Medication-related osteonecrosis of the jaw (MRONJ) has become a well-known side effect of antiresorptive, and antiangiogenic drugs commonly used in cancer management.
- medication-related osteonecrosis of the jaw
- MRONJ
- bisphosphonates
- denosumab
- infection
1. Definition
According to the updated 2014 AAOMS position paper (modified from 2009), in order to distinguish MRONJ, the working definition claims patients may be considered to have MRONJ if all the following characteristics are present:
- 1. Current or previous treatment with antiresorptive or antiangiogenic agents.
- 2. Exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than 8 weeks.
- 3. No history of radiation therapy to the jaws or obvious metastatic disease to the jaws.[7]
Osteonecrosis, or localized death of bone tissue, of the jaws, is a rare potential complication in cancer patients receiving treatments including radiation, chemotherapy, or in patients with tumors or infectious embolic events. In 2003,[9][10] reports surfaced of the increased risk of osteonecrosis in patients receiving these therapies concomitant with intravenous bisphosphonate.[11] Matrix metalloproteinase-2 may be a candidate gene for bisphosphonate-associated osteonecrosis of the jaws, since it is the only gene known to be associated with both bone abnormalities and atrial fibrillation, another side effect of bisphosphonates.[12] In response to the growing base of literature on this association, the United States Food and Drug Administration issued a broad drug class warning of this complication for all bisphosphonates in 2005.[13]
2. Signs and Symptoms
Classically, MRONJ will cause an ulcer or areas of necrotic bone for weeks, months, or even years following a tooth extraction.[14] While the exposed, dead bone does not cause symptoms these areas often have mild pain from the inflammation of the surrounding tissues.[15] Clinical signs and symptoms associated with, but not limited to MRONJ, include:
- Jaw pain and neuropathy[16]
- Loose teeth[17]
- Mucosal swelling[17]
- Erythema
- Suppuration[17]
- Soft tissue ulceration[17] persisting for more than 8 weeks[18]
- Trismus[17]
- Non-healing extraction sockets[17]
- Paraesthesia or numbness in the jaw[19]
- Bad breath
- Exposed necrotic jaw bone[15]
3. Cause
Cases of MRONJ have also been associated with the use of the following two intravenous and three oral bisphosphonates, respectively: zoledronic acid and pamidronate and alendronate, risedronate, and ibandronate.[20][21] Despite the fact that it remains vague as to what the actual cause is, scientists and doctors believe that there is a correlation between the necrosis of the jaw and time of exposure to bisphosphonates.[22] Causes are also thought to be related to bone injury in patients using bisphosphonates as stated by Remy H Blanchaert in an article about the matter.
Risk factors
The overwhelming majority of MRONJ diagnoses, however, were associated with intravenous administration of bisphosphonates (94%). Only the remaining 6% of cases arose in patients taking bisphosphonates orally.[6] Although the total United States prescriptions for oral bisphosphonates exceeded 30 million in 2006, less than 10% of MRONJ cases were associated with patients taking oral bisphosphonate drugs.[23] Studies have estimated that BRONJ occurs in roughly 20% of patients taking intravenous zoledronic acid for cancer therapy and in between 0–0.04% of patients taking orally administered bisphosphonates.[24] Owing to prolonged embedding of bisphosphonate drugs in the bone tissues, the risk for MRONJ is elevated even after stopping the administration of the medication for several years.[25] Patients who stopped taking anti-angiogenic drugs are exposed to the same risk as patients who have never taken the drugs because anti-angiogenic drugs do not normally reside in the body for a long period of time.[8] Risk factors include:[8]
- Dental treatment (e.g. dentoalveolar surgery/procedure that impacts bone) – it is possible for MRONJ to occur spontaneously without any recent invasive dental treatment
- Duration of bisphosphonate drug therapy – increased risk with increased cumulative dose of drug
- Other concurrent medication – use of chronic systemic glucocorticoid increases risk when they are taken in combination with anti-resorptive drugs
- Dental implants
- Drug holidays – no evidence to support a reduction in MRONJ risk if patients stop taking bisphosphonates temporarily/permanently, as drugs can persist in skeletal tissues for many years
- Treatment in the past with anti-resorptive/anti-angiogenic drugs
- Patient being treated for cancer – higher risk
- Patients being treated for osteoporosis/non-malignant bone diseases (e.g. Paget's disease) – lower risk
Research findings
‘The risk of MRONJ after dental extraction was significantly higher in patients treated with ARD (antiresorptive drugs) for oncological reasons (3.2%) than in those treated with ARD for OP (osteoporosis) (0.15%) (p < 0.0001). Dental extraction performed with adjusted extraction protocols decreased MRONJ development significantly. Potential risk indicators such as concomitant medications and pre-existing osteomyelitis were identified.’[26]
Patient risk categories
Low:[8]
- Treatment of osteoporosis or non-malignant bone disease with oral bisphosphonates for <5 years (not taking systemic glucocorticoids)
- Treatment of osteoporosis or non-malignant bone disease with quarterly/yearly infusions of intravenous bisphosphonates for <5 years (not taking systemic glucocorticoids)
