Extracellular vesicles (EVs) are membranous structures, which are secreted by almost every cell type analyzed so far. In addition to their importance for cell-cell communication under physiological conditions, EVs are also released during pathogenesis and mechanistically contribute to this process. Here we summarize their functional relevance in asthma, one of the most common chronic non-communicable diseases. Asthma is a complex persistent inflammatory disorder of the airways characterized by reversible airflow obstruction and, from a long-term perspective, airway remodeling. Overall, mechanistic studies summarized here indicate the importance of different subtypes of EVs and their variable cargoes in the functioning of the pathways underlying asthma, and show some interesting potential for the development of future therapeutic interventions. Association studies in turn demonstrate a good diagnostic potential of EVs in asthma.
1. Introduction
Chronic non-communicable diseases (NCDs) are inflammatory conditions, which are not caused by infectious agents (e.g., bacteria, viruses, parasites). To name a few, these diseases include respiratory disorders such as asthma or chronic obstructive pulmonary disease (COPD), chronic inflammatory bowel diseases, cardiovascular disorders such as coronary artery disease/ischemic heart disease, peripheral vascular disease or stroke, all based on atherosclerosis, inflammatory disease conditions in the skin (e.g., atopic dermatitis, psoriasis), metabolic diseases such as obesity, metabolic syndrome, and diabetes, different forms of cancer, adverse mental outcomes, etc. The burden of NCDs is high in western countries and still rising, in particular in less developed areas. To effectively face this challenge, novel diagnostic and therapeutic approaches should be established based on the growing knowledge on pathobiological mechanisms underlying the development and the clinical course of NCDs.
This specifically applies also to asthma as one of the most prominent NCDs, for which, despite substantial progress, current diagnostic and therapeutic approaches remain suboptimal. One of the major reasons behind this is the heterogeneity of asthma, with a complex etiology and multiple clinical representations, requiring the development of stratified diagnosis and treatment strategies. These can only be achieved on the basis of novel cellular and molecular insights based on innovative methods.
2. Extracellular Vesicles and Asthma: Cellular Level
2.1. Airway Epithelial Cells and Fibroblasts
EVs aChr
eonic involved in asthma-related interactions between different cell types. Adnon-communicable diseases (NCDs) are inflammatory condition
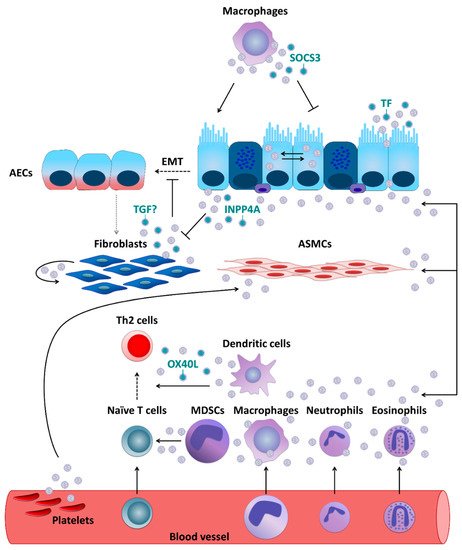
ally, for airway epithelial cells (AECs), the exchange of EV cargo seems to be an important way of communicating with each other, as well as with other cell types. For example, in primary human tracheobronchial cells and cultured Calu-3 cells, as, which are not caused by infectious agents (e.g., bacteria, viruses, parasites). To name a few, these diseases include respiratory
epithelial cell line, the reciprocal transfer of EV-associated proteins and microRNAs (miRNAs) was shown to be sufficient to qualitatively and quantitatively alter the profiles of airway secretions including miRNA cargo of EVs of the target cells and cause mucin hypersecretion. This mechanism may play an important role in epithelial remodeling and other pathologic processes in the airways involved in chronicdisorders such as asthma or chronic obstructive pulmonary disease (COPD), chronic inflammatory bowel diseases, cardiovascular disorders such as coronary artery disease/ischemic heart disease, peripheral vascular disease or stroke, all based on atherosclerosis, inflammatory dis
orders of the respiratory tract, such as asthma, cystic fibrosis, and bronchogenic carcinoma. In a mouse study, it was shown that the composition of the pool of extracellular miRNAs in the lung was very similar to that of the airway epithelium, with 80% of the EVs detected in bronchoalveolar lavage fluid (BALF) being of epithelial origin. However, the number of miRNAs selectively expressed by immune cells, including miR-223 and miR-142a, and hematopoietic cell-derived EVs increasedease conditions in the skin (e.g., atopic dermatitis, psoriasis), metabolic diseases such as obesity, metabolic syndrome, and diabetes, different forms of cancer, adverse mental outcomes, etc. Especially after the development of effective prevention (vaccines) and treatment (e.g., antibiotics) options against infectious diseases over the last decades, NCDs became the most significant
