Cyclic nigerosyl-nigerose (CNN) is an interesting and innovative nanocarrier for anti-cancer drug delivery in the cross-linked forms previously shown. The major advantage of CNN is that its natural structure is a reservoir for oxygen. CNN is a non-reducing cyclic tetrasaccharide with an unusual structure, consisting of four d-glucopyranosyl molecules connected by alternate α-(1-3) and α-(1-6) glycosidic bonds.
- cyclic nigerosyl nigerose
- oxygen delivery
- myocardial infarction
- ischemia
- reperfusion
1. Introduction
The nanocarriers were studied for tissue-specific targeted delivery, triggered release, and co-delivery of synergistic drug combinations to develop new efficient therapeutics [1].
As a result of the high aerobic metabolism, the myocardium already exhibits a high extraction of oxygen in resting conditions compared to other muscle tissues. The heart is an organ with elevated consumption of oxygen in its basal condition, and under several different conditions, e.g., anemia, high altitude exposure, or vessel stenosis, cardiac oxygenation may become critical, leading to tissue hypoxia. In particular, during acute coronary occlusion, cardiac cell death occurs. This is the first phase of acute myocardial infarction (AMI) [2].
Complete and timely restoration of blood flow is mandatory in order to save the ischemic myocardium from irreversible ischemic injury; however, reperfusion induces additional injury, i.e., a
reperfusion injury, and contributes to the final infarct size [3]. The lack of oxygen during ischemia and the subsequent re-introduction of oxygen during reperfusion, which lead to the massive formation of reactive oxygen species (ROS), are the basis of cardiac death and the inflammatory process, typical of ischemia/reperfusion injury. During AMI, a vicious cycle of deleterious events, including oxidative stress and inflammation, induces cardiac damage, impairing both diastolic and systolic function [2,4].
, and contributes to the final infarct size [3]. The lack of oxygen during ischemia and the subsequent re-introduction of oxygen during reperfusion, which lead to the massive formation of reactive oxygen species (ROS), are the basis of cardiac death and the inflammatory process, typical of ischemia/reperfusion injury. During AMI, a vicious cycle of deleterious events, including oxidative stress and inflammation, induces cardiac damage, impairing both diastolic and systolic function [2][4].
It is important to note the apparent paradox that ROS, which are normally considered lesion factors, at low concentrations and at the appropriate time of formation would induce myocardial protection [2,5,6,7,8]. Thus, the production of devices for the controlled delivery of oxygen can be of fundamental importance for reducing ROS lesions and favoring redox-induced protection.
It is important to note the apparent paradox that ROS, which are normally considered lesion factors, at low concentrations and at the appropriate time of formation would induce myocardial protection [2][5][6][7][8]. Thus, the production of devices for the controlled delivery of oxygen can be of fundamental importance for reducing ROS lesions and favoring redox-induced protection.
Protective strategies, such as slow re-flow procedures, have been proposed to limit re-oxygenation-induced lesions. However, this procedure has been abandoned, as it presents the problem of stagnant blood and consequent white blood cell infiltration [2,9]. Moreover, an intermittent re-flow (post-conditioning mode) has been suggested as an alternative strategy. Furthermore, intermittent reperfusion has raised several concerns in clinical settings, and full reperfusion is still the treatment of choice [10]. The ability of nanoparticles to aid in drug delivery by targeting regions affected by organ ischemia has been studied by many authors [11,12,13,14]. However, to the best of our knowledge, only one study has tested a polymer nanoparticle approach for delivering oxygen in a controlled manner to limit I/R injury [15].
