Boswellia serrata counts among the most intensively studied anti-inflammatory medicinal plants with more than 650 publications recorded in the PubMed literature database up to now. In folk medicine lipophilic frankincense extracts are used as alternatives to anti-inflammatory steroidal drugs (i.e., glucocorticoids) or nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of diseases associated with inflammation like rheumatoid arthritis, osteoarthritis, asthma, atopic dermatitis, and inflammatory bowel diseases.
- medicinal plant
- Boswellia serrata
- boswellic acids
- Frankincense Extracts
- bioavailability
- inflammation
1. Frankincense Extracts
Boswellia serrata counts among the most intensively studied anti-inflammatory medicinal plants with more than 650 publications recorded in the PubMed literature database up to now. In folk medicine lipophilic frankincense extracts are used as alternatives to anti-inflammatory steroidal drugs (i.e., glucocorticoids) or nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of diseases associated with inflammation like rheumatoid arthritis, osteoarthritis, asthma, atopic dermatitis, and inflammatory bowel diseases. A detailed overview of a plethora of articles addressing the modes of action of the anti-inflammatory effect both in vitro and in vivo is given in the review of Efferth and Oesch [1].
In summary Boswellia serrata represents one of five species from the genus Boswellia (family: Burseraceae) commonly known as frankincense. This term refers to the oleogum resin of B. serrata Roxb., B. carterii Birdw., B. sacra Flueck, B. papyrifera Hochst, and B. frereana Birdw. The main components of the gum resin of Boswellia serrata are 10% volatile oils, 60% lipophilic resin, and 30% hydrophilic gums [2]. The anti-inflammatory effects are mainly attributed to the boswellic acids, i.e., 11-keto-β-boswellic acid (KBA), 3-O-acetyl-11-keto-β-boswellic acid (AKBA), α-boswellic acid (αBA), β-boswellic acid (βBA), 3-O-acetyl-α-boswellic acid (AαBA), 3-O-acetyl-β-boswellic acid (AβBA) [3]. The chemical structures are presented in Figure 1. Although more than 12 different types of boswellic acids have been identified in the gum resin of B. serrata and B. carterii, mostly the above mentioned six boswellic acids received considerable pharmacological attention [4]. This may be attributed to their high occurrence in lipophilic frankincense extracts, reaching 14 to 25% (m/m) in case of KBA, AKBA, βBA and AβBA [5]. Some additional chemical components include lupeolic acid, roburic acids, tirucallic acids as well as incensole acetate, incensole oxide and isoincensole oxides, just to name a few [4].
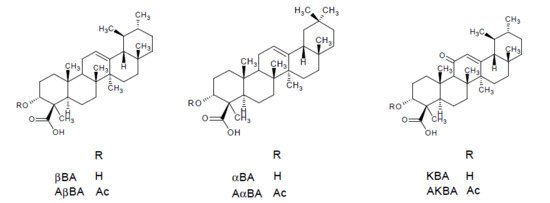
Figure 1. Chemical structures of the most relevant boswellic acids: β-boswellic acid (βBA), α-boswellic acid (αBA), 11-keto-β-boswellic acid (KBA), 3-O-acetyl-β-boswellic acid (AβBA), 3-O-acetyl-α-boswellic acid (AαBA), 3-O-acetyl-11-keto-β-boswellic acid (AKBA).
2. In Vitro IC50 Values
It is believed that the anti-inflammatory effects of boswellic acids are caused by different mechanisms of action including inhibition of key enzymes, kinases and transcription factors involved in the initiation and maintenance of inflammation [4]. For example boswellic acids were reported to inhibit the key enzymes 5-lipoxygenase (5-LO), cyclooxygenase-2 (Cox-2), and microsomal prostaglandin E2 synthase-1 (mPGES-1). The pro-inflammatory prostaglandins (PG) and leukotrienes (LT) produced by the Cox-2 and 5-LO pathways from arachidonic acid significantly contribute to the inflammatory response [5]. Depending on the nature of the target, the potencies of the different boswellic acids varied between IC50 = 0.5 µM (βBA) and 4.1 µM (KBA) for the serine protease cathepsin G inhibition in cell-free assay, between IC50 = 3.0 µM (AKBA) and >10 µM (βBA and AβBA) for suppression of 5-LO activity in human neutrophils stimulated with A23187, and between 3 µM (AKBA) and 10 µM (KBA) for inhibition of mPGES-1 in cell-free assay [5][6]. An even stronger inhibition of mPGES-1 with IC50 = 0.4 µM were reported for 3α-acetoxy-8,24-dienetirucallic acid and 3α-acetoxy-7,24-dienetirucallic acid. mPGES-1 is an inducible enzyme that converts PGH2 formed by Cox-1/2 to the pro-inflammatory PGE2 [7]. Potent suppression of 5-LO in the range of IC50 = 2.9 µM and 5.1 µM was also observed for roburic acid, lupeolic acid as well as tirucallic acids [5]. These molecular targets were also confirmed in in vivo studies as molecular basis for the anti-inflammatory action of frankincense as perfectly summarized in the review of Efferth and Oesch [1].