ly following the induction of allergic airway inflamma cause of death in the world. According to the World Health Organization (
AAI), showing the importance of alterationsWHO), in 2019 the three top causes of death in the
EV miRNA pool for the development of allergic inflammation. Another group reported that EV secretion and production of EV-associated proteins were both higher in the lungs of mice in which AAI was induced compared to theWorld were ischemic heart disease accounting for about 9 million, stroke for more than 6 million deaths, and COPD for more than 3 million deaths in this single year only [1]. For co
ntmpar
ol animals. These EVs, which were released during asthma/AAI by AECs under the influence of type-2 cytokines such as IL-13, triggison, as of mid-April 2021, the worldwide number of COVID-19-related deaths since the very beginning of the pandemic was approaching 3 million [2]. The
bur
ed the proliferation and chemotaxis of undifferentiated macrophagesden of NCDs is high in western countries and still rising, in particular in less developed areas [3,4,5,6].
NTo
t surprisingly, the use of GW4869, an inhibitor of exosome production, resulted in a reduction in the population of proliferating monocytes in the AAI mouse model effectively face this challenge, novel diagnostic and therapeutic approaches should be established based on the growing knowledge on pathobiological mechanisms underlying the development and the
alleviation of varclinical course of NCDs.
Thious asthmatic features.
Addspecifi
tioncally
, primary human fibroblasts were demonstrated to secrete exosomes, which undergo subsequent internalization by normal human bronchial epithelial cells (NHBECs). Moreover, compared to healthy controls, exosomes derived from fibroblasts which were obtained from severe asthmatics showed lower levels of transforming growth factor beta 2 (TGF-β2) and significantly increased the proliferation of NHBECs. These results are intriguing, given that TGF-β is considered to be a major driver of abnormal epithelial-mesenchymal transition (EMT) applies also to asthma as one of the most prominent NCDs, for which, despite substantial progress, current diagnostic and therapeutic approaches remain suboptimal. One of the major reasons behind this is the heterogeneity of asthma, with a complex etiology and multiple clinical representations, requiring the development of stratified diagnosis and treatment strategies [7,8,9,10].
During EMT
, epithelial cells demonstrate enhanced motility and invasive capacity through the downregulation of epithelial markers and higher expression of mesenchymal proteins, being this way a source of migrating myofibroblasts and fibroblasts. In turn, these cells promotehese can only be achieved on the basis of novel cellular and molecular insights based on innovative methods. In this review, we summarize the current knowledge on extracellular
matrix deposition and subepithelial fibrosis, which strongly contributes to the establivesicle (EV)-mediated cell-cell communication obtained in the context of pathobiology and clinical pathology of asthma.
2. Asthma
Asthment of a persistent asthma phenotype. Moreover, fibroblasts themselves can also be recipients of EVs. In vitro experiments using cell lines demonstrated that AECs were able to secrete enzymatically active inositol polyphosphate 4-phosphatase type I A (INPP4A) in EVs and as a soluble free form. INPP4A was then transferred to lung fibroblasts, and inhibition of such transfer resulted in increased fibroblast proliferation. Moreover, in mice with or without AAI neutralization of extracellular INPP4A-induced AHR, with prominenta is a chronic inflammatory disease of the airways characterized by recurrent symptoms of varying intensity and severity, including wheezing, shortness of breath, cough, feeling of tightness in the chest, and others. The symptoms of asthma are underlain by reversible airway obstruction resulting from easily triggered bronchospasm and enhanced mucus secretion. In a longer perspective, disease progression is associated with, or rather results from, airway remodeling, subepithelial fibroblast proliferation, and collagen de including changes in structural cell composition.
