Rechargeable power sources are an essential element of large-scale energy systems based on renewable energy sources. One of the major challenges in rechargeable battery research is the development of electrode materials with good performance and low cost. Carbon-based materials have a wide range of properties, high electrical conductivity, and overall stability during cycling, making them suitable materials for batteries, including stationary and large-scale systems.
- energy storage
- carbon
- nanomaterials
- lead–acid batteries
- lithium-ion batteries
- lithium–sulfur batteries
- sodium-ion batteries
- supercapacitors
1. Introduction
The development of technology, exponentially accelerating in modern times, is closely related to rising energy needs. Moreover, the human population is constantly rising, reaching 7.8 billion in 2020 according to the UN [1]. The changes in population are leading to a further increase in energy demands. This growing demand is not without influence on the natural environment. Recent increases in pollution and CO
2
emissions are causing a gradual, global shift toward more sustainable energy sources than currently widespread fossil fuels. For example, the European Union aims to be climate-neutral by 2050, as presented in the European Commission’s European Green Deal [2]. This transition to renewable energy sources requires efficient energy storage. Energy production by renewable sources is characterized by a variable output and inconsistent behavior. As a result of these properties, there is a growing demand for fast and cheap energy storage, which can be used to capture the excess energy generated during the periods of high production. This stored energy can then be released when required by a drop in production or a rise in energy usage.
In recent times, there has been great interest in developing materials that will allow effective and economically viable energy storage. This goal can be realized by improvements in currently known power sources, as well as by introducing completely new solutions. One of the materials that is often used during development of such technologies is carbon. It is used in various energy storage technologies, including primary and secondary batteries, fuel cells, flow batteries, and capacitors [3,4,5,6,7,8]. Carbon is a very versatile element, capable of forming various types of bonds and compounds, leading to its very rich organic chemistry. At the same time, even in its elemental form, it can create many different allotropic forms with interesting physical and chemical characteristics. Moreover, recent advances in nanotechnology have opened new avenues of carbon usage [9,10,11]. Carbon nanomaterials have very unique properties, further broadening the possibilities of carbon application in energy storage. The versatility of carbon allows it to find use in a wide range of electrochemical power sources.
In recent times, there has been great interest in developing materials that will allow effective and economically viable energy storage. This goal can be realized by improvements in currently known power sources, as well as by introducing completely new solutions. One of the materials that is often used during development of such technologies is carbon. It is used in various energy storage technologies, including primary and secondary batteries, fuel cells, flow batteries, and capacitors [3][4][5][6][7][8]. Carbon is a very versatile element, capable of forming various types of bonds and compounds, leading to its very rich organic chemistry. At the same time, even in its elemental form, it can create many different allotropic forms with interesting physical and chemical characteristics. Moreover, recent advances in nanotechnology have opened new avenues of carbon usage [9][10][11]. Carbon nanomaterials have very unique properties, further broadening the possibilities of carbon application in energy storage. The versatility of carbon allows it to find use in a wide range of electrochemical power sources.
2. Lead–Acid Batteries
Lead–acid batteries are used in automotive, traction, and backup applications and are among the biggest parts of the rechargeable battery market, comparable to lithium-ion batteries. The value of produced lead–acid and lithium batteries has almost been equal in recent years; however, taking into account the total energy, lead–acid batteries account for over 70% of the market [12]. Despite the progress in the construction of newer battery types, lead–acid batteries are still a technology that is very cost-effective, proven, reliable, and easily recyclable [3][13]. Carbon is one of the materials commonly used in lead–acid batteries. There are three main parts of these batteries that can be improved by the usage of elemental carbon: the active mass, the current collectors, and the negative plate as a whole [14][15][16].
First, carbon can be used as an additive to both the negative and the positive active mass (NAM and PAM, respectively). However, the use of carbon in NAM, as part of a so-called “expander”, is much more common and is currently a very widespread practice [13][16]. Such an addition to NAM during its preparation improves the electrochemical properties of the finished battery, including its cycle life, charge efficiency, and specific energy [13][17]. This addition is especially important when the battery is working at high-rate charge/discharge currents. For example, lead–acid batteries with carbon additives show a marked improvement in the high-rate partial stage of charge (HRPSoC) conditions, which are relevant for hybrid vehicle usage [16][18].
presents the increase in the number of completed cycles for batteries with carbon additives in NAM compared to a blank battery without such additives.
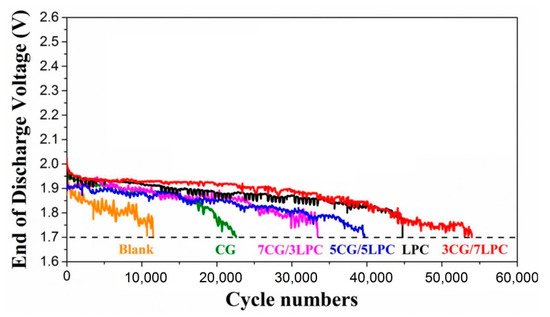
Comparison of number of cycles completed in HRPSoC conditions for lead–acid batteries with various carbon additives, conductive graphene (CG), and lead-doped porous carbon (LPC) with different mass ratios. HRPSoC regime: charge at C/5 for 90 s (voltage limit of 2.35 V), rest for 10 s, discharge at C/5 for 60 s, and rest for 10 s. Adapted from [19] with permission from Elsevier.
There have been a few proposed mechanisms of beneficial carbon influence as an NAM additive, but three are most often recognized [15][16][20][21]. First, carbon can change the structure of the active mass and form a conductive skeleton inside it. When NAM is discharged, metallic lead is transformed into nonconductive lead(II) sulfate(VI). Addition of carbon helps the active mass to retain some conductivity and provides a larger active surface area, even when deeply discharged. The second mechanism is a mechanical restriction of the growth of lead(II) sulfate(VI) crystals. The kinetics of dissolution of these crystals during recharging depends on their size. Bigger crystals have lower surface area compared to their volume; thus, their transformation into lead is slower. During a typical charging process of the battery, some of the larger crystals are not reduced fast enough and remain as lead(II) sulfate(VI). This process of plate sulfation leads to some of the active mass not taking part in charge/discharge processes and gradual loss of capacity of the battery. A carbon additive in NAM relieves this problem, as it becomes incorporated into the pores of the mass, which allows it to physically restrict the available space in them and inhibit the growth of lead(II) sulfate(VI) crystals. This steric hindrance to sulfation improves the cyclability of the battery. In the third mechanism, the carbon additive in NAM can work as a capacitor, storing additional energy in its electric double layer. This process is faster than the typical faradaic process in the active mass. The capacitive effect of carbon additives leads to improved charge acceptance and power when using high-rate currents [15][16][20][21].
Types of carbon material often used as an NAM additive include activated carbon, carbon black, graphitic powder, graphene, or nanomaterials (e.g., nanotubes, graphene, nanofibers) [20][21][22][23][24]. Nanotubes can be single- or multiwalled, and their surface can be modified to further improve their properties [24][25]. Some of the important properties of carbon materials used as additives are surface area, conductivity, affinity to lead, porosity, hydrogen evolution overpotential, and surface chemistry. Typically used carbon additives have specific surface area and capacitance greater by orders of magnitude than the NAM. When working as a capacitor, an addition of 2 wt.% carbon with a specific capacitance of around 200 F/g can provide around 1–2% of the total capacity of the plate, which is enough when charging/discharging for a short time at high-rate currents [17]. The conductivity of the material depends, among other factors, on the presence of connected domains of carbon atoms with a graphite structure. Amorphous carbons with small particle size, e.g., activated carbon, are in general characterized by worse conductivity [16]. Conductivity is not an important parameter regarding the steric effect of additives on sulfation, but the carbon material needs to be able to be properly mixed with the active mass.
