Bone sarcomas are rare cancers which often present with metastatic disease and are still associated with poor survival rates. Studies in the last decade have identified that exosomes, a type of extracellular vesicle released by cells, play an important role in tumour progression and dissemination. Through the transfer of their cargo (RNAs, proteins, and lipids) across cells, they are involved in cellular cross-talk and can induce changes in cellular behaviour. Exosomes have been shown to be important in metastasis organotropism, induction of angiogenesis and vascular permeability, the education of cells towards a pro-metastatic phenotype or the interaction between stromal and tumour cells. Due to the importance exosomes have in disease progression and the high incidence of metastasis in bone sarcomas, recent studies have evaluated the implications of these extracellular vesicles in bone sarcomas.
- bone sarcoma
- exosomes
- metastasis
- osteosarcoma
- Ewing sarcoma
- chondrosarcoma
1. Exosomes
1.1. Exosome Biogenesis, Release, and Uptake
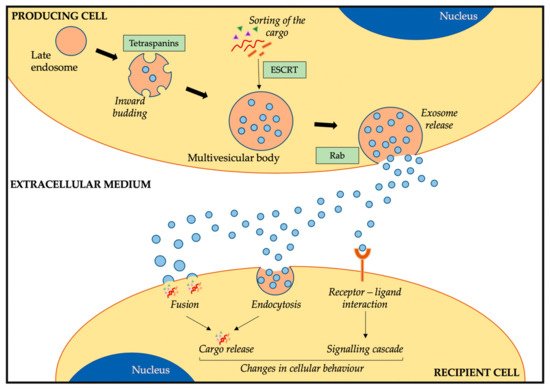
1.2. Exosome Cargo and Composition
1.3. Function of Exosomes
2. The Role of Exosomes in Bone Sarcoma Metastasis
Bone sarcoma metastasis represents the most adverse clinical factor and is associated with poor survival rates [35][1]. This is partly due to the lack of understanding on the molecular mechanisms behind tumour dissemination and metastatic disease [35][36][37][1,74,75]. In both OS and ES, the most common metastatic site is the lung, followed by bone and bone marrow. Interestingly, lung metastasis is associated with better prognosis than non-lung metastasis [35][38][1,76]. Due to the importance of metastasis in these bone sarcomas, many studies have focused on deciphering the pathways behind disease progression. Amongst the different genes identified in OS, we find overexpression of CD155 [39][77], loss of TP53, RB1, and PTEN [40][78], and upregulation of Notch genes [41][79] to be important in metastatic OS. Moreover, different novel treatment strategies are evaluating their efficacy in OS metastatic patients through clinical trials [37][75], with the aim of finding novel treatment strategies for these patients. In ES, similar studies have resulted in several genes linked to metastasis, such as ROR1 [42][80], MSH2, MSH6, RPA2, and RFC2 genes from the mismatch repair pathway [43][81], PPP1R1A [44][82] and TWIST1 proteins [45][83], the Cad11 adhesion molecule [46][84], or ERBB4 via activation of the PI3K-Akt-FAK cascade [47][85]. Moreover, similar to other cancers, hypoxia has been associated with induction of metastasis in OS and ES via regulation of HIF1α through HIF1α [48][86] or overexpression of CXCR4 in ES [49][87], amongst others. Similar to OS, different clinical trials are evaluating the response of metastatic ES patients to different treatment approaches in order to improve survival rates [38][76]. In contrast to OS and ES, CS is usually a non-metastatic disease with locally aggressive tumours [50][88]. However, some evidence suggests integrins are involved in the metastatic organotropism of CS to the lungs [51][52][89,90].2.1. Exosomes in OS
| Origin Cell | Recipient Cell | Cargo | Change | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| OS cells and conditioned media | - | Profiling of proteome and secretome | Exosome proteins involved in migration, adhesion, and angiogenesis | [60][98] | |||||
| Metastatic and non-metastatic OS cell lines | - | Profiling of miRNAs and target genes | miRNA of metastatic OS exosomes target metastasis-associated genes, cell adhesion, and apoptosis | [61][99] | |||||
| Metastatic and non-metastatic OS cell lines | Osteoblasts | ES cell linesmiR-675 (miRNA profiling) |
Osteoblasts, osteoclasts in 3D scaffoldMetastasis-associated exosomes induce migration and invasion in osteoblasts via miR-675/CALN1 axis | [62][100] | |||||
| EZH2 mRNA | (target of interest) | Transfer of EZH2 mRNA to MSC (increase expression), osteoblasts (no change), and osteoclasts (reduction expression) | [82][118] | Metastatic OS cell lines | Macrophages, osteoclasts, endothelial cells | miR-148a, miR-21-5p (RNA profiling) |
Induction of osteoclast-like gene expression (macrophage), increase in bone resorption (osteoclasts) and angiogenesis (endothelial cells) via miRNA transfer | [63][101] | |
| ESCD99neg cell line model | ES cell lines (normal CD99) | Increased miR-34a | Regulation of NFκB via miR-34a through reduction of Notch. Increase in neural differentiation (similar to direct CD99 silencing) | [83][119] | Metastatic and non-metastatic OS cell lines | ||||
| ESCD99neg cell line model | MSC | TGFβ (induction of IL6) |
Internalization of TGFβ induces IL6 production, cell growth, and lung metastasis in vivo | [64][102] | |||||
| Doxorubicin-resistant OS cell lines | Sensitive OS cell lines | MRP1, Pgp(multidrug resistant proteins) | Increase in doxorubicin resistance in recipient cells; increase in MRP1 and Pgp mRNA levels. | [65][103] | |||||
| Bone marrow (conditioned media) | Metastatic and non-metastatic OS cells | uPA (secreted, paracrine loop) | Increase in migration on recipient cells, induction of OS metastasis in vivo | [66][104] | |||||
| CAF | OS cell lines | miR-1228 (miRNA profiling) |
Increase in migration and invasion via miR-1228 transfer | [67][105] | |||||
| MSC | OS cell lines | miR-143 (synthetic introduction) | Reduction of migration via exosome transfer (better than transfection) | [68][106] | |||||
| OS cell lines | - | Profiling of miRNA as OS biomarkers | Better biomarker than ALP or patient stratification according to chemotherapy response | [69][70][107,108], |
2.2. Exosomes in ES
| Origin cell | Recipient cell | Cargo | Change | Ref. |
|---|---|---|---|---|
| ES cell lines | - | EWSR1-FLI1, EZH2, and 10 more mRNAs (mRNA profiling of known ES targets) |
Suitable as circulating biomarkers for ES, detectable in spike-in healthy blood samples | [80][116] |
| ES cell lines | ES cell lines | EWSR1-FLI1 mRNA | Labelled EWSR1-FLI1 transferred to other ES cells, not to OS | [81][117] |
| ES cell lines (normal CD99) | ||||
| miR-199a-3p | (miRNA profiling) miR-199a-3p |
Induction of different gene expression profiles, neural differentiation, and neurogenesis; reduction of cell growth and migration (similar to miR mimic) | [84][120] |