- Treatment of osteoporosis or non-malignant bone disease with denosumab (not taking systemic glucocorticoids)
High:
- Patients being treated for osteoporosis or non-malignant bone disease with oral bisphosphonates/quarterly or yearly infusions of intravenous bisphosphonates for >5 years
- Patients being treated for osteoporosis or non-malignant bone disease with bisphosphonates/denosumab for any length of time as well as being treated with systemic glucocorticoids
- Patients being treated with anti-resorptive/anti-angiogenic drugs/both as part of cancer management
- Previous MRONJ diagnosis
"N.B. Patients who have taken bisphosphonate drugs at any time in the past and those who have taken denosumab in the last nine months are allocated to a risk group as if they are still taking the drug."[8]
Anti-resorptive drugs
Anti-resorptive drugs inhibit osteoclast differentiation and function, slowing down the breakdown of bone.[27] They are usually prescribed for patients with osteoporosis or other metastatic bone diseases[clarification needed], such as Paget's disease, osteogenesis imperfecta and fibrous dysplasia.[28][29] The two main types of anti-resorptive drugs are bisphosphonate and denosumab. These drugs help to decrease the risk of bone fracture and bone pain. Because the mandible has a faster remodeling rate compared to other bones in the body, it is more affected by the effects of these drugs.[30]
- Bisphosphonate
- Bisphosphonates are either administrated orally or intravenously. They reduces bone resorption.[31]
- Mechanism of action: Bisphosphonate binds to the mineral component of the bone and inhibits enzymes (i.e. farnesyl-pyrophosphate synthase) responsible for bone formation, osteoclast recruitment and osteoclast function.[29][31]
- This type of drug has a high affinity for hydroxyapatite[28] and stays in bone tissue for a long period of time,[29] with alendronate, it has a half-life of approximately ten years.[30]
- The risk of a patient having MRONJ after discontinuing this medication is unknown.[30]
- There are suggestions that bisphosphonate may inhibit the proliferation of soft tissue cells and increases apoptosis. This may result in delayed soft tissue healing.[30]
- Examples of bisphosphonates: : Zoledronic acid (Reclast, Zometa), Risedronate (Actonel), Alendronate (Fosamax), Etidronate (Didronel), Ibandronate (Boniva), Pamidronate (Aredia), Tiludronate (Skelid).[32]
- Denosumab
- Denosumab is a monoclonal antibody[33][34] which is administrated subcutaneously. It inhibits osteoclast differentiation and activation, reduces bone resorption, improves bone density and lessens skeletal-related events associated with metastasis.[31]
- Mechanism of action: The drug binds to receptor activator nuclear factor κB ligand (RANKL), preventing the interaction with RANK.[33][31][34]
- It does not bind to bone and its effect on bone diminishes in 9 months.[30]
Anti-angiogenic drugs
Osteonecrosis of the jaw has been identified as one of the possible complications of taking anti-angiogenic drugs; the association of the disease with the medication is known as MRONJ. This has been stated in the Drug Safety Updates by the MHRA.[8] Angiogenesis inhibitors interfere with blood vessel formation by interfering with the angiogenesis signalling cascade. They are used primarily to treat cancer. These cancer-fighting agents tend to hinder the growth of blood vessels that supply the tumour, rather than killing tumour cells directly.[35] They prevent the tumour from growing. For example, bevacizumab/aflibercept is a monoclonal antibody that specifically binds to vascular endothelial growth factor (VEGF), preventing VEGF from binding to receptors on the surface of normal endothelial cells.[36] Sunitinib is a different example of an anti-angiogenic drug; it inhibits cellular signalling by targeting multiple receptor tyrosine kinases. It reduces the blood supply to the tumour by inhibiting new blood vessel formation in the tumor.[37] The tumour may stop growing or even shrink.[38]
4. Pathogenesis
Although the methods of action are not yet completely understood, it is hypothesized that medication-associated osteonecrosis of the jaw is related to a defect in jaw bone healing and remodelling. The inhibition of osteoclast differentiation and function, precipitated by drug therapy, leads to decreased bone resorption and remodelling.[31][39] Evidence also suggests bisphosphonates induce apoptosis of osteoclasts.[40] Another suggested factor is inhibition of angiogenesis due to bisphosphonates; this effect remains uncertain.[41][42][43] Several studies have proposed that bisphosphonates cause excessive reduction of bone turnover, resulting in a higher risk of bone necrosis when repair is needed.[44][45][46] It is also thought that bisphosphonates bind to osteoclasts and interfere with the remodeling mechanism in bone. To be more specific, the drug interferes with the cholesterol biosynthesis pathway through the inhibition of farnesyl diphosphate synthase. Over time, the cytoskeleton of the osteoclasts loses its function and the essential border[clarification needed] needed for bone resorption does not form.[7] Like aminobisphosphonates, bisphosphonates have shown to have antiangiogenic properties. Therefore, effects include an overall decrease in bone recycling/turnover as well as an increased inhibition of the absorptive bone abilities[clarification needed].