The correct preparation of the active mass when mixing the paste and increased hydrogen evolution are two important aspects that need to be considered when using carbon additives. Carbon materials change the physical and chemical properties of the active mass. In order to achieve a mass with appropriate characteristics for pasting plates, some modifications in the pasting process or composition of the mix may be required. Addition of carbon also leads to acceleration of the hydrogen evolution reaction, resulting in losses of the electrolyte during charging of the batteries. These additives in general have lower hydrogen evolution overpotentials than lead; moreover, they can contain impurities and increase the active surface area, further facilitating the oxidation of hydronium ions.
One of the ways to alleviate the hydrogen evolution problem involves new types of composite carbon materials doped with heteroatoms. Modification of the structure of the carbon material by doping with metals with high hydrogen evolution overpotential allows limiting the reaction rate of hydrogen evolution, while retaining the positive influence of carbon [25][26][27]. Doping with nonmetals, e.g., nitrogen or phosphorus, can also provide similar benefits by modifying the electron density on carbon atoms and the strength of their bonds with hydrogen [28][29].
Carbon additives to NAM are a very important part of the lead–acid battery technology. In the past, they solved the problems of premature capacity loss introduced when organic separators were replaced by synthetic materials [13]. Today, their usage allows surpassing the limitations appearing when lead–acid batteries work in an HRPSoC regime. This topic is very important when taking into account the current energy transformation and spread of electric and hybrid vehicles.
Carbon materials can also be used as an additive to PAM. However, this practice is not so widespread, as they are less stable in the working conditions of PAM and can breakdown during the first cycles of life of the battery. Their positive influence on cycle life is also less pronounced than in the case of NAM or even not observable. On the other hand, there is some research indicating an improvement in the behavior of PAM when some carbon additives are used. An increase in cycle life and capacity when carbon fiber was added was reported [30][31]. The additives can reduce PAM softening and shedding, as well as improve conductivity and porosity [30][31][32]. There are fewer studies in this area than in the case of NAM additives. Nonetheless, the research is still ongoing, and introducing new types of carbon materials, including composites and nanomaterials, gives an opportunity to further improve PAM properties [22][33][34]. Additionally, carbon used as a paste additive can be based on biological sources. Improvements in the characteristics of both PAM and NAM were reported when using porous rice husk-based carbon as an additive [35][36].
Another part of a lead–acid battery, namely, current collectors, can also be improved by using carbon materials. Typically, a collector is a grid cast from lead alloy. It can be replaced by a much lighter reticulated carbon material, e.g., reticulated vitreous carbon (RVC), as proposed by Czerwiński et al. [37][38][39]. When used in this role, glassy carbon materials are resistant to corrosion while working in the negative plate [40]. On the other hand, the positive plate of the lead–acid battery experiences conditions causing corrosion of reticulated carbon collectors. To use the carbon collectors in this environment, they need to be protected by a corrosion-resistant layer, for example, a galvanically deposited thin layer of lead [38][41]. This protective layer improves both resistance to corrosion and mechanical properties. Using the reticulated carbon collectors can lead to a decrease in weight, an increase in specific energy and active mass utilization, better mechanical support for the mass, and longer cycle life [14][42][43][44][45].
Different forms of carbon can be used as the reticulated current collector. First experiments mainly concerned RVC [38][39][41]. Further studies investigated different materials, such as pitch-based foams [46][47], graphite foams [45], graphite foils [48], graphite punched sheets [49], polymer-graphite composites [50], honeycomb carbon structures [44][51][52], carbon fiber felt [53], or conductive porous carbon [43]. In general, for the mentioned materials, negative plates showed good results regarding capacity and cycle life. The results for positive plates were more mixed. Better cycle life than in comparable standard batteries could be achieved when the positive collectors were prepared using suitable materials and optimal lead (or lead alloy) coatings [43]. Some of the solutions already available on the market use reticulated carbon collectors, e.g., the Oasis battery produced by Firefly Energy in USA. The use of new types of lighter, reticulated current collectors is a promising solution which improves the main weakness of lead–acid batteries: their low specific capacity. At the same time, this new technology can lead to improvements in other areas, such as cycle life, temperature performance, or environmental impact.
shows the differences between various types of standard and reticulated collectors.

Typical (
), reticulated (
), and honeycomb (reprinted from [44] with permission from Elsevier) (
) current collectors for lead–acid batteries.
The capacitance of carbon materials not only plays an important role when the material is used as an additive to the negative mass, but they can also be used to replace a whole standard negative plate with a capacitor plate [54][55]. The capacitor plate uses the capacitance of the carbon material to store charge instead of using typical faradaic reactions in the active mass. Such a solution delivers more power and better charge acceptance but lowers the capacity of the battery. The capacitor plate can be used instead of the standard negative plate or in parallel with it. The former solution is used in Axion Power’s Pb-C battery, whereas the latter is used in the Ultrabattery developed by the Australian Commonwealth Scientific and Industrial Research Organization. These hybrid batteries were characterized by improved power, lifespan, and high-rate charge acceptance [3][55][56]. The properties of the batteries with capacitor plates improve the prospects of using lead–acid batteries in electric and hybrid vehicles.
The use of carbon allowed drastic improvements of current batteries and helped them achieve their current, strong market position. At the same time, new technologies improving the construction of this type of battery are constantly being developed. The main technologies incorporating carbon were described above: additives to the active mass, reticulated current collectors, and capacitor electrodes. There are different forms of carbons being used in these applications with more advanced materials, e.g., composites or nanomaterials, leading to better tailored properties at an increased cost of manufacturing. Using carbon materials allows the continued use of lead–acid batteries as a cheaper and safer alternative to newer battery types. At the same time, lead–acid batteries can constantly improve their electrochemical parameters and achieve good performance. Their main drawback is currently their low specific energy compared to other battery types. Depending on charge/discharge conditions, their cycle life can also be restricted. Current research on the improvement of lead–acid batteries focuses in great part on increasing their capacity and extending their HRPSoC cyclability, while maintaining their low cost and efficient recycling. The total market value of lead–acid batteries is still growing, even in recent years, especially in developing countries [12]. They have good prospects for the near future, as they are an attractive option for the growing market of storage of energy produced from renewable sources.
3. Lithium-Ion Batteries
Lithium-ion batteries are one of the most widely used secondary batteries with a dynamically increasing market share. The worldwide market for lithium-ion batteries was over 55 billion USD in 2017 [12]. In Li-ion batteries, the positive electrode material is an intercalated lithium compound, e.g., LiCoO
2
, LiMn
2
O
4
, lithium nickel manganese cobalt oxides (NMC, layered compositions with different Co:Ni:Mn ratios), or LiFePO
4 [15,68,69]. Most commercial Li-ion batteries have carbon materials as a negative electrode. When a battery is charged, the lithium ion from the positive electrode get inserted into the negative electrode, while the opposite occurs during discharge. During discharge, the carbon material electrode works as an anode. The basic parameters determining the choice of the anode material are the lithium intercalation and de-intercalation reversibility, chemical, thermal, and electrochemical environment, electrical conductivity, cyclic stability, and cost. Due to its good electrical properties and long cycle life, graphite is the most commonly used anode material [70,71]. The stoichiometry of lithium bonding (one Li
[57][58][59]. Most commercial Li-ion batteries have carbon materials as a negative electrode. When a battery is charged, the lithium ion from the positive electrode get inserted into the negative electrode, while the opposite occurs during discharge. During discharge, the carbon material electrode works as an anode. The basic parameters determining the choice of the anode material are the lithium intercalation and de-intercalation reversibility, chemical, thermal, and electrochemical environment, electrical conductivity, cyclic stability, and cost. Due to its good electrical properties and long cycle life, graphite is the most commonly used anode material [60][61]. The stoichiometry of lithium bonding (one Li
+
can be intercalated per six C atoms—the limiting composition is LiC
6) limits the theoretical specific capacity of graphite to around 370 mAh/g [70,72].