In addition it was reported that 50 µM AKBA completely suppressed the nuclear transcription factor NF-κB in human myeloid KBM-5 cells, another key player in the development and progression of chronic inflammatory diseases [8]. Other boswellic acids lacking either the acetyl group (e.g., KBA) or the keto group (e.g., AβBA) were less potent and inhibited NF-κB only partially. Since the activation of NF-κB is a multi-step process, that involves the activation of the inhibitory κB kinase (IKK) complex, phosphorylation of inhibitors and their degradation, transport of NF-κB to the nucleus, binding to the NF-κB-consensus sequence, and activation of genes, the role of boswellic acids in this cascade was further investigated by Syrovets et al. [9]. They reported that pretreatment of transfected HEK293 cells with 10 µM AαBA and AKBA inhibited the NF-κB activity by 40.9 ± 9.8% and 76.9 ± 7.6%, respectively. Furthermore they demonstrated that AαBA and AKBA concentration-dependently (1–10 µM) inhibited IKK-mediated phosphorylation. Also the production of the pro-inflammatory cytokines TNF-α and IL-1β were inhibited by AαBA and AKBA in concentrations between 5–20 µM in human monocytes [10]. Previously Moussaieff et al. reported that incensole acetate also inhibited NF-κB activation, but this effect was observed at very high concentrations >100 µM [11].
With regard to the effect of frankincense on inflammatory and anti-inflammatory cytokines, Chervier et al. found out that a gum resin extract from B. carterii at a conc. of 10–200 µg/mL in sesame oil simultaneously inhibited the pro-inflammatory T helper (TH)-1 cytokines IL-2 and interferon (IFN)-γ, and promoted the anti-inflammatory TH-2 cytokines IL-4 and IL-10 in murine splenocytes [12].
Of course these pharmacologically active concentrations determined in vitro should be regarded with caution, as they can strongly vary depending on the assay conditions (e.g., species, cell-type, cell-free or cell-based assays, stimuli etc.) [13]. It was also reported that effects observed in cell assays cannot be reproduced under physiologically relevant conditions in the presence of albumin or in whole blood. Hence KBA efficiently suppressed 5-LO product formation in isolated neutrophils but failed to inhibit 5-LO product formation in human whole blood. This is not surprising, as boswellic acids being lipophilic acids are known to be substantially bound to albumin that is abundantly present in plasma (30–40 mg/mL) [13]. It must be also taken into consideration that the effects may vary depending on whether isolated purified phytoconstituents or the whole extract is tested. Nevertheless the abovementioned pharmacological active concentrations determined in common in vitro assays may serve as first indications for the efficacy of boswellic acids with regard to potential molecular drug-target interactions.
But the final pharmacological relevance of these data can only be correctly estimated when taking into consideration the oral bioavailability in human and the pathological role of individual molecular targets in different inflammatory diseases.
3. Oral Bioavailability in Human
The term bioavailability is used to indicate the fraction of an orally administered dose that reaches the systemic circulation as intact drug, taking into account both absorption and local metabolic degradation [14]. Often the terms “absorption” and “bioavailability” are erroneously used and considered interchangeable. However the absorption process represents only one of the steps involved in the passage of the drug from its site of administration into the systemic circulation [14].