Figure 1. Extracellular vesicle- (EV-) mediated communication between cells crucial for asthma pathobiology. If not otherwise stated, EVs are thought to carry their usual content such as microRNAs, proteins, lipids, etc.
2.2. Antigen-Presenting Cells (APCs)
APCs, such a
s dendritic cells (DCs), macrophagesnd extracellular fibrosis [11,
mo12,13,14].
Clin
oic
ytes, and others can communicate through EVsally, asthma is a very heterogeneous disorder with
other types of cells involved in asthma development. A study performed in primary human macrophages and DCs demonstrated that they can secrete exosomes which contain enzymes for leukotriene biosynthesis and thus contribute to chronic inflammation, for example through granulocyte recruitment. Primary human DCs activconsiderable differences in the symptomatology, factors triggering exacerbations, severity, time of onset, demographics, body weight, and other features. Characteristic clinical representations of asthma form so-called phenotypes that are associated with
TSLP, an epithelial cell-derived cytokine, release exosomes expressing OX40 ligand (OX40L)a variety of distinct pathomechanisms named endotypes. Several endotypes have been proposed, which
was able to promote proliferation and differentiation of CD4+can be roughly grouped into those related to T Thelper cell
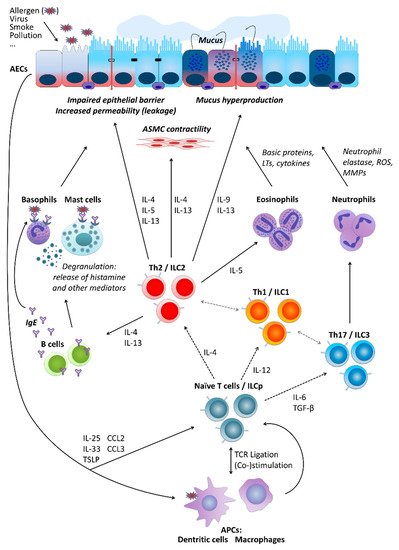
s towards a Th2 phenotype. Resident alveolar macrophages were, in turn, demonstrated to dampen inflammatory signaling in AECs and thus AAI in a mouse model through transcellular delivery of suppressor of cytokine signaling 3 (SOCS3) within EVs. Air pollutants such as particulate matter are well-known contributors to the pathogenesis of chronic inflammatory airway diseases including asthma. In vitro exposure to particulate matter stimulated human m type-2 (Th2) and those related to non-Th2 (e.g., Th1/Th17) immune mechanisms. Since it became evident that Th cytokines can be secreted also by other cell types, e.g., innate lymphoid cells (ILCs), asthma forms are divided into those of a type-2 (mostly allergic) and those of a non-type-2 character, respectively. However, even this paradigm may not cover all possible mechanisms underlying different forms of asthma [8,9,13,15,16].
Pacrophages to release more EVs in a dose-dtly independent manner. Moreover, those EVs were able to induce secretion of pro-inflammatory cytokines, such as IL-6 and tumor necrosis factor-α (TNF-α), by pulmonarly of the pathomechanism behind it, several types of cells are crucially involved in asthma pathogenesis. These include airway epithelial cells.