) limits the theoretical specific capacity of graphite to around 370 mAh/g [60][62].
Li
C
↔ C + xLi
+ xe
.
Non-graphitic carbon materials are also used as the negative electrode material. These materials are characterized by the appearance of amorphous areas together with more crystalline ones. Non-graphitic carbons are broadly classified into soft carbons (graphitizable carbons, where crystallites are stacked in the same direction) and hard carbons (non-graphitizable carbons, where crystallites have disordered orientation) [68]. Schematics of the structure of selected carbon materials are presented in
Non-graphitic carbon materials are also used as the negative electrode material. These materials are characterized by the appearance of amorphous areas together with more crystalline ones. Non-graphitic carbons are broadly classified into soft carbons (graphitizable carbons, where crystallites are stacked in the same direction) and hard carbons (non-graphitizable carbons, where crystallites have disordered orientation) [58]. Schematics of the structure of selected carbon materials are presented in
.
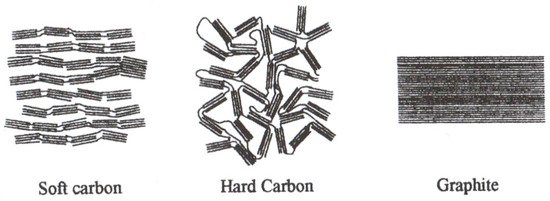
Illustration of carbon types showing different stacking of the graphene layers. Reprinted from [58] with permission from Elsevier.
The main advantages of soft carbons are long cycle life, coulombic efficiency over 90%, and highly reversible specific capacity [70]. One of the first soft carbon materials applied in lithium-ion batteries was coke, which offered a capacity around 180 mAh/g and stability in the presence of propylene carbonate-based electrolytes. Currently, various carbon types are used in negative electrodes e.g., graphitic spheres and natural graphite [15,70,73]. Additionally, the improvement of cyclic stability of soft carbons can be achieved by modifying the surface of the carbon material, e.g., by a silane coating [74]. However, the specific capacity of these materials is similar to that of graphite. These properties result quite often in the use of soft carbons in small, low-power devices (portable electronics) but not in hybrid electric vehicles and electric vehicles.
The main advantages of soft carbons are long cycle life, coulombic efficiency over 90%, and highly reversible specific capacity [60]. One of the first soft carbon materials applied in lithium-ion batteries was coke, which offered a capacity around 180 mAh/g and stability in the presence of propylene carbonate-based electrolytes. Currently, various carbon types are used in negative electrodes e.g., graphitic spheres and natural graphite [57][60][63]. Additionally, the improvement of cyclic stability of soft carbons can be achieved by modifying the surface of the carbon material, e.g., by a silane coating [64]. However, the specific capacity of these materials is similar to that of graphite. These properties result quite often in the use of soft carbons in small, low-power devices (portable electronics) but not in hybrid electric vehicles and electric vehicles.
The second group involves hard carbons. These materials offer higher capacity, typically around 500–1000 mAh/g [15,70]. The relatively high capacity of hard carbons compared to graphite can be explained by the fact that lithium in hard carbons additionally occupies the nearest neighbor sites between pairs of graphene sheets [75]. Another mechanism proposed is that lithium may also bind to the hydrogen-containing regions of carbon [76,77]. Hard carbons show a random alignment of graphene sheets and slow lithium diffusion inside the carbon structure. In turn, the disadvantages of hard carbons are its low initial coulombic efficiency and high loss in initial capacity. In connection with these problems, numerous works on hard carbons modification were carried out [78,79,80,81,82,83]. One of the proposed solutions is carbon surface modification by oxidation. It was observed that a mild oxidation of carbon leads to pore production, which is a crucial process in obtaining higher capacity [78]. Another solution is the application of a soft carbon layer on hard carbon. This modification resulted in a high-power performance and good cycling stability [79]. An improvement of the working parameters of negative electrodes based on hard carbons can also be achieved by using high-porosity carbons. Hu et al. [80] obtained and applied hard carbon spherules as a porous carbon material. A scanning electron microscope (SEM) image illustrating the morphology of such a material is presented in
The second group involves hard carbons. These materials offer higher capacity, typically around 500–1000 mAh/g [57][60]. The relatively high capacity of hard carbons compared to graphite can be explained by the fact that lithium in hard carbons additionally occupies the nearest neighbor sites between pairs of graphene sheets [65]. Another mechanism proposed is that lithium may also bind to the hydrogen-containing regions of carbon [66][67]. Hard carbons show a random alignment of graphene sheets and slow lithium diffusion inside the carbon structure. In turn, the disadvantages of hard carbons are its low initial coulombic efficiency and high loss in initial capacity. In connection with these problems, numerous works on hard carbons modification were carried out [68][69][70][71][72][73]. One of the proposed solutions is carbon surface modification by oxidation. It was observed that a mild oxidation of carbon leads to pore production, which is a crucial process in obtaining higher capacity [68]. Another solution is the application of a soft carbon layer on hard carbon. This modification resulted in a high-power performance and good cycling stability [69]. An improvement of the working parameters of negative electrodes based on hard carbons can also be achieved by using high-porosity carbons. Hu et al. [70] obtained and applied hard carbon spherules as a porous carbon material. A scanning electron microscope (SEM) image illustrating the morphology of such a material is presented in
. The proposed electrode showed an improvement of electrochemical performance and a capacity of almost 390 mAh/g.

SEM image of hard carbon spherules. Reprinted from [70] with permission from Elsevier.
Similar capacity (344 mAh/g) was shown by the material obtained by Rao et al. [81]. Hard carbon in this study was prepared by calcination of polypropylene cyanide. The obtained material showed an initial coulombic efficiency over 87% and a superior cycle stability at different current rates compared to graphite. Li et al. [82] examined a microporous hard carbon prepared from potato starch. The electrochemical tests revealed a good cyclic life and high reversible capacity (around 530 mAh/g). Similarly, nanoporous hard carbon obtained from pyrolyzed sucrose exhibited a specific capacity close to 500 mAh/g (at 0.2 C rate) and an equally good capacity, around 330 mAh/g at 5 C rate [83].
Similar capacity (344 mAh/g) was shown by the material obtained by Rao et al. [71]. Hard carbon in this study was prepared by calcination of polypropylene cyanide. The obtained material showed an initial coulombic efficiency over 87% and a superior cycle stability at different current rates compared to graphite. Li et al. [72] examined a microporous hard carbon prepared from potato starch. The electrochemical tests revealed a good cyclic life and high reversible capacity (around 530 mAh/g). Similarly, nanoporous hard carbon obtained from pyrolyzed sucrose exhibited a specific capacity close to 500 mAh/g (at 0.2 C rate) and an equally good capacity, around 330 mAh/g at 5 C rate [73].
