The oral cavity and gut are the two largest microbial ecosystems. The oral-to-gut and gut-to-oral microbial transmission can regulate pathogenesis, indicating the presence of the oral–gut microbiome axis.
- oral microbiome
- gut microbiome
- oral–gut microbiome axis
- GI disease
- GI cancer
1. Introduction
Gut and oral microbiomes are the two largest microbial ecosystems in the human body [9][1]. Based on the human microbiome project (HMP), among the 15 different body habitats, oral and fecal microbiomes are ecologically rich and taxonomically diverse [3][2]. It is noteworthy that the oral cavity and gut are linked physically as well as chemically. However, most of the research on the oral and gut microbiomes has been conducted separately in an organ-specific manner, rather than in an integrative context. The latest studies have proven the involvement of microbiome in the interorgan networks, such as the gut–brain and gut–lung axes [10,11][3][4]. In this regard, the intestinal colonization of oral microbiota and fecal–oral transmission have been reported to frequently occur and modulate pathophysiological processes in the human body [12,13,14][5][6][7].
2. Oral and Gut Microbiomes: Connection and Segregation
2.1. Oral Cavity and Gut: Connected through GI Tract
The human digestive system consists of the GI tract and the accessory digestive organs, including liver and pancreas. The GI tract is well-lined by the mucous membrane, beginning at the mouth and ending at the gut—more precisely, the anus. Thus, the oral cavity and gut are anatomically continuous regions connected through the GI tract
2.2. Oral Microbiome Composition
According to the human oral microbiome database (HOMD), the oral cavity presents approximately 700 species of microorganisms (from the HOMD website;
; accessed on 20 January 2021).
2.3. Gut Microbiome Composition
The gut is the largest and the most well-characterized microbial ecosystem in the human body, which harbors about 500 to 1000 species in more than 50 different phyla [24]. The gut microbiota, mostly anaerobes, is composed of five major phyla—
The gut is the largest and the most well-characterized microbial ecosystem in the human body, which harbors about 500 to 1000 species in more than 50 different phyla [8]. The gut microbiota, mostly anaerobes, is composed of five major phyla—
Bacteroidetes
,
Firmicutes
,
Actinobacteria
,
Proteobacteria
, and
Verrucomicrobia
—but dominated by two phyla—
Bacteroidetes
and
Firmicutes, which account for more than 90% [25]. At the genus level,
, which account for more than 90% [9]. At the genus level,
Bacteroides is the most abundant [26]. The human gut microbiota is known to be established early in life and can then be changed by age and environments, such as diet and nutrition, similar to the human oral microbiome [27,28]. Thus, both oral and gut microbiomes directly reflect the health status of the host.
is the most abundant [10]. The human gut microbiota is known to be established early in life and can then be changed by age and environments, such as diet and nutrition, similar to the human oral microbiome [11][12]. Thus, both oral and gut microbiomes directly reflect the health status of the host.
2.4. Physiological Functions of Gut Microbiome
It is evident that the gut microbiota plays a crucial role in maintaining physiological homeostasis, primarily metabolism and immunity.
2.5. Physiological Functions of Oral Microbiome
Although the oral cavity is the second largest microbial habitat in the human body, the cumulative knowledge is not sufficient to fully understand the implications of oral microbiome in the human health. It is unquestionable that the oral microbiome is directly associated with dental health [23,47]. The oral microbiome can affect systemic health conditions, not limited to the dental health (see
Although the oral cavity is the second largest microbial habitat in the human body, the cumulative knowledge is not sufficient to fully understand the implications of oral microbiome in the human health. It is unquestionable that the oral microbiome is directly associated with dental health [13][14]. The oral microbiome can affect systemic health conditions, not limited to the dental health (see
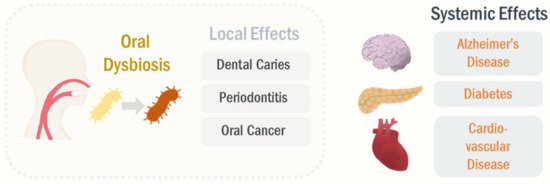
Figure 1.
Local and systemic effects of oral microbiome. The oral dysbiosis can regulate the pathological processes in the oral cavity, such as dental caries, periodontitis, and OSCC. The altered oral microbiota profiles can further modulate systemic diseases, including Alzheimer’s disease, diabetes, and cardiovascular disease, beyond the local impacts.
Oral dysbiosis can induce production of PAMP signals, such as lipopolysaccharide (LPS), resulting in systemic stimulation of innate immune responses and inflammatory transcription factors, including nuclear factor κB [66,67]. These systemic inflammation and immune responses are thought to be one of the primary mechanisms, underlining that the oral microbiome regulates pathogenesis in distal organs. Notably, oral microbiota can translocate to the other organs, which is considered as another mechanism of oral dysbiosis-induced systemic disease [68,69].
