On average, there are 3–5 million severe cases of influenza virus infections globally each year. Seasonal influenza vaccines provide limited protection against divergent influenza strains. Therefore, the development of a universal influenza vaccine is a top priority for the NIH. Here, we report a comprehensive summary of all universal influenza vaccines that were tested in clinical trials during the 2010–2019 decade. Of the 1597 studies found, 69 eligible clinical trials, which investigated 27 vaccines, were included in this review. Information from each trial was compiled for vaccine target, vaccine platform, adjuvant inclusion, clinical trial phase, and results. As we look forward, there are currently three vaccines in phase III clinical trials which could provide significant improvement over seasonal influenza vaccines. This systematic review of universal influenza vaccine clinical trials during the 2010–2019 decade provides an update on the progress towards an improved influenza vaccine.
- Universal Influenza Vaccine
- Clinical Trials
- Influenza
- Influenza Vaccine
- Vaccine Target
Definition
On average, there are 3–5 million severe cases of influenza virus infections globally each year. Seasonal influenza vaccines provide limited protection against divergent influenza strains. Therefore, the development of a universal influenza vaccine is a top priority for the NIH.
1. Introduction
Globally, seasonal influenza virus epidemics are estimated to cause 3–5 million cases of severe infection and result in 290,000–650,000 deaths annually [1,2][1][2]. Mortality is increased in the elderly over 65 years, children under 5 years, and people in developing countries [3,4][3][4]. In the United States alone, influenza virus infects between 9.2–35.6 million people each year, leading to 140,000–710,000 hospitalizations [5]. These annual influenza epidemics result in an estimated total economic loss of $87.1 billion each year due to direct medical costs and indirect costs such as projected lost earnings and loss of life [6]. While the disease burden for seasonal influenza epidemics is substantial, this is significantly increased during influenza pandemics. For example, it is estimated that 24% of the worldwide population was infected during the 2009 H1N1 swine influenza pandemic [7].
A substantial challenge in the development of an effective influenza vaccine is the significant viral population diversity. The current influenza vaccine can be either trivalent or quadrivalent. The trivalent vaccine contains a H1N1, H3N2, and an influenza B strain, with the quadrivalent vaccine including both Yamagata and Victoria influenza B lineage strains [2,8][2][8]. The strains contained in the seasonal influenza vaccine are updated yearly to include those predicted to circulate in the upcoming influenza season. Although the current influenza vaccine is effective at reducing morbidity and mortality due to seasonal influenza infections [9], vaccine effectiveness estimates only range from 10 to 60% [8,10][8][10]. The vaccine effectiveness is lowest when there is poor antigenic match to the circulating influenza strains [8,11][8][11].
Developing a universal influenza vaccine (UIV) that improves cross-protection is a high priority. In 2018, the National Institute of Allergy and Infectious Disease (NIAID) released a strategic plan for the development of a UIV. This plan suggested that the vaccine should (1) be at least 75% effective against symptomatic influenza virus infection, (2) protect against group I and II influenza A viruses, (3) have durable protection that lasts at least 1 year, and (4) be suitable for all age groups [1]. Many strategies have been explored towards the creation of a UIV.
2. Results
2.1. Vaccine Targets
Influenza vaccines typically target specific viral antigens to maximize the immune response to vaccination. Vaccination aims to induce a strong adaptive immune response which results in both T and B cell activation. These immune cells produce cytotoxic T cells and antibodies which can protect against future infection. Vaccines targeting internal viral proteins, such as nucleoprotein (NP) and matrix 1 (M1), can induce strong T cell responses [122][12]. Viral surface (external) antigens, hemagglutinin (HA) and neuraminidase (NA), are targeted by neutralizing antibodies [123][13]. Traditionally, a robust antibody response has been the goal of influenza vaccination and has been the basis upon which vaccines have been tested and licensed [124,125][14][15]. However, these antibodies provide limited protection against divergent influenza strains. Since there are strengths for both internal and external strategies, many vaccines include multiple antigens to induce a strong humoral and cellular immune response. Over the past decade, both internal and external influenza proteins were utilized in UIV clinical trials (Figure 1). Other strategies which target whole virus or attenuated virus through gene deletion have also been investigated. However, recent vaccines have focused on external proteins, specifically HA.