Hard carbons in general are characterized by a faster decrease in capacity compared to soft carbons but higher specific capacity (above 500 mAh/g), especially in the case of highly porous materials. At the same time, the price of hard carbons is only about 20% higher than soft carbons. Nevertheless, due to their high energy and power density, they are widely used in batteries in hybrid-electric and electric vehicles.
Recently, carbon nanomaterials (such as carbon nanotubes, nanofibers, and graphene) have become very promising candidates for use as anodes in lithium-ion batteries. Compared to other materials, carbons nanomaterials have a higher specific theoretical capacity, e.g., 1116 mAh/g for single-walled nanotubes in LiC
2 stoichiometry [84,85,86]. Nanomaterials also offer other advantages, such as stable cyclic behavior and increased lithium ions diffusion rate. The use of carbon nanotube composites with other carbon materials allowed for the improvement of many parameters, such as electrical conductivity, transport properties, and thermal and mechanical stability. On the other hand, the basic disadvantages of nanosized particles are high surface reactivity, low coulombic efficiency during the first cycle, tendency to aggregation during cycling, and higher price [72,87]. Moreover, in practice, it is difficult to achieve the theoretical capacity, and the properties of carbon nanomaterials depend on the preparation methods and pretreatments (e.g., ball milling, acid treatment) [85]. Various morphological modifications of the nanotubes are known, which allow them to reach a capacity of approximately 1000 mAh/g [88,89]. Appropriate modification of parameters such as the tube diameter, wall thickness, or porosity allows improving the working parameters of such anode materials. Even the shape of the nanotubes can be changed. Bamboo-shaped carbon nanotubes produced by using a cresol precursor are characterized by an improved cycle stability and electric conductivity [88]. Quadrangular carbon nanotubes have a high specific capacity and good high-rate performance [89]. High reversible capacity was also found in the case of chemically nano-drilled multiwalled carbon nanotubes [90]. The structures of such materials are presented in
stoichiometry [74][75][76]. Nanomaterials also offer other advantages, such as stable cyclic behavior and increased lithium ions diffusion rate. The use of carbon nanotube composites with other carbon materials allowed for the improvement of many parameters, such as electrical conductivity, transport properties, and thermal and mechanical stability. On the other hand, the basic disadvantages of nanosized particles are high surface reactivity, low coulombic efficiency during the first cycle, tendency to aggregation during cycling, and higher price [62][77]. Moreover, in practice, it is difficult to achieve the theoretical capacity, and the properties of carbon nanomaterials depend on the preparation methods and pretreatments (e.g., ball milling, acid treatment) [75]. Various morphological modifications of the nanotubes are known, which allow them to reach a capacity of approximately 1000 mAh/g [78][79]. Appropriate modification of parameters such as the tube diameter, wall thickness, or porosity allows improving the working parameters of such anode materials. Even the shape of the nanotubes can be changed. Bamboo-shaped carbon nanotubes produced by using a cresol precursor are characterized by an improved cycle stability and electric conductivity [78]. Quadrangular carbon nanotubes have a high specific capacity and good high-rate performance [79]. High reversible capacity was also found in the case of chemically nano-drilled multiwalled carbon nanotubes [80]. The structures of such materials are presented in
and
.
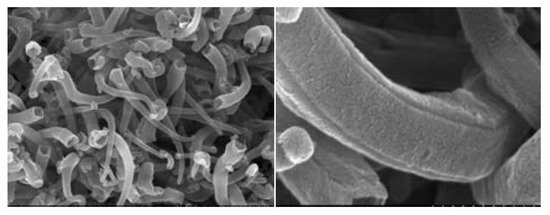
SEM images showing morphology of quadrangular carbon nanotubes. Republished with permission from the Royal Society of Chemistry, from [79]; permission conveyed through Copyright Clearance Center, Inc. (Danvers, MA, USA).
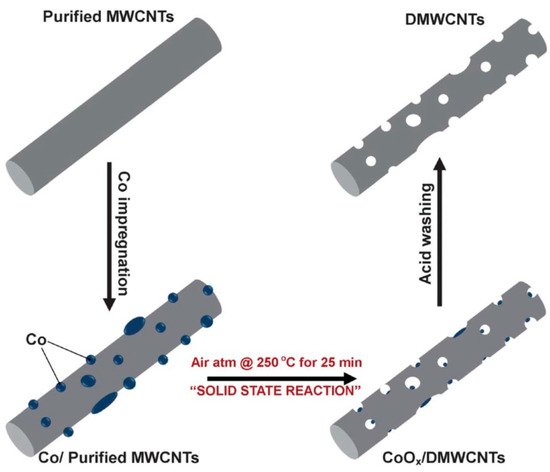
Schematic structure and procedure of preparing drilled multiwalled carbon nanotubes (DMWCNTs) from multiwalled carbon nanotubes (MWCNTs) Republished with permission from the Royal Society of Chemistry, from [80]; permission conveyed through Copyright Clearance Center, Inc.
A considerable number of studies investigated composites of carbon nanomaterials (nanotubes, nanofibers) with semi-metals, metals, and metal oxides such as Si, Ti, TiO, MnO, Fe, Fe
2
O
3
, Fe
3
O
4
, Co, Ni, Cu, Ge, Nb
2
O
7
, Sn, SnSb, and SnO
2 [91,92,93,94,95,96,97,98,99]. The use of composite material allows for the improvement of many parameters. It has been found that the use of a nickel and titanium contact in a free-standing single-walled carbon nanotube electrode increased both the reversible lithium ion capacity and the rate capacity [92]. Another example is the Fe
[81][82][83][84][85][86][87][88][89]. The use of composite material allows for the improvement of many parameters. It has been found that the use of a nickel and titanium contact in a free-standing single-walled carbon nanotube electrode increased both the reversible lithium ion capacity and the rate capacity [82]. Another example is the Fe
3
O
4–carbon nanotube composite presented by Wu et al. [95], which exhibited a high reversible capacity, high rate capability and remarkable capacity retention. Graphene is yet another material of interest for use as the anode material in lithium-ion batteries with a specific capacity at the level of 780–1120 mAh/g, depending on its structure [100,101,102]. Anode materials based on graphene sheets exhibit a high specific capacity (around 1000 mAh/g) and long cycling capability, up to 3000 cycles [100,103,104]. Graphene composite materials are also known to be used as anodes. Good anode properties, highly reversible capacity, and small loss from initial capacity were exhibited by graphene composites with silicon [93], SnO [105], or Fe
–carbon nanotube composite presented by Wu et al. [85], which exhibited a high reversible capacity, high rate capability and remarkable capacity retention. Graphene is yet another material of interest for use as the anode material in lithium-ion batteries with a specific capacity at the level of 780–1120 mAh/g, depending on its structure [90][91][92]. Anode materials based on graphene sheets exhibit a high specific capacity (around 1000 mAh/g) and long cycling capability, up to 3000 cycles [90][93][94]. Graphene composite materials are also known to be used as anodes. Good anode properties, highly reversible capacity, and small loss from initial capacity were exhibited by graphene composites with silicon [83], SnO [95], or Fe
3
O
4 [106].
[96].
illustrates how the introduction of nanomaterials and composite materials can lead to marked improvements in characteristics of the anode.