Despite the widespread use of frankincense, only view preliminary pharmacokinetic studies were conducted. In order not to exceed the framework of this review, the focus was set on the pharmacokinetic studies carried out in at least six humans determining more than one boswellic acid. Two studies of Gerbeth et al. [15] and Sterk et al. [16] fulfilled these requirements, the results of which are summarized in Table 1. In the study of Gerbeth et al. the subjects included in a prospective, randomized, placebo-controlled double-blind pilot clinical trial on the effect of Boswellia on cerebral edema were asked to take the Boswellia medication at home [17]. No instructions were given regarding intake of medication or standardization of food. The blood samples were taken during weekly routine medical check-ups, irrespective of the time of medication intake [15]. The randomized, open, single-dose two-way cross over study of Sterk et al. was devoted to investigate the effect of concomitant standardized high-fat meal intake on the bioavailability of boswellic acids [16].
Table 1. Overview on the plasma concentrations reported for the individual boswellic acids in pharmacokinetic studies carried out on at least six humans.
| Dosage of Boswellia Extract | Concentrations of Boswellic Acids in Plasma [µM] | Ref. | |||||
|---|---|---|---|---|---|---|---|
| KBA | AKBA | βBA | AβBA | αBA | AαBA | ||
| 3 × 4 capsules à 350 mg/day for 1 week (n = 14) (in total: KBA 63.6 mg, AKBA 80,4 mg, βBA 2236.8 mg, AβBA 228 mg, αBA 969.6 mg, AαBA 73.2 mg) | 0.01–0.52 | 0–0.03 | 0.19–26.20 | 0.26–12.31 | 0.08–10.59 | 0.14–5.99 | [15] |
| 3 × 282 mg/day—fasted state | 0.17 [0.05–0.52] |
0.01 [0.002–0.08] |
0.4 [0.10–3.9] |
ND | ND | ND | [16] |
| 3 × 282 mg/day—fed state (n = 12) (in total: KBA 48.12 mg, AKBA 28.71 mg, βBA 143.4 mg, AβBA 82.71 mg, αBA 103.71 mg, AαBA 26.25 mg) | 0.48 [0.21–0.9] |
0.06 [0.03–0.52] |
2.5 [0.91–4.7] |
ND in most subjects | 0.69 [0.1–2.9] |
0.24 [0.09–0.8] |
|
Values are expressed as range or as mean [range]. ND = not detected.
Compared to the high doses of Boswellia gum resin extracts that have been orally administered in the above studies, boswellic acids, especially KBA and AKBA, revealed very low bioavailability associated with a very high pharmacokinetic variability. The highest plasma levels could be determined for βBA that is also present at the highest concentration in the extract. Also at steady state the concentrations of the six boswellic acids determined after the oral administration of 4 × 786 mg/day for 10 days in one patient did not exceed 0.3, 0.1, 10.1, 2.4, 3.5 and 4.0 μM for KBA, AKBA, βBA, AβBA, αBA, and AαBA, respectively [18]. This might be attributed to the low aqueous solubility of boswellic acids, their high lipophilicity, gastrointestinal instability, low intestinal absorption, high accumulation within the enterocytes and intestinal metabolism by CYP enzymes as well as saturable kinetics [19]. These observations made in vivo are substantiated by low Papp values <3.00 × 10−6 cm/s determined for β-boswellic acids in the classical Caco-2 model using Hank’s balanced salt solution (HBSS) buffer without sink conditions [19]. However, when adapting the Caco-2 model to physiological conditions by the use of modified fasted state simulated intestinal fluid on the apical side and the addition of bovine serum albumin to the basolateral side in order to simulate sink conditions Papp values of 4.47 (βBA), 6.18 (AβBA), 5.52 (αBA) and 4.72 (AαBA) × 10−6 cm/s could be determined. These Papp values indicate moderate permeability according to Yee [20]. Also the Papp values of KBA and AKBA could be improved yielding Papp values of 29.54 and 17.83 × 10−6 cm/s, respectively, suggesting high permeability. The nevertheless low plasma concentrations of KBA in vivo can be explained by the extensive phase I metabolism observed for KBA and other non-acetylated boswellic acids (βBA and αBA) in human liver microsomes [21][22]. An explanation for the low plasma levels of AKBA might be the initially lower content of that boswellic acid in the extract combined with a greater distribution in different compartments [23]. The initial assumption that AKBA could be deacetylated to KBA following oral administration could not be verified [21].
