Crystallization-driven self-assembly (CDSA) represents a highly versatile method for the production of well-defined block copolymer micelles in solution giving access to numerous tailor-made one-, two- and three-dimensional assemblies with controlled length, length distribution, shape, and corona chemistries. One special example of micelles derived by CDSA are the so-called patchy micelles, which possess a corona made of alternating nanometer-sized compartments. These patchy micelles show superior interfacial activity making them excellent candidates for the use as compatibilizers or metal (oxide) nanoparticle templates.
- crystallization-driven self-assembly (CDSA)
- crystalline-core micelles
- patchy micelles
1. Surface Compartmentalized Micelles
The solution self-assembly of block copolymers (BCPs) has paved the way to a vast number of micellar assemblies of various shapes (e.g. spheres, cylinders, vesicles, platelets, core-shell, core-shell-corona, and compartmentalized (core or corona) structures) and hierarchical superstructures, as well as hybrids with fascinating applications in drug delivery and release, as emulsifiers/blend compatibilizers, in nanoelectronics, as responsive materials (temperature, pH, light), templates for nanoparticles, in heterogeneous catalysis, etc. [1][2][3][4][5][6][1,2,3,4,5,6]. A key prerequisite for controlling/programming the solution self-assembly is the synthesis of well-defined diblock and triblock (linear, star-shaped, ABA- or ABC-type) copolymers via controlled or living polymerization techniques, such as living anionic polymerization, reversible addition−fragmentation chain transfer, nitroxide-mediated, and atom transfer radical polymerization [5][6][7][8][9][5,6,7,8,9]. In general, anisotropic polymer micelles can be divided into three main categories: multicompartment core micelles (MCMs), surface-compartmentalized micelles, and a combination of both [2]. MCMs are generally defined as micellar assemblies with a solvophilic corona and a microphase-separated solvophobic core. According to the suggestion of Laschewsky et al., a key feature of multicompartment micelles is that the various sub-domains in the micellar core feature substantially different properties to behave as separate compartments [10][11][10,11]. MCMs are commonly prepared via hierarchical self-assembly of suitable building blocks, which provide “sticky patches” [12][13][14][15][12,13,14,15]. Depending on the number and geometrical arrangement (linear, triangular, tetrahedral, etc.) of the “sticky patches”, as well as the volume fraction of the solvophilic block, various spherical, cylindrical, sheet-like, and vesicular MCMs are accessible [16][17][18][19][20][21][22][23][24][25][16,17,18,19,20,21,22,23,24,25]. For a deeper insight into this highly relevant topic, the reader is referred to recent extensive reviews on MCMs [26][27][28][29][30][31][26,27,28,29,30,31]. Surface-compartmentalized micelles are subdivided into micelles with a Janus-type (two opposing faces with different chemistry or polarity) or patch-like, microphase-separated corona, featuring several compartments of different chemistry or polarity (denoted as patchy micelles), as illustrated in FigureFigure 1 1 for cylindrical micelles. Here, block co-micelles with a block-like arrangement of several (>2) surface compartments along the cylindrical long axis can be regarded as a special case of patchy micelles. It is noted that AB-type diblock co-micelles also represent Janus-type micelles, where the two opposing faces are arranged perpendicular to the cylindrical long axis. The broken symmetry of Janus particles offers efficient and distinctive means of targeting complex materials by hierarchical self-assembly and realize unique properties and applications, like particulate surfactants, optical nanoprobes, biosensors, self-propulsion, and many more [32][33][34][35][36][37][38][39][40][41][32,33,34,35,36,37,38,39,40,41].

Figure 1.
Schematic depiction of a cylindrical (
a
) Janus micelle, (
b
) block co-micelle, and (
c
) patchy micelle.