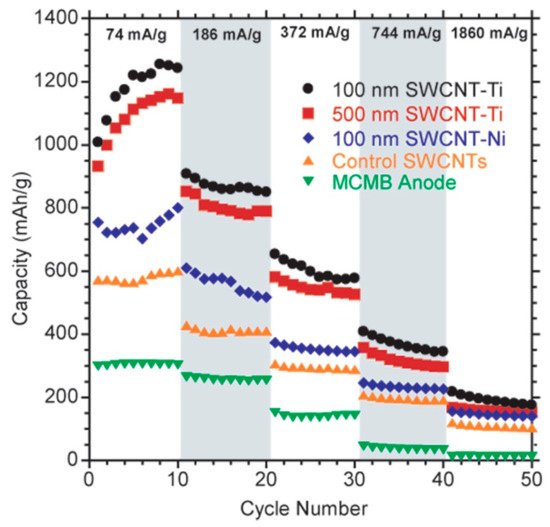
Rate cycle performance of various electrodes: single-walled nanotubes (SWCNT) (orange), SWCNT composite with Ni (blue), SWCNT composite with Ti (100 nm nanotube size—black; 500 nm—red), and standard graphite mesocarbon microbead (MCMB) (green). Reprinted with permission from [82]. Copyright 2010 American Chemical Society.
In recent years, lithium titanate with a spinel structure (LTO) has been studied as a potential anode material characterized by a good cycling stability [107,108]. Over the last 10 years, there has been a continuous increase in the number of studies from about 50 publications per year in 2010 to almost 300 in 2020 [13]. Carbon materials are also used as additives to LTO anodes. Carbon nanotubes and graphene used as additives provide good contact between particles and conductivity at the interfaces [109,110,111]. Additional solutions for improving electrochemical properties and capacity retention are carbon additives with a large surface area such as carbon black, mesoporous carbon, and amorphous carbon [112,113,114].
In recent years, lithium titanate with a spinel structure (LTO) has been studied as a potential anode material characterized by a good cycling stability [97][98]. Over the last 10 years, there has been a continuous increase in the number of studies from about 50 publications per year in 2010 to almost 300 in 2020 [99]. Carbon materials are also used as additives to LTO anodes. Carbon nanotubes and graphene used as additives provide good contact between particles and conductivity at the interfaces [100][101][102]. Additional solutions for improving electrochemical properties and capacity retention are carbon additives with a large surface area such as carbon black, mesoporous carbon, and amorphous carbon [103][104][105].
Another new research area involves lithium-ion capacitors, a combination of a a faradaic lithium-ion battery anode and a supercapacitor cathode, which offers rapid charging–discharging capability and long cycle life. In this promising technology, carbon materials can be used in both the positive and the negative electrode [115,116,117]. Lithium-ion capacitors combine high energy density with high power density and excellent durability. Due to this, an important potential application for lithium-ion capacitors is represented by energy recovery systems in industrial machinery and transportation systems. Hybrid ion capacitors can be successfully used in regenerative braking energy harvesting from trains, heavy automobiles, and ultimately electric and hybrid-electric vehicles.
Another new research area involves lithium-ion capacitors, a combination of a a faradaic lithium-ion battery anode and a supercapacitor cathode, which offers rapid charging–discharging capability and long cycle life. In this promising technology, carbon materials can be used in both the positive and the negative electrode [106][107][108]. Lithium-ion capacitors combine high energy density with high power density and excellent durability. Due to this, an important potential application for lithium-ion capacitors is represented by energy recovery systems in industrial machinery and transportation systems. Hybrid ion capacitors can be successfully used in regenerative braking energy harvesting from trains, heavy automobiles, and ultimately electric and hybrid-electric vehicles.
The carbon materials used during the earlier phases of lithium-ion battery development were mainly graphite and soft and hard carbons. With maturing of the technology and the introduction of new available techniques and concepts, other carbon materials were employed. In particular, the use of carbon nanomaterials is very promising. They can lead to large improvements of the capacity and the cycle life of lithium-ion batteries. Their successful and economically viable introduction into mass-produced battery models would provide lithium-ion batteries with a very dominant position in the current market. Some of the disadvantages of currently used lithium-ion batteries are their temperature limitations, limited safety, and comparatively high cost. To address these problems, many potential solutions are being researched, including technologies beyond the scope of this review, e.g., solid-state electrolytes or new electrode compositions. The recent growth of portable electronic devices and electric vehicles creates a very attractive market for lithium-ion batteries with proper characteristics and provides strong incentives for developing further improvements in these batteries.
References
- United Nations Department of Economic and Social Affairs. Available online: (accessed on 11 March 2021).
- An Official Website of the European Union. Available online: (accessed on 11 March 2021).
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A review. J. Energy Storage 2018, 15, 145–157.
- Chen, S.; Qiu, L.; Cheng, H.M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120, 2811–2878.
- Pethaiah, S.S.; Kumar, J.A.; Kalyani, P. Improvement in the Discharge Characteristics of Zinc–Carbon Primary Cells: A Comparative Study with Various Carbon Additives. Ionics 2011, 17, 339–342.
- Li, Y.; Wu, X.; Liu, C.; Wang, S.; Zhou, P.; Zhou, T.; Miao, Z.; Xing, W.; Zhuo, S.; Zhou, J. Fluorinated Multi-Walled Carbon Nanotubes as Cathode Materials of Lithium and Sodium Primary Batteries: Effect of Graphitization of Carbon Nanotubes. J. Mater. Chem. A 2019, 7, 7128–7137.
- Jayakumar, A. A Comprehensive Assessment on the Durability of Gas Diffusion Electrode Materials in PEM fuel cell stack. Front. Energy 2019, 13, 325–338.
- Banerjee, R.; Bevilacqua, N.; Mohseninia, A.; Wiedemann, B.; Wilhelm, F.; Scholta, J.; Zeis, R. Carbon Felt Electrodes for Redox Flow Battery: Impact of Compression on Transport Properties. J. Energy Storage 2019, 26, 100997.
- Mobasser, S.; Firoozi, A.A. Review of Nanotechnology Applications in Science and Engineering. J. Civ. Eng. Urban. 2016, 6, 84–93.
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey Jr., J.T.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of Carbon Nanomaterials for Advanced Polymernanocomposites: A Comparison Study Between CNT and Graphene. Prog. Polym. Sci. 2017, 67, 1–47.
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784.
- Pillot, C. Current Status and Future Trends of the Global Li-ion Battery Market; Avicenne Energy: London, UK, 2018.
- Pavlov, D. Lead-Acid Batteries: Science and Technology; Elsevier B. V: Amsterdam, The Netherlands, 2011; pp. 22–23.
- Lach, J.; Wróbel, K.; Wróbel, J.; Podsadni, P.; Czerwiński, A. Applications of Carbon in Lead-Acid Batteries: A Review. J. Solid State Electrochem. 2019, 23, 693–705.
- Hao, Z.; Xu, X.; Wang, H.; Liu, J.; Yan, H. Review on the Roles of Carbon Materials in Lead-Carbon Batteries. Ionics 2018, 24, 951–965.
- Moseley, P.T.; Rand, D.A.J.; Peters, K. Enhancing the Performance of Lead-Acid Batteries with Carbon—In Pursuit of an Understanding. J. Power Sources 2015, 295, 268–274.
- Moseley, P.T.; Rand, D.A.J.; Davidson, A.; Monahov, B. Understanding the Functions of Carbon in the Negative Active-Mass of the Lead–Acid Battery: A Review of Progress. J. Energy Storage 2018, 19, 272–290.