Oral dysbiosis can induce production of PAMP signals, such as lipopolysaccharide (LPS), resulting in systemic stimulation of innate immune responses and inflammatory transcription factors, including nuclear factor κB [17][18]. These systemic inflammation and immune responses are thought to be one of the primary mechanisms, underlining that the oral microbiome regulates pathogenesis in distal organs. Notably, oral microbiota can translocate to the other organs, which is considered as another mechanism of oral dysbiosis-induced systemic disease [19][20].
3. Interconnection between Oral and Gut Microbiomes: Oral–Gut Microbiome Axis
3.1. Oral-to-Gut Microbial Translocation
The oral and gut microbiomes are well-segregated due to the presence of the oral–gut barrier, physical distance as well as chemical hurdles, such as gastric acid and bile [20,30,77]. However, the impairment of the oral–gut barrier can allow interorgan translocation and communication. Oral microbes can overcome the physical and/or chemical barriers between the oral cavity and gut under certain circumstances and potentially translocate into the gut.
The oral and gut microbiomes are well-segregated due to the presence of the oral–gut barrier, physical distance as well as chemical hurdles, such as gastric acid and bile [21][22][23]. However, the impairment of the oral–gut barrier can allow interorgan translocation and communication. Oral microbes can overcome the physical and/or chemical barriers between the oral cavity and gut under certain circumstances and potentially translocate into the gut.
3.2. Fecal-to-Oral Microbial Translocation
Enteric microorganisms can be transmitted by fecal–oral routes through direct contact or indirect exposure via contaminated fluids and foods [89]. The human hand microbiota profile was highly overlapped with oral and gut microbiome patterns, suggesting that the human hand is a carrier for fecal-to-oral microbial transmission [14].
Enteric microorganisms can be transmitted by fecal–oral routes through direct contact or indirect exposure via contaminated fluids and foods [24]. The human hand microbiota profile was highly overlapped with oral and gut microbiome patterns, suggesting that the human hand is a carrier for fecal-to-oral microbial transmission [7].
In addition to intrapersonal transmission, the fecal–oral route is considered as an important mechanism for human-to-human transmission of pathogens as well. The oral and gut microbiomes are closely connected through both oral-to-gut and fecal-to-oral routes (see
).
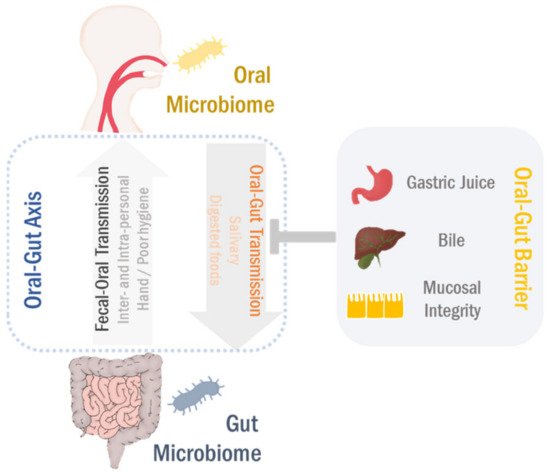
Figure 2. Oral–gut microbiome axis. The oral microbiota can translocate to the gut in conditions of the oral–gut barrier disruption. Likewise, the gut microbes transmit to the oral cavity in both intra- and interpersonal manners, particularly related to poor hygienic conditions. This bidirectional interaction between oral and gut microbiomes develops the microbial ecosystems in both habitats through either competition or cooperation, eventually regulating the pathophysiological processes in the gastrointestinal (GI) tract.
4. Oral–Gut Microbiome Axis in Human GI Diseases and Cancers
The oral pathogen(s) can interfere with intestinal barrier function and invade the gut mucosa, which induces the intestinal dysbiosis and chronic inflammation, consequently leading to IBD pathogenesis. Notably, IBD patients as well as colitis-induced mice displayed alterations in their salivary microbiota compositions, which were associated with inflammatory responses, indicating that the oral–gut microbial interactions could be bidirectional [124,125].
The oral pathogen(s) can interfere with intestinal barrier function and invade the gut mucosa, which induces the intestinal dysbiosis and chronic inflammation, consequently leading to IBD pathogenesis. Notably, IBD patients as well as colitis-induced mice displayed alterations in their salivary microbiota compositions, which were associated with inflammatory responses, indicating that the oral–gut microbial interactions could be bidirectional [25][26].
Colorectal cancer (CRC) is one of the most common cancer types and the second leading cause of cancer mortality worldwide [126]. IBD is the most well-established risk factor for development and progression of CRC [127]. Thus, IBD and CRC share etiological factors in pathogenesis, including distinct changes in the gut microbiome [128,129].
Colorectal cancer (CRC) is one of the most common cancer types and the second leading cause of cancer mortality worldwide [27]. IBD is the most well-established risk factor for development and progression of CRC [28]. Thus, IBD and CRC share etiological factors in pathogenesis, including distinct changes in the gut microbiome [29][30].