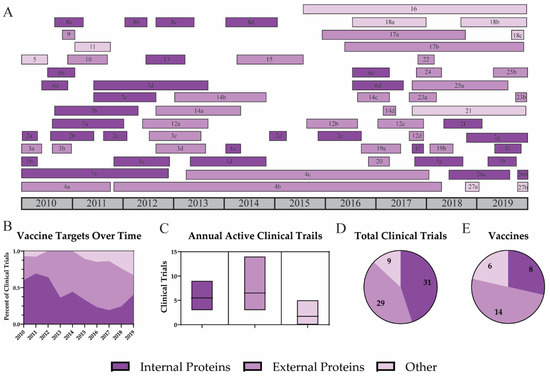
Figure 1. Targets for universal influenza vaccine strategies. A timeline of all universal influenza vaccine clinical trials is shown for vaccines which target internal proteins (dark purple), external proteins (purple), or other (light purple) antigens (A). Trends for vaccine targets are shown by the percent of active clinical trials each year (B). Average clinical trials for each target (C), the total number of clinical trials (D), and the number of vaccines directed against each target are reported (E).
2.2. Vaccine Platforms
Antigens are presented to the immune system in different ways depending on the vaccine platform. Most seasonal influenza vaccines utilize attenuated or inactivated wild-type viruses. These viruses display the external influenza proteins and stimulate strong antibody responses [127][16]. Although this strategy has been utilized since 1945, it has consistently shown low efficacy for protection against mismatched influenza strains [10,128][10][17]. Therefore, a variety of vaccine platforms were investigated during the last decade to further improve influenza vaccination. Although many vaccine platforms have been investigated, no single platform has thus far been demonstrated to show superior protection against influenza.
2.3. Adjuvants
An ideal UIV will provide highly effective and long-lasting protection. This can be difficult to achieve when targeting internal proteins or using poorly immunogenic vaccine platforms. Adjuvants are compounds that stimulate the immune system and improve vaccine efficacy [132][18]. This is commonly achieved by oil-in-water emulsions, which recruit immune cells to the site of vaccination [134][19]. Another common group of adjuvants are toll-like receptor (TLR) agonists. These adjuvants bind and activate cellular host pathways, which leads to increased immune activation [135][20]. New adjuvants continue to be discovered and explored, but few are licensed for use in the United States [136][21].
2.4. Clinical Trial Phases
In the US, new drugs and vaccines must complete four phases of clinical trials to be licensed and marketed for public use. Phase I trials investigate the safety and dosage of the vaccine. Typically, phase I trials have limited numbers of participants and do not assess efficacy due to low statistical power [139][22]. Phase II trials assess the dose response, efficacy, and side effects of the new vaccine. These trials include more study participants and can last longer than phase I trials. Occasionally, phases I and II can be combined into one clinical trial, phase I/II. Phase III trials include a large sample size and assess participants for vaccine efficacy and adverse reactions. At this point, the new vaccine or drug may be approved for the market [139][22]. Lastly, phase IV clinical trials involve post-marketing surveillance of the efficacy and safety of the new vaccine. Importantly, not all clinical trial results are reported or published. It is common for results to be posted several years after the completion of a trial (Figure 2). Over the past decade, only half of completed trials reported their findings (Figure 2E). This delay is consistent regardless of clinical trial phase (Figure 2D).
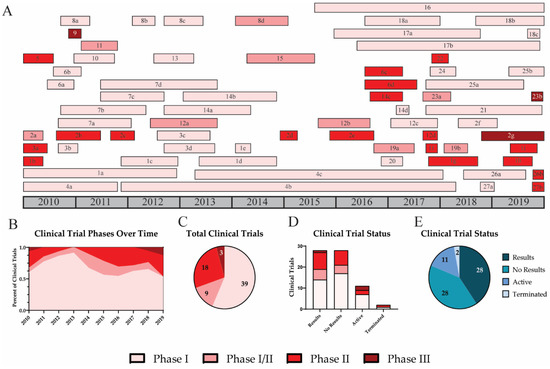
Figure 2. Clinical trial phases and results for universal influenza vaccines. A timeline for universal influenza vaccine clinical trials is shown for phase I (light pink), I/II (pink), II (red), and III (dark red) (A). Trends for vaccines in various clinical trial phases are shown by the percent of active clinical trials each year (B). The number of clinical trials in each phase is shown (C). Result status for trials in each clinical phase is shown (D). The total number of trials completed with results (dark blue), completed with no results (blue), active (grey blue), or terminated (light blue) is indicated (E).
As expected, most UIV clinical trials performed over the past decade were phase I trials (57.4%) (Figure 2). Of the 27 vaccines, 11 have progressed past phase I (40.7%); however, only 3 vaccines (11%) have been tested in phase III clinical trials. The first phase III trial investigated Inflexal V, a trivalent adjuvanted virus-like particle (VLP) vaccine [70][23]. This study included 205 children between 6 and 36 months and was completed in November 2010 [69][24]. All participants were immunized with a single full dose (0.5 mL) or with two doses (0.25 mL) of the Inflexal V vaccine. Results suggest that both vaccine groups demonstrated improved seroprotection and seroconversion rates. Participants who received two 0.25 mL doses 4 weeks apart showed higher seroprotection rates for H1N1 (99.0), H3N2 (99.0), and influenza B (92.2). For H1N1 and H3N2, the two-dose regimen resulted in higher seroconversion and geometric mean titer (GMT) fold increases than the single-shot regimen. Half of participants from each group experienced non-serious adverse events including pyrexia, malaise, rhinitis, cough, otitis media acute, as well as adverse events at the injection site including erythema, induration, pain, or hemorrhage.