Considering the pharmacologically active concentration in vitro, only βBA achieved sufficient high plasma concentration in the range of the IC50 values determined for serine protease cathepsin G and mPGES-1 inhibition, favoring a role of βBA as the most relevant anti-inflammatory boswellic acid [24]. At the same time pharmacological relevance of the putative targets of AKBA in vivo remains unclear. Hence most of the pharmacological effects of this boswellic acid, considered as the most potent one, were observed at relatively high concentrations in vitro, which revealed to be far above the plasma levels achieved after oral application of frankincense [25][26]. Unfortunately all pharmacokinetic studies focused on the determination of boswellic acids in plasma, so that no pharmacokinetic data are available for other potentially effective frankincense ingredients like roburic, lupeolic and tirucallic acids.
Based on that background it seems that bioavailability represents a major hurdle in the translation of the pharmacological potential of boswellic acids into therapeutic effects. Therefore several attempts were made to enhance the bioavailability of boswellic acids. As shown by Sterk et al. the bioavailability of boswellic acids could be significantly enhanced by concomitant intake of a fat-meal due to the solubilizing effect of bile acids [16]. Also the plasma concentration of KBA but not AKBA could be increased in 15 male volunteers who were administered a single dose of 800 mg Boswellia extract in fed conditions compared to the plasma levels obtained under fasted conditions. According to this study a significant but clinically infeasible dose increase of 10–15 fold the applied dose is required, to achieve pharmacologically relevant plasma concentrations for AKBA [27].
Further attempts to enhance the bioavailability focused on increasing the solubility of boswellic acids by developing new formulations based on the preparation of boswellic acid phosphatidyl choline complexes [28] and Boswellia serrata extract/phospholipid/pluronic f127 (1:1:1 w/w/w) formulations [29]. The latter formulation resulted in 26- and 14-fold higher plasma levels of KBA and AKBA, respectively, following the administration of 240 mg/kg to rats compared to the non-formulated extract. Also in the brain, 5-fold higher levels of AKBA compared with the non-formulated extract were determined eight hours after oral administration [29]. Casperome™, another formulation of Boswellia serrata extract and Phytosome® (soy lecithin) at a 1:1 ratio revealed increased plasma levels and up to 35-fold higher concentrations of KBA and AKBA in the brain and 17-fold higher boswellic acid levels in poorly vascularized organs in rats [30]. Other formulations reported to lead to significant increase in the bioavailability of boswellic acids include Aflapin (composed of Boswellia serrata extract enriched in AKBA and non-volatile oil portion of B. serrata gum resin) [31], AKBA loaded poly-lactic-co-glycolic acid-nanoparticles [32], other nanotechnological formulations [33], as well as micellar delivery forms [34].
Obviously bioavailability seems to represent the major hurdle in the translation of the preclinical potential of frankincense extracts and boswellic acids into therapeutic effects [23]. Therefore it is worth to take a closer look at the efficacy of frankincense extracts in various preliminary human clinical trials that have been carried out.
4. Clinical Trials
A frequent problem of clinical trials carried out with traditional herbal remedies is their suboptimal or questionable quality, which hampers reliable statements on their clinical activity [1]. Therefore the focus will be set on randomized, double-blind, placebo-controlled clinical trials that used frankincense extract without other medications or other food supplements in diseases associated with inflammation, in order to securely trace back the observed effects to frankincense. An overview of these studies is given in Table 2. Unfortunately almost all clinical trials focused on clinical outcomes without determining inflammatory mediators. According to these studies clinically measurable improvements could be achieved with frankincense extracts enriched with AKBA in osteoarthritis [35][36][37][38][39]. Before Kimmatkar et al. reported decrease in knee pain, increased knee flexion and increased walking distance following the intake of 333 mg of Boswellia serrata oleogum resin with a minimum of 40% total boswellic acids three times a day for 8 weeks [40]. In contrast, a multi-centre controlled trial revealed no measurable effects of H15™ (3600 mg Boswellia serrata extract) in 37 outpatients with rheumatoid arthritis and chronic polyarthritis under constant therapy with steroids and anti-rheumatic drugs [41]. Although it may be assumed, that administration of H15™ to patients already treated with steroids would lead to additional effects, no further trials were conducted on patients with rheumatoid arthritis [10].