2.3. Granulocytes and Mast Cells
Likewi (AECse,) human eosinophils were found to be able to secrete exosomes, the production of which was higher by cells deriving from asthmatics. In addition, exosomes secreted by the eosinophils of patients withforming together with local macrophages the first point of contact for external influences entering the airways, for instance, allergens (type-2/atopic forms of asthma) or cigarette smoke (neutrophilic asthma could, in an autocrine manner, modify several specific eosinophil functions related to asthma pathogenesis including an increase in reactive oxygen species and nitric oxide synthesis and an augmentation of eosinophil migration and adhesion, suggesting that they could fundamentally contribute to the development and maintenance of asthma. Further, asthmatic eosinophil-derived exosomes could enhance the apoptosis of primary AECs and delay the repair of established epithelial damage, as well as increase the proliferbelonging to non-type-2 disease forms). Cytokines secreted by AECs (e.g., thymic stromal lymphopoietin, TSLP; interleukin-25, IL-25; and IL-33) in response to stimulation influence of downstream cells including, among others, antigen-presenting cells (APCs) and T cells. Depending on the type of stimulation, T cells differentiate towards Th2 cells secreting cytokines driving allergic forms of the disease or Th17 and Th1 cells contributing to non-type-2 asthma endotypes. As type-2 cytokines, IL-4 triggers the differentiation of primary bronchial smooth muscle cefurther Th2 cells and perpetuate airway inflammation status . Upon in vitro stimulation with LPS, horse neutrophil-derived exosomes, carrying proteins associated with immune response and positive regulation of cell communication, were rapidly internalized by equine the production of immunoglobulin E (IgE) by B cells, IL-13 activates mast cells and basophils as well as stimulates airway smooth muscle (ASM) cells and enhanced their proliferative capabilities. The effects of neutrophil-derived exosomes on ASM proliferation [might play an important role in the neutrophil-mediated progression of asthma and promotion of airway remodeling in severe and corticosteroid-insensitive patients with asthma. Based on in vitro data obtained using human contractility and thus airway hyper-responsiveness (AHR) and hyperplasia of goblet cells and mucus production, IL-5 activates eosinophils, IL-9 further contributes to increased mucus production and enhanced proliferation of mast cells, exosomes were also suggested to partially contribute to mast cell-mediated pro-. Mediators secreted by mast cells and basophils result in allergic inflammatory modulation of ASM cells, although it was undisputed that soluble, extra-exosomal factors such as IL-8 were critthe respiratory tract accompanied by a respective clinical picture. IL-17 released by Th17 cells, in turn, stimulates neutrophil activation, which leads to severe endothelial injury typical for the effect.
2.4. Platelets
Monon-type-2 neutr
eo
ver, platphilic asthma () [9,14,17,18,19].
Figure 1. Basic cellular mechanisms underlying type-2 and non-type-2 asthma. For a more detailed description, please refer to the main text, Section 2. “Asthma”. AECs, airway epithelial cells; ASMC, airway smooth muscle cells; LTs, leukotrienes; ROS, reactive oxygen species; MMPs, matrix metalloproteinases; IL, interleukin; ILC, innate lymphoid cells; ILCp, ILC precursors; Th (cells), T helper (cells); IgE, immunoglobulin E; TGF-β, transforming growth factor beta; TSLP, thymic stromal lymphopoietin; CCL, C-C motif chemokine ligand; TCR, T cell receptor; APCs, antigen-presenting cells.
3. Extracellular Vesicles
A kely fets, which are known toature that has significantly contributed to the pathophysiology of asthma as well, can exert their effects through EVs. Plasma EVs, a substantial portion of which is of platelet origin, isolated from asthmatics were found to be able to reduce the endothelium-dependent relaxation in response to bradykinin and increase the acetylcholine-induced contraction of the mouse trachea, which is suggestive of their potential role in ASM dysfunction typical for asthma. Moreover, the levels of circulating platelet microevolution of multicellular organisms and especially higher levels of complexity is represented by the ability of intercellular communication such as transfer of soluble molecules between cells and/or direct cell-cell contact. After the discovery that cells release so-called apoptotic bodies during programmed cell death, it was shown already in the mid-60s that physiologically active cells also release extracellular particles (PMPs) were reported to be increased in asthmatics.
3. Conclusions and Perspectives
Curr, at that time referred to as the
ntly available studies, whatever their nature, iso-called “platelet dust” [20].
Howe
., in vitro, in vivo on animals, in human studies, etc., clearly demonstrate the existence of EV communication between cells known as the crucial players in asthma pathology and, moreover, they also strongly suggest an importance of EV-mediated communication mechanisms for the pathobiology of the disease. This includes the mediation of etiopathogenic effects of environmental factors, e.g., microbver, within the last two decades, EVs have turned out to be more prominent and functionally important than initially expected and emerged as an interesting and promising research field. Virtually all cell types analyzed so far release EVs, which can roughly be classified into two major groups: endosomal derived exosomes and microvesicles (MVs; also referred to as microparticles or
pollutants, and the role of EVsectosomes), the latter directly budding from
external originsthe plasma membrane [21,22,23,24,
e25].
