert, R.; Baron, G. Reaction and diffusion in a gel membrane reactor containing immobilized cells. Biotechnol. Bioeng. 1992, 40, 322–328.
- Nath, S.; Chand, S. Mass Transfer and Biochemical Reaction in Immobilized Cell Packed Bed Reactors: Correlation of Experiment with Theory. J. Chem. Technol. Biotechnol. 1996, 66, 286–292.
- Nie, Z.; Zheng, Y.; Wang, M.; Han, Y.; Wang, Y.; Luo, J.; Niu, D. Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresour. Technol. 2013, 148, 325–333.
- Boonsupa, W.; Chumchuere, S.; Chaovarat, M. Physicochemical properties and antioxidant activity of banana vinegar produced using one-stage and two-stage fermentation. Agric. Nat. Resour. 2019, 53, 298–305.
- Sun, D.-W. Advances in Vinegar Production, 1st ed.; Bekatorou, A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351208475.
- Solieri, L.; Giudici, P. Acetic Acid Bacteria Taxonomy from Early Descriptions to Molecular Techniques. In Vinegars of the World; Springer: Berlin/Heidelberg, Germany, 2009; pp. 41–59.
- Garrido-Vidal, D.; Pizarro, C.; González-Sáiz, J.M. Study of Process Variables in Industrial Acetic Fermentation by a Continuous Pilot Fermentor and Response Surfaces. Biotechnol. Prog. 2003, 19, 1468–1479.
- Davies, C.V.; Gerard, L.M.; Ferreyra, M.M.; Schvab, M.D.C.; Solda, C.A. Bioactive compounds and antioxidant activity analysis during orange vinegar production. Food Sci. Technol. 2017, 37, 449–455.
- Maal, K.B.; Shafiei, R. A Thermotolerant Acetobacter Strain Isolated from Iranian Peach Suitable for Industrial Microbiology. Asian J. Biol. Sci. 2011, 4, 244–251.
- Ndoye, B.; Lebecque, S.; Dubois-Dauphin, R.; Tounkara, L.; Guiro, A.-T.; Kere, C.; Diawara, B.; Thonart, P. Thermoresistant properties of acetic acids bacteria isolated from tropical products of Sub-Saharan Africa and destined to industrial vinegar. Enzym. Microb. Technol. 2006, 39, 916–923.
- García Garibay, M.; Quinteros Ramirez, R.; López Munguía, A. Biotecnología Alimentaria; Editorial LIMUSA | (Grupo Noriega Editores): México, MX, USA, 2007.
- Fregapane, G.; Rubio-Fernández, H.; Salvador, M. Influence of fermentation temperature on semi-continuous acetification for wine vinegar production. Eur. Food Res. Technol. 2001, 213, 62–66.
- Kersters, K.; Lisdiyanti, P.; Komagata, K.; Swings, J. The Family Acetobacteraceae: The Genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia. Prokaryotes 2006, 5, 163–200.
- Trček, J.; Barja, F. Updates on quick identification of acetic acid bacteria with a focus on the 16S–23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int. J. Food Microbiol. 2015, 196, 137–144.
- Dias, D.R.; Silva, M.S.; De Souza, A.C.; Magalhães-Guedes, K.T.; Ribeiro, F.S.D.R.; Schwan, R.F. Vinegar Production from Jabuticaba Fruits (Myrciaria jaboticaba) Using Immobilized Acetic Acid Bacteria. Food Technol. Biotechnol. 2016, 54, 351–359.
- Saeki, A. Application of gluconobacter oxydans subsp. sphaericus IFO 12467 to vinegar production. J. Ferment. Bioeng. 1993, 75, 232–234.
- Pinto, L.; Malfeito-Ferreira, M.; Quintieri, L.; Silva, A.; Baruzzi, F. Growth and metabolite production of a grape sour rot yeast-bacterium consortium on different carbon sources. Int. J. Food Microbiol. 2019, 296, 65–74.
- Boonsupa, W. Chemical Properties, Antioxidant Activities and Sensory Evaluation of Berry Vinegar. Walailak J. Sci. Technol. 2018, 16, 887–896.
- Fatima, B.; Mishra, A.A. Optimization of Process Parameter for the Production of vinegar from babana peel and coconut water. Int. J. Sci. Eng. Technol. 2015, 3, 817–823.
- Özen, M.; Özdemir, N.; Filiz, B.E.; Budak, N.H.; Kök-Taş, T. Sour cherry (Prunus cerasus L.) vinegars produced from fresh fruit or juice concentrate: Bioactive compounds, volatile aroma compounds and antioxidant capacities. Food Chem. 2020, 309, 125664.