Protective strategies, such as slow re-flow procedures, have been proposed to limit re-oxygenation-induced lesions. However, this procedure has been abandoned, as it presents the problem of stagnant blood and consequent white blood cell infiltration [2][9]. Moreover, an intermittent re-flow (post-conditioning mode) has been suggested as an alternative strategy. Furthermore, intermittent reperfusion has raised several concerns in clinical settings, and full reperfusion is still the treatment of choice [10]. The ability of nanoparticles to aid in drug delivery by targeting regions affected by organ ischemia has been studied by many authors [11][12][13][14]. However, to the best of our knowledge, only one study has tested a polymer nanoparticle approach for delivering oxygen in a controlled manner to limit I/R injury [15].
Cyclic nigerosyl-nigerose (CNN) is an interesting and innovative nanocarrier for anti-cancer drug delivery in the cross-linked forms previously shown [16]. The major advantage of CNN is that its natural structure is a reservoir for oxygen.
CNN is a non-reducing cyclic tetrasaccharide with an unusual structure, consisting of four d-glucopyranosyl molecules connected by alternate α-(1-3) and α-(1-6) glycosidic bonds (
). The presence of two oxydryl groups oriented to the cavity of the molecules leads to a more hydrophilic polarity of the CNN cavity in comparison with the well-known cyclodextrin one. The smaller cavity is much more prone to hosting small molecules of suitable size and polarity.
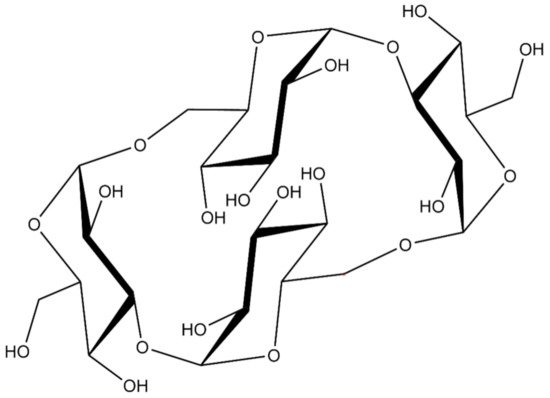
Figure 1.
The structure of cyclic nigerosyl-nigerose (CNN).
2. In Vitro Oxygen Release Kinetics
The pH value of cyclic nigerosyl-nigerose solution, either in the presence or in the absence of oxygen, was 6.0, and the osmolarity was about 300 mOsm. Hemolysis testing was performed (see CNN samples at all the concentrations tested), indicating good biocompatibility and a tonicity value suitable for the cell experiments. The in vitro oxygen release kinetics from CNN solution (NaCl 0.9%
w
/
v
) in the two receiving phases, at 37 °C, are shown in
. As can be seen, O
2
concentration reached a maximum level after 15 h both in saline solution and in a cell culture medium.
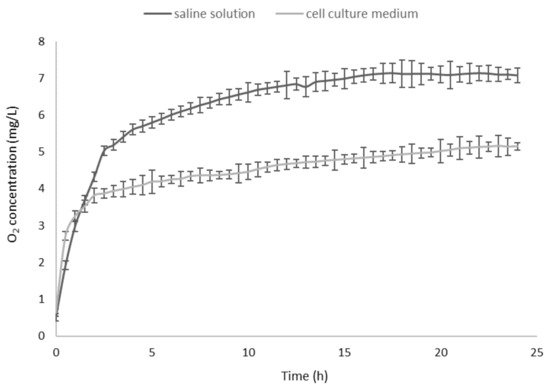
In vitro oxygen release kinetics from oxygen-loaded cyclic nigerosyl-nigerose solution (NaCl 0.9%
w/
v) in the two receiving phases (NaCl 0.9%
w/
vand cell culture medium).
A sustained oxygen release was observed in both of the receiving phases, reaching an oxygen concentration of 7.08 mg/L and 5.15 mg/L, in the saline solution and the cell culture medium, respectively.
The in vitro release profile of oxygen from the oxygen-loaded CNN glycerol solution containing dextran-70 (6%
w
/
v
) was compared to that of a common plasma volume expander (5%
w
/
v
glucose solution containing dextran-70 (6%
w
/
v
)) saturated with oxygen (
).