- Ebner, E.; Burow, D.; Panke, J.; Börger, A.; Feldhoff, A.; Atanassova, P.; Valenciano, J.; Wark, M.; Rühl, E. Carbon Blacks for Lead-Acid Batteries in Micro-Hybrid Applications—Studied by Transmission Electron Microscopy and Raman Spectroscopy. J. Power Sources 2013, 222, 554–560.
- Wang, L.; Zhang, H.; Zhang, W.; Cao, G.; Zhao, H.; Yang, Y. Enhancing Cycle Performance of Lead-Carbon Battery Anodes by Lead-Doped Porous Carbon Composite and Graphite Additives. Mater. Lett. 2017, 206, 113–116.
- Blecua, M.; Fatas, E.; Ocon, P.; Gonzalo, B.; Merino, C.; de la Fuente, F.; Valenciano, J.; Trinidad, F. Graphitized Carbon Nanofibers: New Additive for the Negative Active Material of Lead Acid Batteries. Electrochim. Acta 2017, 257, 109–117.
- Mahajan, V.; Bharj, R.S.; Bharj, J. Role of Nano-Carbon Additives in Lead-Acid Batteries: A Review. Bull. Mater. Sci. 2019, 42, 21.
- Marom, R.; Ziv, B.; Banerjee, A.; Cahana, B.; Luski, S.; Aurbach, D. Enhanced Performance of Starter Lighting Ignition Type Lead-Acid Batteries with Carbon Nanotubes as an Additive to the Active Mass. J. Power Sources 2015, 296, 78–85.
- Hu, H.Y.; Xie, N.; Wang, C.; Wu, F.; Pan, M.; Li, H.F.; Wu, P.; Wang, X.D.; Zeng, Z.; Deng, S.; et al. Enhancing the Performance of Motive Power Lead-Acid Batteries by High Surface Area Carbon Black Additives. Appl. Sci. 2019, 9, 186.
- Logeshkumar, S.; Manoharan, R. Influence of Some Nanostructured Materials Additives on the Performance of Lead Acid Battery Negative Electrodes. Electrochim. Acta 2014, 144, 147–153.
- Jiang, Y.; Zhu, H.; Yu, C.; Cao, X.; Cheng, L.; Li, R.; Yang, S.; Dai, C. Effects of Carbon Additives on the HRPSoC Performance of Lead Carbon Batteries and Their Low Temperature Performance. Int. J. Electrochem. Sci. 2017, 12, 10882–10893.
- Wang, L.; Zhang, H.; Zhang, W.; Guod, H.; Cao, G.; Zhao, H.; Yang, Y. A New Nano Lead-Doped Mesoporous Carbon Composite as Negative Electrode Additives for Ultralong-Cyclability Lead-Carbon Batteries. Chem. Eng. J. 2018, 337, 201–209.
- Hong, B.; Jiang, L.; Xue, H.; Liu, F.; Jia, M.; Li, J.; Liu, Y. Characterization of Nano-Lead-Doped Active Carbon and its Application in Lead-Acid Battery. J Power Sources 2014, 270, 332–341.
- Wang, F.; Hu, C.; Lian, J.; Zhou, M.; Wang, K.; Yan, J.; Jiang, K. Phosphorus-Doped Activated Carbon as a Promising Additive for High Performance Lead Carbon Batteries. RSC Adv. 2017, 7, 4174–4178.
- Hong, B.; Yu, X.; Jiang, L.; Xue, H.; Liu, F.; Li, Y.; Liu, Y. Hydrogen Evolution Inhibition with Diethylenetriamine Modification of Activated Carbon for a Lead-Acid Battery. RSC Adv. 2014, 4, 33574–33577.
- Mandal, S.; Thangarasu, S.; Thong, P.T.; Kim, S.C.; Shim, J.Y.; Jung, H.Y. Positive Electrode Active Material Development Opportunities through Carbon Addition in the Lead-Acid Batteries: A Recent Progress. J. Power Sources 2021, 485, 229336.
- Ball, R.J.; Evans, R.; Thacker, E.L.; Stevens, R. Effect of Valve Regulated Lead/Acid Battery Positive Paste Carbon Fibre Additive. J. Mater. Sci. 2003, 38, 3013–3017.
- Hao, H.; Chen, K.; Liu, H.; Wang, H.; Liu, J.; Yang, K.; Yan, H. A Review of the Positive Electrode Additives in Lead-Acid Batteries. Int. J. Electrochem. Sci. 2018, 13, 2329–2340.
- Banerjee, A.; Ziv, B.; Shilina, Y.; Levi, E.; Luski, S.; Aurbach, D. Single-Wall Carbon Nanotube Doping in Lead-Acid Batteries: A New Horizon. ACS Appl. Mater. Interfaces 2017, 9, 3634–3643.
- Shen, H.; Jin, Y.; Zhao, Z.; Sun, Y.; Huang, B.; Wang, J. Preparation of Graphite-Based Lead Carbon Cathode and its Performance of Batteries. Micro Nano Lett. 2019, 14, 915–918.
- Shi, J.; Lin, N.; Wang, Y.; Liu, D.; Lin, H. The Application of Rice Husk-Based Porous Carbon in Positive Electrodes of Lead Acid Batteries. J. Energy Storage 2020, 30, 101392.
- Yin, J.; Lin, N.; Lin, Z.; Wang, Y.; Chen, C.; Shi, J.; Bao, J.; Lin, H.; Feng, S.; Zhang, W. Hierarchical Porous Composite for High-Performance Lead-Carbon Battery Towards Renewable Energy Storage. Energy 2020, 193, 116675.
- Czerwiński, A. Sposób Galwanicznego Nanoszenia Ołowiu lub Tlenku Ołowiowego na Przewodzące Materiały Węglowe. RP Patent 167796, 12 May 1995.
- Czerwiński, A.; Żelazowska, M. Electrochemical Behavior of Lead Deposited on Reticulated Vitreous Carbon. J. Electroanal. Chem. 1996, 410, 55–60.
- Czerwiński, A.; Żelazowska, M. Electrochemical Behavior of Lead Dioxide Deposited on Reticulated Vitreous carbon (RVC). J. Power Sources 1997, 64, 29–34.
- Czerwiński, A.; Obrębowski, S.; Kotowski, J.; Rogulski, Z.; Skowroński, J.; Bajsert, M.; Przystałowski, M.; Buczkowska-Biniecka, M.; Jankowska, E.; Baraniak, M.; et al. Hybrid Lead-Acid Battery with Reticulated Vitreous Carbon as a Carrier- and Current-Collector of Negative Plate. J. Power Sources 2010, 195, 7530–7534.
- Gyenge, E.; Jung, J.; Mahato, B. Electroplated Reticulated Vitreous Carbon Current Collectors for Lead–Acid Batteries: Opportunities and Challenges. J. Power Sources 2003, 113, 388–395.
- Czerwiński, A.; Rogulski, Z.; Obrębowski, S.; Lach, J.; Wróbel, K.; Wróbel, J. Positive Plate for Carbon Lead-Acid Battery. Int. J. Electrochem. Sci. 2014, 9, 4826–4839.
- Czerwiński, A.; Wróbel, J.; Lach, J.; Wróbel, K.; Podsadni, P. The Charging-Discharging Behavior of the Lead-Acid Cell with Electrodes Based on Carbon Matrix. J. Solid State Electrochem. 2018, 22, 2703–2714.
- Kirchev, A.; Serra, L.; Dumenil, S.; Brichard, G.; Alias, M.; Jammet, B.; Vinit, L. Carbon Honeycomb Grids for Advanced Lead-Acid Batteries. Part III: Technology Scale-Up. J. Power Sources 2015, 299, 324–333.