- Zhao, H.; Zhou, X.; Huang, Y.; Wuyun, T.; Li, F.; Zhu, G.; Luo, Y. Two Types of New Natural Materials for Fruit Vinegar in Prunus Plants. MATEC Web Conf. 2017, 100, 4006.
- Ubeda, C.; Callejón, R.M.; Troncoso, A.M.; Moreno-Rojas, J.M.; Peña, F.; Morales, M.L. A comparative study on aromatic profiles of strawberry vinegars obtained using different conditions in the production process. Food Chem. 2016, 192, 1051–1059.
- Song, N.; Cho, S.; Baik, S. Microbial community, and biochemical and physiological properties of Korean traditional black raspberry (Robus coreanus Miquel) vinegar. J. Sci. Food Agric. 2016, 96, 3723–3730.
- Prieto, C.; Saenz, C.; Silva, P.; Loyola, E. Balsamic Type Vinegar from Colored Ecotypes of Cactus Pear (Opuntia Ficus-Indica). Acta Hortic. 2009, 811, 123–126.
- Molelekoa, T.J.; Regnier, T.; Da Silva, L.S.; Augustyn, W.A. Potential of marula (Sclerocarya birrea subsp. caffra) waste for the production of vinegar through surface and submerged fermentation. S. Afr. J. Sci. 2018, 114, 1–6.
- Conner, H.A.; Allgeier, R.J. Vinegar: Its History and Development. Adv. Virus Res. 1976, 20, 81–133.
- García-García, I.; Santos-Dueñas, C.; Jiménez-Ot, J.; Jiménez Hornero, J. Vinegar Engineering. In Vinegars of the World; Springer: Berlin/Heidelberg, Germany, 2009; pp. 97–120.
- Tessaro, D.; Larsen, A.C.; Dallago, R.C.; Damasceno, S.G.; Sene, L.; Coelho, S.R.M. Avaliação das fermentações alcoólica e acética para produção de vinagre a partir de suco de laranja. Acta Sci. Technol. 2010, 32, 201–205.
- Ordoudi, S.A.; Mantzouridou, F.; Daftsiou, E.; Malo, C.; Hatzidimitriou, E.; Nenadis, N.; Tsimidou, M.Z. Pomegranate juice functional constituents after alcoholic and acetic acid fermentation. J. Funct. Foods 2014, 8, 161–168.
- Hornedo-Ortega, R.; Fernandez, M.A.; Cerezo, A.B.; Garcia-Garcia, I.; Troncoso, A.M.; Garcia-Parrilla, M.C. Influence of Fermentation Process on the Anthocyanin Composition of Wine and Vinegar Elaborated from Strawberry. J. Food Sci. 2017, 82, 364–372.
- Durán-Guerrero, E. Volatile Compounds, Polyphenols and Sensory Quality in the Production of Tomato Vinegar. J. Food Nutr. Res. 2017, 5, 391–398.
- Maal, B.K.; Shafiei, R.; Kabiri, N.; Maal, K.B.; Shafiei, R.; Kabiri, N. Production of apricot vinegar using an Isolated acetobacter strain from Iranian apricot. World Acad. Sci. Eng. Technol. 2010, 4, 810–813.
- Tesfaye, W.; Garcia-Parrilla, M.C.; Troncoso, A.M. Set Up and Optimization of a Laboratory Scale Fermentor for the Production of Wine Vinegar. J. Inst. Brew. 2000, 106, 215–220.
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-García, I. Gluconic acid: Properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization. Process. Biochem. 2016, 51, 1891–1903.
- Sainz, F.; Navarro, D.; Mateo, E.; Torija, M.; Mas, A. Comparison of d-gluconic acid production in selected strains of acetic acid bacteria. Int. J. Food Microbiol. 2016, 222, 40–47.
- Raspor, P.; Goranovič, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124.
- De Ory, I.; Romero, L.E.; Cantero, D. Maximum yield acetic acid fermenter. Bioprocess Biosyst. Eng. 1999, 21, 187–190.
- De Ory, I.; Romero, L.E.; Cantero, D. Operation in semi-continuous with a closed pilot plant scale acetifier for vinegar production. J. Food Eng. 2004, 63, 39–45.
- Turhan, E.Ü.; Canbas, A. Chemical and Sensory Properties of Vinegar from Dimrit Grape by Submerged and Surface Method. GIDA J. Food 2016, 1–7.
More