For the preparation of patchy micelles and polymersomes from amorphous BCPs, three main strategies can be applied: (i) self-assembly of ABC triblock terpolymers in selective solvents for the incompatible A and C blocks [42][43][44][45][46][47][48][42,43,44,45,46,47,48]; (ii) co-assembly of AB and CD diblock copolymers with selective interactions between the B and C blocks (e.g. hydrogen bonding, ionic interactions, solvophobic interactions) [49][50][51][52][49,50,51,52], resulting in patchy micelles with an insoluble mixed B/C core; and (iii) co-assembly of AB and BC diblock copolymers [53][54][55][56][53,54,55,56] where the B block forms the insoluble core. However, mostly spherical micelles or polymersomes with a patchy corona have been reported and only a few reports describe the preparation of one-dimensional (worm-like, cylindrical) assemblies with a patch-like compartmentalized corona, even though theoretical work on mixed polymer brushes predict their existence [57][58][59][60][61][57,58,59,60,61]. One of the rare but highly intriguing examples are PtBA–b–PCEMA–b–PGMA (poly(tert-butyl acrylate)–block–poly(2-cinnamoyloxyethyl methacrylate)–block–poly(glyceryl monomethacrylate)) and PnBA–b–PCEMA–b–PtBA (PnBA: poly(n-butyl acrylate)) triblock terpolymers [42][43][45][42,43,45]. For self-assembly, the triblock terpolymers were first dissolved in a good solvent for all blocks (CH2Cl2, CHCl3, or THF), followed by the addition of methanol (non-solvent for the middle block) to induce micelle formation. As an intermediate, cylindrical micelles with a patchy corona were formed first, with the PtBA blocks forming small circular patches in a corona mainly consisting of PGMA or PnBA. Upon further decreasing the solvent quality for the PtBA block (addition of MeOH), these cylinders can form double and triple helices via hierarchical self-assembly. This concept has also been applied to triblock terpolymers with a poly(2-hydroxyethyl methacrylate) middle block, having the potential for further modification by esterification of the pendant hydroxy functions [42]. Besides, crystallization-driven self-assembly (CDSA) is a highly versatile tool for the preparation of well-defined cylindrical micelles of controlled length and length distribution, and has proven as a valuable method for the preparation of patchy cylindrical micelles.
2. Crystallization-Driven Self-Assembly (CDSA)
As pointed out in the introduction, the preparation of one-dimensional (1D) cylindrical (or worm-like) micelles with controlled dimensions, low-length dispersities, and tailored corona structures and functionalities still remains a challenge in the self-assembly of fully amorphous BCPs. Besides, the introduction of a crystallizable block, which adds an additional and strong driving force for micelle formation, has turned out to be a highly efficient route to solve these issues. Consequently, the self-assembly of such BCPs, bearing crystallizable blocks, is termed crystallization-driven self-assembly (CDSA) [1][62][63][1,62,63]. This field was pioneered by studies on poly(ferrocenyl dimethylsilane) (PFS)-containing BCPs and is gaining increasing importance for the preparation of well-defined 1D and two-dimensional (2D) assemblies, especially since the discovery of living CDSA (Figure 2) [63][64][65][66][67][63,64,65,66,67]. Analogous to the living polymerization of monomers, CDSA can proceed in a living manner, employing small micellar fragments as seeds (Figure 2a: seeded growth) for the addition of unimers (molecularly dissolved BCPs with a crystallizable block). In this approach, the micellar seeds, also termed “stub-like” micelles, are produced by vigorous sonication of long, polydisperse cylindrical micelles prepared by conventional CDSA. Owing to its living nature, the length of the produced cylindrical micelles shows a linear dependence on the unimer/seed ratio employed, and length dispersities are very low (Lw/Ln typically well below 1.1; where Ln is the number average and Lw the weight average micelle length).