- Jang, Y.I.; Dudney, N.J.; Tiegs, T.N.; Klett, J.W. Evaluation of the Electrochemical Stability of Graphite Foams as Current Collectors for Lead Acid Batteries. J. Power Sources 2006, 161, 1392–1399.
- Ma, L.W.; Chen, B.Z.; Chen, Y.; Yuan, Y. Pitch-Based Carbon Foam Electrodeposited with Lead as Positive Current Collectors for Lead Acid Batteries. J. Appl. Electrochem. 2009, 39, 1609–1615.
- Chen, Y.; Chen, B.Z.; Shi, X.C.; Xu, H.; Shang, W.; Yuan, Y.; Xiao, L.P. Preparation and Electrochemical Properties of Pitch-Based Carbon Foam as Current Collectors for Lead Acid Batteries. Electrochim. Acta 2008, 53, 2245–2249.
- Lannelongue, J.; Cugnet, M.; Guillet, N.; Kirchev, A. Electrochemistry of Thin-Plate Lead-Carbon Batteries Employing Alternative Current Collectors. J. Power Sources 2017, 352, 194–207.
- Hariprakash, B.; Gaffoor, S.A. Lead-Acid Cells with Lightweight, Corrosion-Protected, Flexible-Graphite Grids. J. Power Sources 2007, 173, 565–569.
- Zhang, S.; Zhang, H.; Cheng, J.; Zhang, W.; Cao, G.; Zhao, H.; Yang, Y. Novel Polymer-Graphite Composite Grid as a Negative Current Collector for Lead-Acid Batteries. J. Power Sources 2016, 334, 31–38.
- Kirchev, A.; Kircheva, N.; Perrin, M. Carbon Honeycomb Grids for Advanced Lead-Acid Batteries. Part I: Proof of Concept. J. Power Sources 2011, 196, 8773–8788.
- Kirchev, A.; Dumenil, S.; Alias, M.; Christin, R.; de Mascarel, A.; Perrin, M. Carbon Honeycomb Grids for Advanced Lead-Acid Batteries. Part II: Operation of the Negative Plates. J. Power Sources 2015, 279, 809–824.
- Christie, S.; Wong, Y.S.; Titelman, G.; Abrahamson, J. Lead-Acid Battery Construction. US Patent 9543589, 10 January 2017.
- Lam, L.T.; Louey, R. Development of Ultra-Battery for Hybrid-Electric Vehicle Applications. J. Power Sources 2006, 158, 1140–1148.
- Cooper, A.; Furakawa, J.; Lam, L.; Kellaway, M. The UltraBattery—A New Battery Design for a New Beginning in Hybrid Electric Vehicle Energy Storage. J. Power Sources 2009, 188, 642–649.
- Monahov, B. The beneficial Role of Carbon in the Negative Plate of Advanced Lead-Carbon Batteries. Ecs Trans. 2012, 41, 45–69.
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002; pp. 35.6–35.21.
- Broussely, M.; Pistoia, G. Industrial Applications of Batteries, 1st ed.; Elsevier: London, UK, 2007; pp. 2–28.
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367.
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capigila, C. Review on Recent Progress of Nanostructured Anode Materials for Li-ion Batteries. J. Power Sources 2014, 257, 421–443.
- Ratynski, M.; Hamankiewicz, B.; Krajewski, M.; Boczar, M.; Ziolkowska, D.; Czerwinski, A. Impact of Natural and Synthetic Graphite Milling Energy on Lithium-Ion Electrode Capacity and Cycle Life. Carbon 2019, 145, 82–89.
- Park, C.M.; Choi, W.; Hwa, Y.; Kim, J.H.; Jeong, G.; Sohn, H.J. Characterizations and Electrochemical Behaviors of Disproportionated SiO and its Composite for Rechargeable Li-ion Batteries. J. Mater. Chem. 2010, 20, 4854–4860.
- Pistoia, G. Lithium Batteries—New Materials, Developments and Perspectives; Elsevier: New York, NY, USA, 1994; pp. 1–97.
- Chen, Z.; Wang, Q.; Amine, K. Improving the Performance of Soft Carbon for Lithium-Ion Batteries. Electrochim. Acta 2006, 51, 3890–3894.
- Sato, K.; Noguchi, M.; Demachi, A.; Oki, N.; Endo, M. A Mechanism of Lithium Storage in Disordered Carbons. Science 1994, 264, 556–558.
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593.
- Papanek, P.; Radosavljevic, M.; Fischer, J.E. Lithium Insertion in Disordered Carbon-Hydrogen Alloys: Intercalation vs Covalent Binding. Chem. Mater. 1996, 8, 1519–1526.
- Fujimoto, H.; Tokumitsu, K.; Mubuchi, A.; Chinnasamy, N.; Kasuh, T. The Anode Performance of the Hard Carbon for the Lithium Ion Battery Derived from the Oxygen-Containing Aromatic Precursors. J. Power Sources 2010, 195, 7452–7456.
- Wang, J.; Liu, J.L.; Wang, Y.G.; Wang, C.X.; Xia, Y.Y. Pitch Modified Hard Carbons as Negative Materials for Lithium-Ion Batteries. Electrochim. Acta 2012, 74, 1–7.
- Hu, J.; Li, H.; Huang, X. Influence of Micropore Structure on Li-Storage Capacity in Hard Carbon Spherules. Solid State Ion. 2005, 176, 1151–1159.
- Rao, X.; Lou, Y.; Chen, J.; Lu, H.; Cheng, B.; Wang, W.; Fang, H.; Li, H.; Zhong, S. Polyacrylonitrile Hard Carbon as Anode of High Rate Capability for Lithium Ion Batteries. Front. Energy Res. 2020, 8, 3.
- Li, W.; Chen, M.; Wang, C. Spherical Hard Carbon Prepared from Potato Starch Using as Anode Material for Li-ion Batteries. Mater. Lett. 2011, 65, 3368–3370.
- Yang, J.; Zhou, X.Y.; Li, J.; Zou, Y.L.; Tang, J.J. Study of Nano-Porous Hard Carbons as Anode Materials for Lithium Ion Batteries. Mater. Chem. Phys. 2012, 135, 445–450.
- Zhao, J.; Buldum, A.; Han, J.; Ping, J. First-Principles Study of Li-Intercalated Carbon Nanotube Ropes. Phys. Rev. Lett. 2000, 85, 1706–1709.
- Meunier, V.; Kephart, J.; Roland, C.; Bernholc, J. Ab Initio Investigations of Lithium Diffusion in Carbon Nanotube Systems. Phys. Rev. Lett. 2002, 88, 075506–01.
- Schauermann, C.M.; Ganter, M.J.; Gaustad, G.; Babbitt, C.W.; Raffaelle, R.P.; Landi, B.J. Recycling Single-Wall Carbon Nanotube Anodes from Lithium Ion Batteries. J. Mater. Chem. 2012, 22, 12008–12015.
- Yu, Y.; Cui, C.; Qian, W.; Xie, Q.; Zheng, C.; Kong, C.; Wei, F. Carbon Nanotube Production and Application in Energy Storage. Asia-Pac. J. Chem. Eng. 2013, 8, 234–245.
- Lv, R.; Zou, L.; Gui, X.; Kang, F.; Zhu, Y.; Zhu, H.; Wei, J.; Gu, J.; Wang, K.; Wu, D. High-Yield Bamboo-Shaped Carbon Nanotubes from Cresol for Electrochemical Application. Chem. Commun. 2008, 2046–2048.