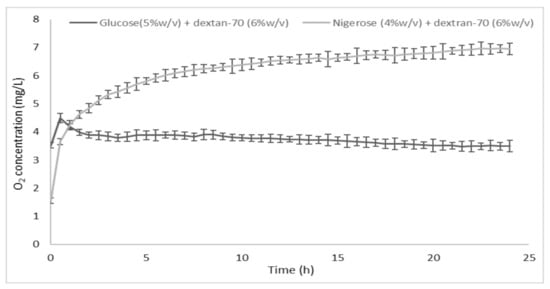
In vitro oxygen release kinetics from the oxygen-loaded cyclic nigerosyl-nigerose glycerol solution containing dextran-70 (6%
w/
v), compared to a 5%
w/
vglucose solution containing dextran-70 (6%
w/
v).
The characteristics of the oxygen-loaded CNN samples are reported in
.
Table 1.
Characteristics of oxygen-loaded cyclic nigerosyl-nigerose samples.
|
Cyclic Nigerosyl-Nigerose (4% w/v) in NaCl (0.9% w/v) |
Cyclic Nigerosyl-Nigerose (4% w/v) in Glycerol (2% w/v) + Dextran-70 (6% w/v) |
Glucose (5% w/v) + Dextran-70 (6% w/v) |
|
|---|---|---|---|
|
pH |
6.00 |
6.05 |
5.85 |
|
Osmolarity (mOsm) |
310 |
300 |
280 |
|
Viscosity (cP) |
1.07 |
2.30 |
3.05 |
3. Dose–Response and Growth Curve in Normoxic Conditions
We report in
results obtained with different concentrations (0.2, 2, 10, and 20 μg/mL) of oxygenated (O
2
-CNN, upper panels) and non-oxygenated (N
2
-CNN, lower panels) nanocarriers, in normoxic conditions. The exposure of H9c2 to different concentrations of CNN nanocarriers for 2 hours did not affect cell vitality, regardless of the presence of oxygen.
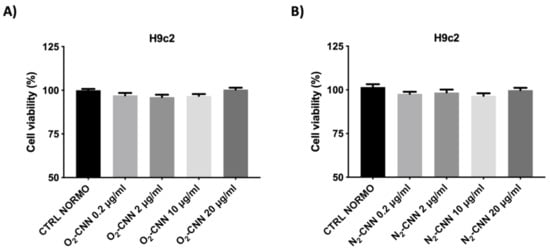
Dose–response of H9c2 in normoxic conditions. (
): Treatment with O
-CNN (0.2, 2, 10, and 20 μg/mL) compared to the untreated control group (CTRL). (
): Treatment with N
-CNN (0.2, 2, 10, and 20 μg/mL) compared to the untreated control group (CTRL). Data were expressed as mean ± SE. Data were normalized to the mean value under control conditions (CTRL NORMO) and expressed as a percentage.
To evaluate the effect of CNN on cellular growth, we have treated two different cell lines (H9c2 and HMEC) at a concentration of 10 μg/mL, for varying periods of time (24, 48, 72, 96, and 120 h). Both the H9c2 and HMEC proliferations were unaffected by either O
2
-CNN or N
2
-CNN given for 5 days at 10 μg/mL (
A,B).
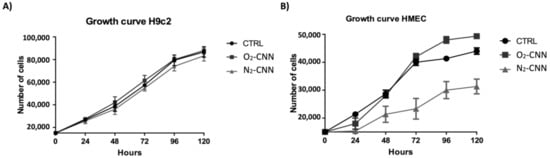
Figure 5.
Growth curves of the two cell models. (
A
): Treatment of H9c2 with O
2
-CNN or N
2
-CNN at 10 μg/mL compared to the untreated control group (CTRL). (
B
): Treatment of HMEC with O
2
-CNN or N
2
-CNN at 10 μg/mL compared to the untreated control group (CTRL). Data were expressed as mean ± SE.