With regard to chronic inflammatory diseases no firm conclusion can be drawn regarding the efficacy of Boswellia serrata oleogum resin in the treatment of ulcerative colitis and chronic colitis because of the methodological weakness of the two studies conducted without blinding and randomization [42][43]. The greatest number of patients was included in the study of Gerhardt et al. [44] suggesting comparable efficacy of Boswellia serrata resin extract with mesalazine in Morbus Crohn. Another study with relatively small numbers of patients suggests promising effects of Boswellia serrata resin extract in the treatment of collagenous colitis [45]. But since no more studies were carried out since then, no valid data exist that support the use of frankincense extracts as monotherapy for chronic inflammatory bowel diseases [46].
Unfortunately only one study was carried out on the effect of Boswellia serrata extract in the treatment of asthma bronchiale, which however provided promising results [47]. Moreover Kirste et al. [17] confirmed previous observations made by Streffer at al. [48] as well as Boeker and Winking [49] regarding positive effects of concomitant administration of frankincense extract on cerebral edema associated with radiochemotherapy in patients with malignant glioma.
Table 2. Overview on the randomized, double-blind, placebo-controlled clinical trials addressing the efficacy of frankincense extracts in different diseases associated with inflammation.
| Disease | Study Design | Dosage | Observations | References |
|---|---|---|---|---|
| Osteoarthritis knee | Pilot, randomized, double-blind, placebo-controlled on 48 newly diagnosed or untreated osteoarthritis patients | Self-administration of two tablets à 169.33 mg Boswellia serrata extract enriched in AKBA and βBA (AKBA 53.27 mg, βBA 20.83 mg, KBA 7.11 mg, AβBA 6.06 mg) for 120 days | ↓ pain and stiffness ↑ motility of knee joints ↓ hs-CRP |
[36] |
| Osteoarthritis knee | Pilot, randomized, double-blind, placebo-controlled on 60 patients with mild to moderate osteoarthritis | Aflapin 100 mg per day for 30 days (Aflapin contains Boswellia serrata extract enriched in AKBA with non-volatile oil of Boswellia serrata) | Clinically and statistically significant improvement in pain scores and physical function scores already after 5 days of treatment by ↓ 5-LO and ↓ TNFα | [37] |
| Osteoarthritis knee | randomized, double-blind, placebo-controlled on 60 patients with mild to moderate symptoms | Aflapin 100 mg per day compared to 100 mg 5-Loxin per day for 90 days | Clinically and statistically significant improvement in pain scores and physical functional scores 7 days after start of treatment ↓ TNFα induced cartilage degrading synovial fluid matrix metalloproteinase-3 and ↓ TNFα induced intercellular adhesion molecule (ICAM)-1 expression Aflapin better than 5-Loxin |
[38] |
| Osteoarthritis knee | randomized, double-blind, placebo-controlled on 75 patients with mild to moderate symptoms | 100 mg or 250 mg 5-Loxin (Boswellia serrata extract enriched with 30% AKBA) for 90 days | dose dependant clinically and statistically significant improvement in pain scores and physical functional scores 7 days after start of treatment ↓ TNFα induced synovial fluid matrix metalloproteinase-3 |
[39] |
| Osteoarthritis knee | randomized, double-blind, placebo-controlled on 30 patients | 3 × 333 mg WokVel™ per day for 8 weeks (Boswellia serrata oleogum resin with a minimum of 65% organic acids or a minimum of 40% total boswellic acids) | ↓ knee pain, ↑ knee flexion, ↑ walking distance and ability to climb stairs. After withdrawal of treatment symptoms returned |
[40] |
| Morbus Crohn | randomized double-blind, verum-controlled parallel group on 83 patients | ↓ Crohn’s Disease Activity Index (CDAI) by 90 in the H15 group and by 53 score points after therapy with mesalazine. Difference not statistically significant | [44] | |
| Collagenous Colitis | randomized, double-blind, placebo-controlled multicenter trial on 25 patients | 3 × 400 mg Boswellia serrata resin extract (H15™) per day for 6 weeks | Proportion of patients in clinical remission was higher in the Boswellia group compared to placebo group (63.6% vs. 26.7%). No significant difference in histology or quality of life | [45] |
| Bronchial asthma | double-blind, placebo-controlled on 40 patients | 3 × 300 mg Boswellia serrata oleogum resin extract (S-compound™) per day for 6 weeks | Improvement of disease reflected in disappearance of physical symptoms and different signs as well as decrease in eosinophilic count in 70% of the Boswellia group compared to 27% of the placebo group. | [47] |
| Brain tumors | Prospective pilot, randomized, placebo-controlled double-blind study on 44 patients | 3 × 4 × H15 (350 mg Boswellia serrata extract) starting with the first day of radiotherapy | reduction >75% of cerebral edema in 60% of the patients receiving Boswellia compared to 26% of patients in placebo group | [17] |
↓ stands for decrease and ↑ stands for increase.