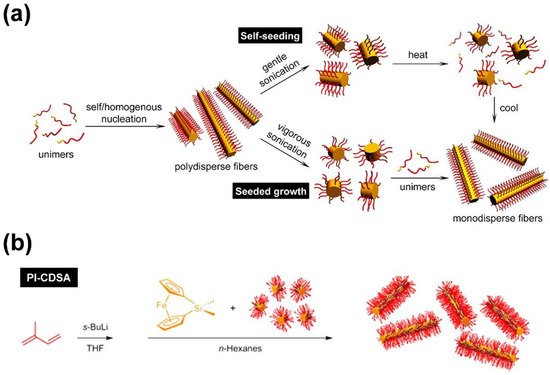
Figure 2. (a) Concepts for living CDSA, enabling the production of cylindrical micelles with defined length and narrow length distribution. Self-seeding employing seeds produced by thermal treatment of micelle fragments (top) and seeded growth using small micellar fragments (“stub”-like micelles) as seeds (bottom). (b) Living polymerization-induced CDSA (PI-CDSA) utilizing micellar seeds during anionic polymerization of the PFS block. After complete conversion, the reaction was quenched with 4-tert-butylphenol. (a) Reproduced from [68][76] with permission of the American Chemical Society (ACS).
Living CDSA can also be realized by using spherical CCMs as seeds [69][68], by self-seeding [70][71][72][69,70,71] (Figure 2a), and even directly by polymerization-induced CDSA (Figure 2b) [73][74][75][72,73,74], i.e., via polymerization in the presence of seed micelles. The self-seeding approach also uses small micellar fragments that are heated in dispersion to a specific annealing temperature (Ta), where most of the crystalline core is molten/dissolved and only a very minor fraction of crystallites survive. These act as seeds in the subsequent CDSA upon cooling (Figure 2a: self-seeding), and the length of the micelles can be controlled by a proper choice of Ta. If Ta is too low, the crystalline cores will not melt/dissolve, and the length distribution of the employed micellar fragments remains unchanged. On the other hand, if Ta is too high, the crystalline cores will melt/dissolve completely, and no crystallites will survive that could act as seeds. As a result, in between these two limiting cases, an increase in micelle length with increasing Ta is observed, as the fraction of surviving crystallites (seeds) decreases with Ta. This range of self-seeding temperatures can be very restricted, making length control difficult. Another drawback of these seed-based protocols is the low amount of cylindrical micelles that can be produced, as commonly rather dilute solutions have to be used. This can be overcome by the living polymerization-induced CDSA approach, enabling the production of uniform cylindrical micelles with concentrations up to ca. 10–20% (w/w solids) within a few hours. In a recent report, it was shown that living CDSA can even be stimulated by light, utilizing the photo-induced cis-trans isomerization in oligo(p-phenylenevinylene) (OPV)-based BCPs [76][75].
Living CDSA has paved the way to a myriad of 1D and 2D micellar assemblies of controlled dimensions, including patchy and block co-micelles (both will be addressed in the next sections) [65][69][77][78][79][80][65,68,77,78,79,80], branched micelles [68][76], platelet-like micelles and co-micelles [81][82][83][84][85][86][81,82,83,84,85,86], and hierarchical assemblies [81][87][88][89][90][91][81,87,88,89,90,91]. Next to BCPs with a PFS block, a variety of other crystallizable polymer blocks were employed in CDSA, e.g. polyethylene (PE) [69][92][93][94][68,92,93,94], poly(ethylene oxide) [95], polyesters (poly(ε-caprolactone) (PCL) or poly(L-lactide) (PLLA)) [86][96][97][98][99][100][101][86,96,97,98,99,100,101], polycarbonate [102], poly(2-iso-propyl-2-oxazoline) (PiPrOx) [103][104][103,104], liquid crystalline polymers [72][105][71,105], poly(vinylidene fluoride) [106], polypeptoids [107][108][107,108], and various conjugated polymers (e.g., poly(3-hexyl thiophene) (P3HT) and OPV) [76][109][110][111][112][113][75,109,110,111,112,113].