4. Discussion
This study strongly indicates that oxygenated CNN is beneficial during an H/R protocol, and limits cell death in two relevant cellular models (i.e., cardiomyocyte-like, H9c2, and endothelial, HMEC) when applied before the hypoxic insult. When applied after hypoxia, at the beginning of re-oxygenation, its protective properties are limited to high concentrations, and in the H9c2 model only. Moreover, our results demonstrate that neither O
2
-CNN nor N
2
-CNN has toxic effects, and they do not affect cell vitality or growth in normoxia, in two different cellular models. When given in the post-hypoxic phase, O
2
-CNN was protective only at high concentration, and only in one cellular model, underlining the different susceptibility to re-oxygenation injury of the cardiac and the endothelial cell lines.
Despite the success and recent improvement of interventional coronary reperfusion strategies, morbidity and mortality from AMI are still considerable. Myocardial infarct size is a major determinant of prognosis in heart failure patients. Myocardial ischemia/reperfusion elicits various types of cardiomyocyte death and coronary microvascular damage [17]. Therefore, cardioprotective strategies aimed at reducing infarct size that protect both cardiomyocytes and microvessels are much needed [2,3,10,14]. Here, the addition of nanocarriers charged with oxygen prior to hypoxia was protective for both cardiomyocyte-like (H9c2) and endothelial (HMEC) cell lines. When given after hypoxia, they were protective for H9c2 only, but were not damaging to HMEC. Future studies should focus first on clinically relevant animal models [18], and then on patients in severe need of adjunct cardioprotection. Hopefully, these oxygen formulations may be included in the clinical armamentarium, either in programmed intervention (programmed revascularization), or in acute ischemia/reperfusion (elective angioplasty in AMI), as they can provide protection either before or after an ischemic event. Importantly, they might protect both cardiomyocytes and microvessels, therefore boosting the potential for cardioprotection.
Despite the success and recent improvement of interventional coronary reperfusion strategies, morbidity and mortality from AMI are still considerable. Myocardial infarct size is a major determinant of prognosis in heart failure patients. Myocardial ischemia/reperfusion elicits various types of cardiomyocyte death and coronary microvascular damage [17]. Therefore, cardioprotective strategies aimed at reducing infarct size that protect both cardiomyocytes and microvessels are much needed [2][3][10][14]. Here, the addition of nanocarriers charged with oxygen prior to hypoxia was protective for both cardiomyocyte-like (H9c2) and endothelial (HMEC) cell lines. When given after hypoxia, they were protective for H9c2 only, but were not damaging to HMEC. Future studies should focus first on clinically relevant animal models [18], and then on patients in severe need of adjunct cardioprotection. Hopefully, these oxygen formulations may be included in the clinical armamentarium, either in programmed intervention (programmed revascularization), or in acute ischemia/reperfusion (elective angioplasty in AMI), as they can provide protection either before or after an ischemic event. Importantly, they might protect both cardiomyocytes and microvessels, therefore boosting the potential for cardioprotection.
The release of oxygen has been the focus of extensive research, in particular for the reduction of cell death in hypoxic tissues [15,19,20,21,22,23]. The advantage of CNN is that the reduced size of the cavity allows it to host small molecules as gases. Recently, CNN in the form of a cross-linked polymer has been used, with excellent results, in the preparation of a new formulation of doxorubicin [16], a widely used anticancer drug.
The release of oxygen has been the focus of extensive research, in particular for the reduction of cell death in hypoxic tissues [15][19][20][21][22][23]. The advantage of CNN is that the reduced size of the cavity allows it to host small molecules as gases. Recently, CNN in the form of a cross-linked polymer has been used, with excellent results, in the preparation of a new formulation of doxorubicin [16], a widely used anticancer drug.