The second UIV tested in a phase III clinical trial was M-001. This vaccine is a synthetic recombinant protein containing common linear influenza epitopes [31][25]. As discussed above, the adjuvanted M-001 vaccine has shown promising immunogenicity and the phase III trial was scheduled for primary completion in May 2020 [31,137][25][26].
The third vaccine tested in a phase III clinical trial is NanoFlu. This vaccine is a recombinant HA protein delivered in a nanoparticle with a saponin-based Matrix-M adjuvant [107][27]. Although results for the phase II trial have not been posted, a press release from Novavax stated that NanoFlu induced superior HAI antibody responses against homologous and drifted strains compared to the seasonal influenza vaccine. A phase III clinical trial involving 2650 participants over 65 years of age was scheduled for primary completion in December 2019.
3. Discussion
This systematic review documents UIVs that were tested in clinical trials from January 2010 to December 2019. Although many papers have discussed strategies for UIVs, few review papers address the translation of UIV strategies to clinical trials [140,141][28][29]. This is the first systematic review of UIVs in clinical trials.
The definition of a “universal” influenza vaccine is highly debated [125,141][15][29]. In 2018, the NIAID announced that a UIV should (1) be at least 75% effective, (2) protect against group I and II IAV, (3) have durable protection that lasts at least 1 year, and (4) be suitable for all age groups [1]. Since this standard was put forward towards the end of the decade, our definition of a UIV remains broader than the NIAID requirements. Here, we have defined a UIV as a vaccine that aims to induce better cross-protection than seasonal influenza vaccines. Therefore, “supra-seasonal vaccines” which cover a large subset of influenza strains and vaccines against specific subtypes of influenza have been included in this analysis.
The influenza diversity targeted by each vaccine varied. Only 37% of universal vaccines were designed to protect against both influenza A and B viruses. Other strategies focused on IAV (22%) or a single subtype of IAV (41%). Importantly, no vaccines focused on influenza B virus (IBV) alone. Furthermore, the current NIAID requirements for a universal influenza vaccine do not require cross-protection against IBV. Notably, the CDC reports that IBV is responsible for 72% of influenza cases reported for children and young adults each year [142][30]. Overall, approximately 26% of annual influenza cases can be attributed to IBV [143][31]. The significant burden of IBV should be addressed in the design of universal influenza vaccines.
Some limitations to this review should be noted. First, information about clinical trials can be limited until the results are published. Specifically, not all clinical trial summaries include information on vaccine design and mechanism. In these cases, previous publications and press releases for the vaccines were consulted. Additionally, most results reported safety information and homologous vaccine efficacy, providing limited information on the cross-reactivity of each vaccine. Second, we searched clinical trials registered through ClinicalTrials.gov, which could potentially exclude some studies. There are other clinical trial databases such as EU Clinical Trials Register, however, the ClinicalTrials.gov database reports more accurate and updated information for clinical trials [144][32].
Despite limited information, this review provides a comprehensive summary of the UIVs tested in clinical trials. Indeed, this is the first comprehensive review to also discuss efficacy and trends in vaccine development for influenza. The field of influenza vaccine development is ever progressing. This is reflected in new vaccine targets and platforms such as HA stalk and nanoparticles. Researchers over the past decade have produced many promising influenza vaccines, each with strengths and limitations. The efficacy of a vaccine may induce strong protection against matched strains, but an effective UIV must induce strong cross-protection as well. This review identifies vaccines that report efficacy against matched strains alone. Importantly, these vaccines may provide cross-protection if delivered in combination with vaccines targeting other influenza subtypes. However, this would require further research and investigation.
4. Conclusions
Influenza virus remains a major global pathogen despite the general widespread use of seasonal vaccines due to varying efficacy to drifted strains. A UIV remains a top priority for the NIH and World Health Organization. This review provides an update on the progress towards a better influenza vaccine. With this information, researchers and clinicians can remain informed about the status and limitations of universal influenza vaccines. The search for a UIV has been a long process and scientists have dedicated their lives to this pursuit. Advances in technology and molecular biology have made this daunting tasks much more possible. However, as we have seen so many times, viruses with high levels of genetic diversity will require the production of an exceptional vaccine, which will most likely requirement constant refinement and improvement.
References
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354.
- WHO. Influenza (Seasonal). Available online: (accessed on 12 November 2018).
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300.