Oral dysbiosis potentially aggravates chronic liver diseases via shifts in the gut microbiome.
In a gnotobiotic mouse model, certain types of intestinal bacteria, such as
Escherichia coli
and
Streptococcus faecalis, can significantly increase liver tumorigenesis, indicating the direct involvement of the gut microbiota in HCC pathogenesis [174].
, can significantly increase liver tumorigenesis, indicating the direct involvement of the gut microbiota in HCC pathogenesis [31].
5. Conclusions
It has been well-appreciated that the gut and oral dysbioses are associated with numerous diseases [5,8,55,56]. To date, most of the research on microbiome-associated diseases have been conducted with respect to a single organ-specific microbiome, with less concern for an interorgan microbial communication. The oral cavity and gut are the two largest microbial habitats in the human body [9]. Cumulative evidence supports that the oral microbiota can change the overall gut microbial ecosystem through direct translocation and/or rather indirectly, by secretomes of oral bacteria [12,201,202]. Gut-to-oral microbial transmission can occur as well, particularly under certain circumstances, such as poor hygienic and immunocompromised conditions [14,90,92]. Collectively, the bidirectional crosstalk between oral and gut microbiomes can develop the oral–gut microbiome axis, which plays a crucial role in regulating pathogenesis of various human diseases, primarily in the GI system.
It has been well-appreciated that the gut and oral dysbioses are associated with numerous diseases [32][33][15][16]. To date, most of the research on microbiome-associated diseases have been conducted with respect to a single organ-specific microbiome, with less concern for an interorgan microbial communication. The oral cavity and gut are the two largest microbial habitats in the human body [1]. Cumulative evidence supports that the oral microbiota can change the overall gut microbial ecosystem through direct translocation and/or rather indirectly, by secretomes of oral bacteria [5][34][35]. Gut-to-oral microbial transmission can occur as well, particularly under certain circumstances, such as poor hygienic and immunocompromised conditions [7][36][37]. Collectively, the bidirectional crosstalk between oral and gut microbiomes can develop the oral–gut microbiome axis, which plays a crucial role in regulating pathogenesis of various human diseases, primarily in the GI system.
References
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323.
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214.
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013.
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290.
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422.
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive transmission of microbes along the gastrointestinal tract. Elife 2019, 8.
- Shaffer, M.; Lozupone, C. Prevalence and Source of Fecal and Oral Bacteria on Infant, Child, and Adult Hands. mSystems 2018, 3.
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118.
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14.
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179.
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666.
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012, 18, 109–120.
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500.
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143.
- Chu, H.; Mazmanian, S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013, 14, 668–675.
- Yu, J.C.; Khodadadi, H.; Baban, B. Innate immunity and oral microbiome: A personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019, 10, 43–50.
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463.
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Fernandez Moro, C.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194.
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42.
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338.
- Tennant, S.M.; Hartland, E.L.; Phumoonna, T.; Lyras, D.; Rood, J.I.; Robins-Browne, R.M.; van Driel, I.R. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect. Immun. 2008, 76, 639–645.
- de Graaf, M.; Beck, R.; Caccio, S.M.; Duim, B.; Fraaij, P.; Le Guyader, F.S.; Lecuit, M.; Le Pendu, J.; de Wit, E.; Schultsz, C. Sustained fecal-oral human-to-human transmission following a zoonotic event. Curr. Opin. Virol. 2017, 22, 1–6.
- Said, H.S.; Suda, W.; Nakagome, S.; Chinen, H.; Oshima, K.; Kim, S.; Kimura, R.; Iraha, A.; Ishida, H.; Fujita, J.; et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014, 21, 15–25.
- Rautava, J.; Pinnell, L.J.; Vong, L.; Akseer, N.; Assa, A.; Sherman, P.M. Oral microbiome composition changes in mouse models of colitis. J. Gastroenterol. Hepatol. 2015, 30, 521–527.
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103.
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009, 29, 2727–2737.
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395–411.
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016, 3, 130–143.
- Mizutani, T.; Mitsuoka, T. Effect of intestinal bacteria on incidence of liver tumors in gnotobiotic C3H/He male mice. J. Natl. Cancer Inst. 1979, 63, 1365–1370.
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904.
- Ray, K. The oral-gut axis in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 532.
- du Teil Espina, M.; Gabarrini, G.; Harmsen, H.J.M.; Westra, J.; van Winkelhoff, A.J.; van Dijl, J.M. Talk to your gut: The oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol. Rev. 2019, 43, 1–18.
- Ayele, B.H.; Geleto, A.; Ayana, D.A.; Redi, M. Prevalence of feco-oral transmitted protozoan infections and associated factors among university students in Ethiopia: A cross-sectional study. BMC Infect. Dis. 2019, 19, 499.
- Gaetti-Jardim, E., Jr.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018, 90, 45–52.