The absence of oxygen, and its subsequent abrupt re-introduction, are considered the main mechanisms of ischemia/reperfusion injury. On one hand, the addition of this formulation—which releases oxygen slowly—before ischemia may represent a sort of pre-conditioning that triggers protection and builds a reservoir of oxygen that can support cell vitality during the absence of blood flow. On the other hand, the administration of this formulation immediately after ischemia may represent a sort of post-conditioning, protecting from reperfusion injury. The slow release of oxygen may limit reperfusion injury. Of note, while in pre-treatment both the 10 and 20 μg/mL concentrations were protective in both cell lines, only the higher concentration was protective in post-treatment, and in H9c2 only. Although we do not know the reasons for these differences, they open the possibility that endothelial cells are more sensitive to oxidative stress, and that different concentrations of O
2
-CNN formulation may be used in different clinical conditions. These results are interesting for the future use of CNN in clinical settings. The controlled release of oxygen could induce the activation of protective cascades, similar to conditioning, thus explaining their protective activity.
The ability to release oxygen would find numerous applications in the clinical setting, as well as at the cardiovascular level. One possible area could be the use of these oxygen carrier molecules as substitutes for hemoglobin. It is likely that, in patients with coronary disease, blood transfusion may in certain circumstances do more harm than good, even in anemic patients [24]. Therefore, substitutes for hemoglobin that overcome many of the problems associated with transfusion are much needed. Importantly, besides oxygen, the CNN may also deliver nitric oxide (NO), overcoming the putative impairment of NO effectiveness attributed to the transfused hemoglobin as well.
In conclusion, we have demonstrated here that oxygen-charged CNN has the potential to be beneficial in the context of hypoxia/re-oxygenation in vitro, limiting the cell damage in different cell lines and in a timing-specific manner. Whether CNN has beneficial effects in the context of ischemia/reperfusion in vivo remains to be ascertained. Future studies will determine the context in which these nanotools can be better exploited.
References
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, E837.
- Pagliaro, P.; Penna, C. Redox signaling and cardioprotection: Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 1974–1995.
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209.
- Pagliaro, P.; Moro, F.; Tullio, F.; Perrelli, M.G.; Penna, C. Cardioprotective pathways during reperfusion: Focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal. 2011, 14, 833–850.
- Tullio, F.; Angotti, C.; Perrelli, M.G.; Penna, C.; Pagliaro, P. Redox balance, and cardioprotection. Basic Res. Cardiol. 2013, 108, 392.
- Evans, C.W.; Iyer, K.S.; Hool, L.C. The potential for nanotechnology to improve delivery of therapy to the acute ischemic heart. Nanomedicine 2016, 11, 817–832.
- Penna, C.; Rastaldo, R.; Mancardi, D.; Raimondo, S.; Cappello, S.; Gattullo, D.; Losano, G.; Pagliaro, P. Post–conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP–sensitive K+ channel and protein kinase C activation. Basic Res. Cardiol. 2006, 101, 180–189.
- Tsutsumi, Y.M.; Yokoyama, T.; Horikawa, Y.; Roth, D.M.; Patel, H.H. Reactive oxygen species trigger ischemic and pharmacological postconditioning: In vivo and in vitro characterization. Life Sci. 2007, 81, 1223–1227.
- Vinten-Johansen, J.; Granfeldt, A.; Mykytenko, J.; Undyala, V.V.; Dong, Y.; Przyklenk, K. The multidimensional physiological responses to postconditioning. Antioxid. Redox Signal. 2011, 14, 791–810.
- Martins-Marques, T.; Hausenloy, D.J.; Sluijter, J.P.G.; Leybaert, L.; Girao, H. Intercellular Communication in the Heart: Therapeutic Opportunities for Cardiac Ischemia. Trends Mol. Med. 2020, 27, 248–262.
- Serviddio, G.; Bellanti, F.; Sastre, J.; Vendemiale, G.; Altomare, E. Targeting mitochondria: A new promising approach for the treatment of liver diseases. Curr. Med. Chem. 2010, 17, 2325–2337.