g., those present in cow’s milk or secreted by certain bacteria. Some of the studies already characterized components of the cargo of EVs and identified molecules responsible for asthma-related effects, mainly small RNAs, Although exosomes and MVs show differences, such as their biogenesis and release pathways, they also share many bio-physicochemical properties, including size range, density as well as certain surface proteins
, and lipid mediators. Based on this continuously expanding knowledge, the high diagnostic potential (for a summary of the differential characteristics of EVs
has beensee ) [26,27,28,29,30,31,32,33]. Th
ighlighted in a variety of studies. It has been shown that EV-based asthma diagnostics effectively targeting miRNA, proteins, or lipids could be performed in different types of biomaterials such as bronchoalveolar lavage fluid (BALF), sputum, nasal lavage fluid (NLF)ese features barely allow distinguishing between the individual subpopulations in detail. Instead of referring to the individual subpopulations, the term EV should therefore be preferred in the nomenclature, which encompasses vesicles released by cells in their entirety [34],
ho
r serum. Considering the access to biomaterials and the methodological rationale, the analytical approach based owever, in the current review we will retain the terminology used in the
analysis of serum miRNAs seems particularly promising, irrespective of whether and how the miRNAome patteroriginal publications to which we refer.
Table 1. Basic characteristics of extracellular vesicles of different types [26,27,31,32,33].
| |
Exosomes |
Microvesicles |
Apoptotic Bodies |
| Alternative nomenclature |
- |
Microparticles, ectosomes |
- |
| Size |
10–150 nm |
100–1000 nm |
800–5000 nm |
| Origin |
Intraluminal vesicles within multivesicular bodies |
Plasma membrane and cellular content |
Plasma membrane, fragmented cell |
| Formation mechanism |
Fusion of multivesicular bodies with plasma membrane |
Outward blebbing of plasma membrane |
Shrinkage and programmed death of the cell |
| Release |
Constitutive and/or cell activation |
Constitutive and/or cell activation |
Apoptosis |
| Time of release |
≥10 min |
<1 s |
- |
| Composition |
Protein, lipids, coding RNA, noncoding RNA, DNA |
Protein, lipids, cell organelles, coding RNA, noncoding RNA, DNA |
Cell organelles, proteins, nuclear fractions, coding RNA, noncoding RNA, DNA |
| Enriched protein markers |
CD81, CD63, Alix, Tsg101 |
Selectins, integrin, CD40 |
Caspase 3, histones |
Sin
ce EVs
in blood and lung correspond to each other. These approaches will certainly be expanded in the future to more precisely identify asthma phenotypes particularly by means of non- or minimally-invasive diagnostic sampling techniques. Finally, several in vitro and animal studies reviewed in this article already show that EVs, not only, but especially those secreted by MSCs, can exert beneficial immunomodulatory effects with anti-asthmatic capacity, suggesting a promising potential for EV-based therapeutic approaches. Although procedures regarding targeting specific cell types and the level of EV (cargo) degradation still need to be further optimized, it seems that modified or designed EVs with a higher propensity to fuse with (endosomal) membranes will offer even betterplay an important role in cell-cell communication, they are not simply empty lipid bins but rather contain various biomolecules such as diverse RNA types, proteins, lipids and metabolites by which they have the potential to regulate the function of recipient cells. With respect to EV-mediated signaling, non-coding RNAs were studied in depth during the last decade. In particular, the role of microRNAs (miRNAs) turned into the focus of research, due to their well-established role in the regulation of gene expression [35,36,37]. Int
herapeutic abilities. Another approach being developed as a possible anti-asthmatic therapeutic strategy may involve the use of inhibitors of EV production, which have been shown to exhibit anti-AAI effects in some studies. However, further basic and clinical studies are needed, which undoubtedly will lead to diagnostic and therapeutic innovations basederestingly, the way in which EVs avoid degradation while entering the cell compartment by endocytosis and subsequent cargo release via membrane fusion suggests that EVs exploit mechanisms similar to those observed in certain viral infections, such as endosomal acidification [38,39].
In olin their results.
Te with this
, entry is based on Alashkar Alhamwe et al. Extracellular Vesicles and Asthma—More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22(9), 4984; https://doi.org/10.3390/ijms22094984 (https://www.mdpi.com/1422-0067/22/9/4984/htm). For the full contentrecent studies also implicated EVs in the progression of human disease, including
the supporting references, please, refer to the full version of the articlecancer and infectious diseases (for a summary see [40,41]).