5. Conclusions
Definitely frankincense extracts are counted among the most studied herbal medicinal plants that are not authorized as herbal medicinal products. Nevertheless several outstanding issues remain that are essential for estimating the therapeutic efficacy. These issues relate to:
-
possible role of boswellic acids with too high IC50 values that are not achieved in vivo in providing therapeutic effects
-
pharmacokinetic properties of other promising frankincense ingredients i.e., tirucallic, lupeolic and roburic acid
-
the influence of other extract ingredients on the pharmacological activity and efficacy
-
effect of the pharmacological assays applied and experimental conditions on the outcoming results with regard to pharmacological activity and bioavailability
-
the need for more well designed and high quality clinical trials to better underline positive/negative effects already observed
Alltogether Boswellia serrata reflects perfectly the general problems plant research suffers from. While a lot of data exist on possible molecular targets and pharmacologically relevant concentrations in vitro mostly for purified and isolated phytoconstituents, much less information is available on the pharmacokinetics of the tentative active ingredients of medicinal plants and even less information on clinical efficacy and the translation of in vitro data to the clinic. Therefore thought must be given to new methodological approaches that allow a better evaluation of the therapeutic potential of medicinal plants at a very early stage of research.
References
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2020.
- Herrmann, A.; König, S.; Lechtenberg, M.; Sehlbach, M.; Vakhrushev, S.Y.; Peter-Katalinic, J.; Hensel, A. Proteoglycans from Boswellia serrata Roxb. and B. carterii Birdw. and identfication of a proteolytic plant basic secretory protein. Glycobiology 2019, 22, 1424–1439.
- Ammon, H.P.T. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. Adv. Exp. Med. Biol. 2016, 928, 291–327.
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101.
- Koeberle, A.; Henkel, A.; Verhoff, M.; Tausch, L.; König, S.; Fischer, D.; Kather, N.; Seitz, S.; Paul, M.; Jauch, J.; et al. Triterpene Acids from Frankincense and Semi-Synthetic Derivatives That Inhibit 5-Lipoxygenase and Cathepsin G. Molecules 2018, 23, 506.
- Simoneit, U. Anti-Inflammatory Actions of Boswellic Acids: Identification and Critical Evaluation of Molecular Targets and Signaling Pathways. Ph.D. Thesis, Eberhard Karls University, Tuebingen, Germany, 24 April 2009.
- Verhoff, M.; Seitz, S.; Paul, M.; Noha, S.M.; Jauch, J.; Schuster, D.; Werz, O. Tetra- and Pentacyclic Triterpene Acids from the Ancient Anti-inflammatory Remedy Frankincense as Inhibitors of Microsomal Prostaglandin E2Synthase-1. J. Nat. Prod. 2014, 77, 1445–1451.
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inibits invasion and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B regulated gene expression. J. Immunol. 2006, 176, 3127–3140.
- Syrovets, T.; Büchele, B.; Krauss, C.; Laumonier, Y.; Simmet, T. Acetylboswellic acids inhibt lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinase. J. Immunol. 2005, 174, 498–506.
- Ammon, H.P.T. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases. Advances in Experimental Medicine and Biology 928, 1st ed.; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 291–326.
- Moussaieff, A.; Shohami, E.; Kashman, Y.; Fride, E.; Schmitz, M.L.; Renner, F.; Fiebich, B.L.; Munoz, E.; Ben-Neriah, Y.; Mechoulam, R. Incensole Acetate, a novel anti-inflammatory compound isoalted from Boswellia resin, inhibits nuclear factor-κB activation. Mol. Pharmacol. 2007, 72, 1657–1664.