- Lakshmanan, R.; Ukani, G.; Rishi, M.T.; Maulik, N. Trimodal rescue of hind limb ischemia with growth factors, cells, and nanocarriers: Fundamentals to clinical trials. Can. J. Physiol. Pharmacol. 2017, 95, 1125–1140.
- Bar, A.; Cohen, S. Inducing Endogenous Cardiac Regeneration: Can Biomaterials Connect the Dots? Front. Bioeng. Biotechnol. 2020, 8, 126.
- Davidson, S.M.; Andreadou, I.; Barile, L.; Birnbaum, Y.; Cabrera-Fuentes, H.A.; Cohen, M.V.; Downey, J.M.; Girao, H.; Pagliaro, P.; Penna, C.; et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc. Res. 2019, 115, 1156–1166.
- Femminò, S.; Penna, C.; Bessone, F.; Caldera, F.; Dhakar, N.; Cau, D.; Pagliaro, P.; Cavalli, R.; Trotta, F. α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers 2018, 10, 211.
- Caldera, F.; Argenziano, M.; Trotta, F.; Dianzani, C.; Gigliotti, L.; Tannous, M.; Pastero, L.; Aquilano, D.; Nishimoto, T.; Higashiyama, T.; et al. Cyclic nigerosyl-1,6-nigerose-based nanosponges: An innovative pH and time-controlled nanocarrier for improving cancer treatment. Carbohydr. Polym. 2018, 194, 111–121.
- Sezer, M.; van Royen, N.; Umman, B.; Bugra, Z.; Bulluck, H.; Hausenloy, D.J.; Umman, S. Coronary Microvascular Injury in Reperfused Acute Myocardial Infarction: A View from an Integrative Perspective. J. Am. Heart Assoc. 2018, 7, e009949.
- Bøtker, H.E.; Hausenloy, D.; Andreadou, I.; Antonucci, S.; Boengler, K.; Davidson, S.M.; Deshwal, S.; Devaux, Y.; Di Lisa, F.; Di Sante, M.; et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 2018, 113, 39.
- Cavalli, R.; Akhter, A.K.; Bisazza, A.; Giustetto, P.; Trotta, F.; Vavia, P. Nanosponge formulations as oxygen delivery systems. Int. J. Pharm. 2010, 402, 254–257.
- Cavalli, R.; Bisazza, A.; Giustetto, P.; Civra, A.; Lembo, D.; Trotta, G.; Guiot, C.; Trotta, M. Preparation and characterization of dextran nanobubbles for oxygen delivery. Int. J. Pharm. 2009, 381, 160–165.
- Cavalli, R.; Bisazza, A.; Rolfo, A.; Balbis, S.; Madonnaripa, D.; Caniggia, I.; Guiot, C. Ultrasound-mediated oxygen delivery from chitosan nanobubbles. Int. J. Pharm. 2009, 378, 215–217.
- Magnetto, C.; Prato, M.; Khadjavi, A.; Giribaldi, G.; Fenoglio, I.; Jose, J.; Gulino, G.R.; Cavallo, F.; Quaglino, E.; Benintende, E.; et al. Ultrasound-activated decafluoropentane-cored and chitosan-shelled nanodroplets for oxygen delivery to hypoxic cutaneous tissues. RCS Adv. 2014, 4, 38433–38441.
- Prato, M.; Magnetto, C.; Jose, J.; Khadjavi, A.; Cavallo, F.; Quaglino, E.; Panariti, A.; Rivolta, I.; Benintende, E.; Varetto, G.; et al. 2H, 3H-decafluoropentane-based nanodroplets: New perspectives for oxygen delivery to hypoxic cutaneous tissues. PLoS ONE 2015, 10, e0119769.
- Doyle, B. There will be blood. JACC Cardiovasc. Interv. 2009, 2, 54–55.