- Zhou, J.; Song, H.; Fu, B.; Wu, B.; Chen, X. Synthesis and High-Rate Capability of Quadrangular Carbon Nanotubes with One Open end as Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 2794–2800.
- Oktaviano, H.S.; Yamada, K.; Waki, K. Nano-Drilled Multiwalled Carbon Nanotubes: Characterizations and Application for LIB Anode Materials. J. Mater. Chem. 2012, 22, 25167.
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon Nanotubes for Lithium Ion Batteries. Energy Environ. Sci. 2009, 2, 638–654.
- DiLeo, R.A.; Castiglia, A.; Ganter, M.J.; Rogers, R.E.; Cress, C.D.; Raffaelle, R.P.; Landi, B.J. Enhanced Capacity and Rate Capability of Carbon Nanotube Based Anodes with Titanium Contacts for Lithium Ion Batteries. ACS Nano 2010, 4, 6121–6131.
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable Silicon-Carbon Nanocables Sandwiched between Reduced Graphene Oxide Sheets as Lithium Ion Battery Anodes. ACS Nano 2013, 7, 1437–1445.
- Zhang, X.; Ji, L.; Toprakci, O.; Liang, Y.; Alcoutlabi, M. Electrospun Nanofiber-Based Anodes, Cathodes, and Separators for Advanced Lithium-Ion Batteries. Polym. Rev. 2011, 51, 239–264.
- Wu, Y.; Wei, Y.; Wang, J.; Jiang, K.; Fan, S. Conformal Fe3O4 Sheath on Aligned Carbon Nanotube Scaffolds as High-Performance Anodes for Lithium Ion Batteries. Nano Lett. 2013, 13, 818–823.
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon Nanotubes in Li-ion Batteries: A Review. Mater. Sci. Eng. B 2016, 213, 12–40.
- Chen, S.; Shen, L.; van Aken, P.A.; Maier, J.; Yu, Y. Dual-Functionalized Double Carbon Shells Coated Silicon Nanoparticles for High Performance Lithium-Ion Batteries. Adv. Mater. 2017, 1605650.
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational Design of Three-Dimensional Graphene Encapsulated with Hollow Nanocomposite as Outstanding Anode Material for Lithium Ion and Sodium Ion Batteries. ACS Nano 2017, 11, 11602–11616.
- Lin, C.; Hu, L.; Cheng, C.; Sun, K.; Guo, X.; Shao, Q.; Li, J.; Wang, N.; Guo, Z. Nano-TiNb2O7/Carbon Nanotubes Composite Anode for Enhanced Lithium-Ion Storage. Electrochim. Acta 2018, 260, 65–72.
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li Storage Properties of Disordered Graphene Nanosheets. Chem. Mater. 2009, 21, 3136–3142.
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-Based Electrochemical Energy Conversion and Storage: Fuel Cells, Supercapacitors and Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402.
- Hwang, H.J.; Koo, J.; Park, M.; Park, N.; Kwon, Y.; Lee, H. Multilayer Graphynes for Lithium Ion Battery Anode. J. Phys. Chem. C 2013, 117, 6919–6923.
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large Reversible Capacity of High Quality Graphene Sheets as an Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2010, 55, 3909–3914.
- Wang, Z.L.; Xu, D.; Wang, H.G.; Wu, Z.; Zhang, X.B. In Situ Fabrication of Porous Graphene Electrodes for High-Performance Energy Storage. ACS Nano 2013, 7, 2422–2430.
- Vinayan, B.P.; Ramaprabhu, S. Facile Synthesis of SnO2 Nanoparticles Dispersed Nitrogen Doped Graphene Anode Material for Ultrahigh Capacity Lithium Ion Battery Applications. J. Mater. Chem. A 2013, 1, 3865–3871.
- Hu, A.; Chen, X.; Tang, Y.; Tang, Q.; Yang, L.; Zhang, S. Self-assembly of Fe3O4 Nanorods on Graphene for Lithium Ion Batteries with High Rate Capacity and Cycle Stability. Electrochem. Commun. 2013, 28, 139–142.
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A Comprehensive Review of Li4Ti5O12-based Electrodes for Lithium-Ion Batteries: The latest Advancements and Future Perspectives. Mater. Sci. Eng. R 2015, 98, 1–71.
- Fu, R.; Zhou, X.; Fan, H.; Blaisdell, D.; Jagadale, A.; Zhang, X.; Xiong, R. Comparison of Lithium-Ion Anode Materials Using an Experimentally Verified Physics-Based Electrochemical Model. Energies 2017, 10, 2174.
- Scopus Abstract & Citation Database. Available online: (accessed on 20 April 2021).
- Liu, J.; Wei, A.X.; Chen, M.; Xia, X. Rational synthesis of Li4Ti5O12/N-C Nanotube Arrays as Advanced High-Rate Electrodes for Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 3857–3863.
- Wei, A.; Li, W.; Bai, X.; Zhang, L.; Liu, Z.; Wang, Y. A Facile One-Step Solid-State Synthesis of a Li4Ti5O12/Graphene Composite as an Anode Material for High-Power Lithium-Ion Batteries. Solid State Ion. 2019, 329, 110–118.
- Wei, A.; Mu, J.; He, R.; Bai, X.; Liu, Z.; Zhang, L.; Liu, Z.; Wang, Y. Preparation of Li4Ti5O12/Carbon Nanotubes Composites and LiCoO2/ Li4Ti5O12 Full-Cell with Enhanced Electrochemical Performance for High-Power Lithium-Ion Batteries. J. Phys. Chem. Solids 2020, 138, 109303.
- Lan, C.K.; Bao, Q.; Huang, Y.H.; Duh, J.G. Embedding nano-Li4Ti5O12 in Hierarchical Porous Carbon Matrixes Derived from Water Soluble Polymers for Ultra-Fast Lithium Ion Batteries Anodic Materials. J. Alloys Compd. 2016, 673, 336–348.
- Yao, N.Y.; Liu, H.K.; Liang, X.; Sun, Y.; Feng, X.Y.; Chen, C.H.; Xinag, H.F. Li4Ti5O12 Nanosheets Embedded in Three-Dimensional Amorphous Carbon for Superior-Rate Battery Applications. J. Alloys Compd. 2019, 771, 755–761.
- Stenina, I.; Shaydullin, R.; Kulova, T.; Kuzmina, A.; Tabachkova, N.; Yoroslavtsev, A. Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes. Energies 2020, 13, 3941.
- Shen, L.; Lv, H.; Chen, S.; Kopold, P.; van Aken, P.A.; Wu, X.; Maier, J.; Yu, Y. Peapod-like Li3VO4/N-Doped Carbon Nanowires with Pseudocapacitive Properties as Advanced Materials for High-Energy Lithium-Ion Capacitors. Adv. Mater. 2017, 29, 1700142.
- Sun, X.; Zhang, X.; Liu, W.; Wang, K.; Li, C.; Li, Z.; Ma, Y. Electrochemical Performances and Capacity Fading Behaviors of Activated Carbon/Hard Carbon Lithium Ion Capacitor. Electrochim. Acta 2017, 235, 158–166.
- Decaux, C.; Lota, G.; Raymoundo-Piñero, E.; Frąckowiak, E.; Béguin, F. Electrochemical Performance of a Hybrid Lithium-Ion Capacitor with a Graphite Anode Preloaded from Lithium Bis(Trifluoromethane)Sulfonimide-Based Electrolyte. Electrochim. Acta 2012, 86, 282–286.