- Chevrier, M.R.; Ryan, A.E.; Lee, D.Y.W.; Zhongze, M.; Wu-Yan, Z.; Via, C.S. Boswellia carterii Extract Inhibits TH1 Cytokines and Promotes TH2 Cytokines In Vitro. Clin. Diagn. Lab. Immunol. 2005, 12, 575–580.
- Siemoneit, U.; Pergola, C.; Jazzar, B.; Northoff, H.; Skarke, C.; Jauch, J.; Werz, O. On the Interference of Boswellic Acids with 5-lipoxygenase: Mechanistic Studies In Vitro and Pharmcological Relevance. Eur. J. Pharmacol. 2009, 606, 246–254.
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400.
- Gerbeth, K.; Meins, J.; Kirste, S.; Momm, F.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Determination of major boswellic acids in plasma by high-pressure liquid chromatography/mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 998–1005.
- Sterk, V.; Büchele, B.; Simmet, T. Effect of Food Intake on the Bioavailability of Boswellic Acids from a Herbal Preparation in Healthy Volunteers. Planta Med. 2004, 70, 1155–1160.
- Kirste, S.; Treier, M.; Wehrle, S.J.; Becker, G.; Abdel-Tawab, M.; Gerbeth, K.; Hug, M.J.; Lubrich, B.; Grosu, A.L.; Momm, F. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors. Cancer 2011, 117, 3788–3795.
- Buechele, B.; Simmet, T. Analysis of 12 different pentacyclic triterpene acids from frankincense in plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 795, 355–362.
- Sharma, T.; Jana, S. Investigation of Molecular Properties that Influence the Permeability and Oral Bioavailability of Major β-Boswellic Acids. Eur. J. Drug Metab. Pharmacokinet. 2019, 45, 243–255.
- Yee, S. In Vitro Permeability across Caco-2 Cells (Colonic) Can Predict In Vivo (Small Intestinal) Absorption in Man: Fact or Myth. Pharm. Res. 1997, 14, 763–766.
- Kruger, P.; Daneshfar, R.; Eckert, G.P.; Klein, J.; Volmer, D.A.; Bahr, U.; Müller, W.E.; Karas, M.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Metabolism of Boswellic Acids In Vitro and In Vivo. Drug Metab. Dispos. 2008, 36, 1135–1142.
- Gerbeth, K.; Hüsch, J.; Fricker, G.; Werz, O.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. In Vitro metabolism, permeation, and brain availability of six major boswellic acids from Boswellia serrata gum resins. Fitoterapia 2013, 84, 99–106.
- Du, Z.; Liu, Z.; Ning, Z.; Liu, Y.; Song, Z.; Wang, C.; Lu, A. Prospects ob boswellic acids as potential pharmaceutics. Planta Med. 2015, 81, 259–271.
- Siemoneit, U.; Koeberle, A.; Rossi, A.; Dehm, F.; Verhoff, M.; Reckel, S.; Maier, T.J.; Jauch, J.; Northoff, H.; Bernhard, F.; et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br. J. Pharmacol. 2010, 162, 147–162.
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and moelcular targets. Curr. Med. Chem. 2006, 13, 3359–3369.
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to non-steroidal anti-inflammatory drugs (NSAIDs): A critical review. Curr. Med. Chem. 2009, 16, 4274–4296.
- Skarke, C.; Kucka, K.; Tausch, L.; Werz, O.; Rossmanith, T.; Barrett, J.S.; Harder, S.; Holtmeier, W.; Schwarz, J.A. Increased bioavailability of 11-keto-beta-boswellic acid following single oral dose frankincense extract administration after a standardized meal in healthy male volunteers: Modeling and simulation considerations for evaluating drug exposures. J. Clin. Pharmacol. 2012, 52, 1592–1600.
- Sharma, A.; Gupta, N.K.; Dixit, V.K. Complexation with phosphatidyl choline as a strategy for absorption enhancement of boswellic acid. Drug Deliv. 2010, 17, 587–595.
- Hüsch, J.; Gerbeth, K.; Fricker, G.; Setzer, C.; Zirkel, J.; Rebmann, H.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Effect of Phospholipid-Based Formulations of Boswellia serrata Extract on the Solubility, Permeability, and Absorption of the Individual Boswellic Acid Constituents Present. J. Nat. Prod. 2012, 75, 1675–1682.