- Lafond, K.E.; Nair, H.; Rasooly, M.H.; Valente, F.; Booy, R.; Rahman, M.; Kitsutani, P.; Yu, H.; Guzman, G.; Coulibaly, D.; et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLoS Med. 2016, 13, e1001977.
- Rolfes, M.A.; Foppa, I.M.; Garg, S.; Flannery, B.; Brammer, L.; Singleton, J.A.; Burns, E.; Jernigan, D.; Olsen, S.J.; Bresee, J.; et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir. Viruses 2018, 12, 132–137.
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096.
- Van Kerkhove, M.D.; Hirve, S.; Koukounari, A.; Mounts, A.W. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: A meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respir. Viruses 2013, 7, 872–886.
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–2019 Influenza Season. Mmwr Recomm. Rep. 2018, 67, 1–20.
- Foppa, I.M.; Cheng, P.Y.; Reynolds, S.B.; Shay, D.K.; Carias, C.; Bresee, J.S.; Kim, I.K.; Gambhir, M.; Fry, A.M. Deaths averted by influenza vaccination in the U.S. during the seasons 2005/06 through 2013/14. Vaccine 2015, 33, 3003–3009.
- CDC. CDC Seasonal Flu Vaccine Effectiveness Studies. Available online: (accessed on 31 August 2020).
- Lewnard, J.A.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines 2018, 6, 28.
- Spitaels, J.; Roose, K.; Saelens, X. Influenza and Memory T Cells: How to Awake the Force. Vaccines 2016, 4, 33.
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.-L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320.
- Grant, E.; Wu, C.; Chan, K.F.; Eckle, S.; Bharadwaj, M.; Zou, Q.M.; Kedzierska, K.; Chen, W. Nucleoprotein of influenza A virus is a major target of immunodominant CD8+ T-cell responses. Immunol. Cell Biol. 2013, 91, 184–194.
- Krammer, F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol. J. 2015, 10, 690–701.
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin. Vaccine Immunol. CVI 2017, 24, 1.
- Barberis, I.; Myles, P.; Ault, S.K.; Bragazzi, N.L.; Martini, M. History and evolution of influenza control through vaccination: From the first monovalent vaccine to universal vaccines. J. Prev. Med. Hyg. 2016, 57, E115–E120.
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21.
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114.
- Gnjatic, S.; Sawhney, N.B.; Bhardwaj, N. Toll-like receptor agonists: Are they good adjuvants? Cancer J. 2010, 16, 382–391.
- CDC. Adjuvants Help Vaccines Work Better. Available online: (accessed on 13 January 2020).
- NIH. What Are Clinical Trials and Studies? Available online: (accessed on 13 January 2020).
- Mischler, R.; Metcalfe, I.C. Inflexal V a trivalent virosome subunit influenza vaccine: Production. Vaccine 2002, 20 (Suppl. S5), B17–B23.
- Crucell Holland BV. Immunogenicity and Safety of a Single 0.5 mL Dose of Inflexal V With a 0.25 mL 2-dose Regimen of Inflexal V. Available online: (accessed on 13 January 2020).
- Atsmon, J.; Caraco, Y.; Ziv-Sefer, S.; Shaikevich, D.; Abramov, E.; Volokhov, I.; Bruzil, S.; Haima, K.Y.; Gottlieb, T.; Ben-Yedidia, T. Priming by a novel universal influenza vaccine (Multimeric-001)-a gateway for improving immune response in the elderly population. Vaccine 2014, 32, 5816–5823.
- Atsmon, J.; Kate-Ilovitz, E.; Shaikevich, D.; Singer, Y.; Volokhov, I.; Haim, K.Y.; Ben-Yedidia, T. Safety and Immunogenicity of Multimeric-001—A Novel Universal Influenza Vaccine. J. Clin. Immunol. 2012, 32, 595–603.
- Novavax. Novavax Announces Positive Phase 2 NanoFlu Results in Older Adults. In Sets the Stage for Phase 3 Clinical Trial in 2019; Cohen, A., Ed.; Sam Brown Inc.: Wayne, PA, USA, 2019.
- Valkenburg, S.A.; Leung, N.H.L.; Bull, M.B.; Yan, L.M.; Li, A.P.Y.; Poon, L.L.M.; Cowling, B.J. The Hurdles From Bench to Bedside in the Realization and Implementation of a Universal Influenza Vaccine. Front. Immunol. 2018, 9, 1479.
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228.
- CDC. Weekly U.S. Influenza Surveillance Report. Available online: (accessed on 27 August 2020).
- Heikkinen, T.; Ikonen, N.; Ziegler, T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 1519–1524.
- Fleminger, J.; Goldacre, B. Prevalence of clinical trial status discrepancies: A cross-sectional study of 10,492 trials registered on both ClinicalTrials.gov and the European Union Clinical Trials Register. PLoS ONE 2018, 13, e0193088.