- Hüsch, J.; Bohnet, J.; Fricker, G.; Skarke, C.; Artaria, C.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Enhanced absorption of boswellic acids by a lecithin delivery form (Phytosome®) of Boswellia extract. Fitoterapia 2013, 84, 89–98.
- Sengupta, K.; Kolla, J.N.; Krishnaraju, A.V.; Yalamanchili, N.; Rao, C.V.; Golakoti, T.; Raychaudhuri, S.; Raychaudhuri, S.P. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: A novel Boswellia serrata extract. Mol. Cell. Biochem. 2011, 354, 189–197.
- Bairwa, K.; Jachak, S.M. Development and optimisation of 3-Acetyl-11-keto-β-boswellic acid loaded poly-lactic-co-glycolic acid-nanoparticles with enhanced oral bioavailability and In Vivo anti-inflammatory activity in rats. J. Pharm. Pharmacol. 2015, 67, 1188–1197.
- Mehta, M.; Satijy, S.; Nanda, A.; Garg, M. Nanotechnolgies for boswellic acids. Am. J. Drug Discov. Dev. 2014, 4, 1–11.
- Meins, J.; Behnam, D.; Abdel-Tawab, M. Enhanced absorption of boswellic acids by a micellar solubilized delivery form of Boswellia extract. NFS J. 2018, 11, 12–16.
- Yu, G.; Xiang, W.; Zhang, T.; Zeng, L.; Yang, K.; Li, J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 1–16.
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother. Res. 2019, 33, 1457–1468.
- Vishal, A.A.; Mishra, A.; Raychaudhuri, S.P. A Double Blind, Randomized, Placebo Controlled Clinical Study Evaluates the Early Efficacy of Aflapin® in Subjects with Osteoarthritis of Knee. Int. J. Med. Sci. 2011, 8, 615–622.
- Sengupta, K.; Krishnarju, A.V.; Vishal, A.A.; Mishra, A.; Trimurtulu, G.; Sharma, K.V.S.; Raychaudhuri, S.K.; Raychaudhuri, S.P. Comparative efficacy and tolerability of 5-Loxin and Aflapin against osteoarthritis of the knee: A double blind randomized, placebo controlled clinical study. Int. J. Med. Sci. 2010, 7, 366–379.
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double-blind randomized placebo controlled clinical study of the efficacy of and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85.
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee: A randomized double blind placebo controlled trial. Phytomedicine 2003, 10, 3–7.
- Sander, O.; Herborn, G.; Rau, R. Is H15 (extract of Boswellia serrata “incense”) an efficient supplementation to established drug therapy of rhemuamtoid arthritis? Results of a double-blind pilot trial. Z. Rheumatol. 1998, 57, 11–16.
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Lüdtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res. 1997, 2, 37–43.
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Lüdtke, R.; Safayhi, H.; Ammon, H.P.T. Effects of Boswellia serrata gum resin in patients with chronic colitis. Planta Med. 2001, 67, 391–395.
- Gerhardt, H.; Seifert, F.; Buvari, P.; Vogelsang, H.; Repges, R. Therapy of active Crohn Disease with Boswellis serrata extract H15. Z. Gastroenterol. 2001, 39, 11–17.
- Madisch, A.; Miehlke, S.; Eichele, O.; Mrwa, J.; Bethke, B.; Kuhlisch, E.; Bästlein, E.; Wilhelms, G.; Morgner, A.; Wigginghaus, B.; et al. Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int. J. Color. Dis. 2007, 22, 1445–1451.
- Parian, A.; Limketkai, B.N. Dietary supplement therapies for inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Curr. Pharm. Dis. 2016, 22, 180–188.
- Gupta, I.; Gupta, V.; Parihar, A.; Gupta, S.; Lüdtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with bronchial asthma: Results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res. 1998, 3, 511–514.
- Streffer, J.; Bitzer, M.; Schabet, M.; Dichgans, J.; Weller, M. Response of radiochemotherapy-associated cerebral edema to a phytotherapeutic agent, H15. Neurology 2001, 56, 1219–1221.
- Boeker, D.K.; Winking, M. The role of boswellic acids in therapy of malignant glioma. Dt. Aerztebl. 1997, 94, 1197–1199.