One theory is that because bisphosphonates are preferentially deposited in bone with high turnover, it is possible that the levels of bisphosphonate within the jaw are selectively elevated. To date, there have been no reported cases of bisphosphonate-associated complications within bones outside the craniofacial skeleton.[13]
5. Diagnosis
A diagnosis of bisphosphonate-associated osteonecrosis of the jaw relies on three criteria:[6]
- the patient possesses an area of exposed bone in the jaw persisting for more than 8 weeks,
- the patient must present with no history of radiation therapy to the head and neck
- the patient must be taking or have taken bisphosphonate medication.
According to the updated 2009 BRONJ Position Paper published by the American Association of Oral and Maxillofacial Surgeons, both the potency of and the length of exposure to bisphosphonates are linked to the risk of developing bisphosphonate-associated osteonecrosis of the jaw.[47] In the 2014 AAOMS update[7] on MRONJ, a staging and treatment strategies table was created:
| MRONJ Staging | Criteria* (>8 weeks) | Treatment Strategies** | Picture |
|---|---|---|---|
| At risk | No apparent necrotic bone in patients who have been treated with either oral or IV bisphosphonates | No treatment indicated, Patient education | N/A |
| Stage 0 | No clinical evidence of necrotic bone, but non-specific clinical findings, radiographic changes and symptoms | Systemic management, including the use of pain medication and antibiotics | N/A |
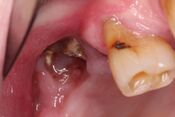
| Stage 1 | Exposed and necrotic bone, or fistulae that probes to bone, in patients who are asymptomatic and have no evidence of infection | Antibacterial mouth rinse, Clinical follow-up on a quarterly basis, Patient education and review of indications for continued bisphosphonate therapy |  |
| Stage 2 | Exposed and necrotic bone, or fistulae that probes to bone, associated with infection as evidenced by pain and erythema in the region of the exposed bone with or without purulent drainage | Symptomatic treatment with oral antibiotics, Oral antibacterial mouth rinse, Pain control, Debridement to relieve soft tissue irritation and infection control |  |
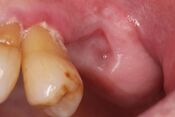
| Stage 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and one or more of the following: exposed and necrotic bone extending beyond the region of alveolar bone,(i.e., inferior border and ramus in the mandible, maxillary sinus and zygoma in the maxilla) resulting in pathologic fracture, extra-oral fistula, oral antral/oral nasal communication, or osteolysis extending to the inferior border of the mandible of sinus floor | Antibacterial mouth rinse, Antibiotic therapy and pain control, Surgical debridement/resection for longer term palliation of infection and pain |   |
6. Prevention
Tooth extraction is the major risk factor for development of MRONJ. Prevention including the maintenance of good oral hygiene, comprehensive dental examination and dental treatment including extraction of teeth of poor prognosis and dentoalveolar surgery should completed prior to commencing any medication which is likely to cause osteonecrosis (ONJ). Patients with removable prostheses should be examined for areas of mucosal irritation. Procedures which are likely to cause direct osseous trauma, e.g. tooth extraction, dental implants, complex restoration, deep root planning, should be avoided in preference of other dental treatments. There are limited data to support or refute the benefits of a drug holiday for osteoporotic patients receiving antiresorptive therapy. However, a theoretical benefit may still apply for those patients with extended exposure histories (>4 yr), and current recommendations are for a 2 month holiday for those at risk.[7] There was low quality evidence suggesting taking antibiotics prior to the dental extraction, as well as the use of post operative techniques for wound closure lowered the risk of patients developing medication-related osteonecrosis of the jaw compared with the usual standard care received for regular dental extractions. Post operative wound closure has been suggested to prevent the contamination of the underlying bone. More evidence is needed to assess the use of antibiotics prior to treatment and the use of wound closure to prevent contamination of the bone, as the quality of evidence evaluated was low.[49]
7. Management
MRONJ is an adverse reaction which can occur as a result of medicines used to treat cancer and osteoporosis.[49] Some medications which induce these effects are bisphosphonates, denosumab and antiangiogenic agents. They involve the destruction of bone in a progressive manner, particularly associated with the mandible or maxilla. The overall effects depend on which drug is being used, the dose and the duration of taking this drug. MRONJ is associated with significant severe disease, negative affects on the quality of life and remains to be increasingly challenging to treat.[50] It is debate whether the various management techniques used for MRONJ are effective.[51] The management of people taking the drugs of concern undergo initial management and continuing management. Before either of these are considered the patient must be as dentally fit as possible. Treatment usually involves antimicrobial mouth washes and oral antibiotics to help the immune system fight the attendant infection, and it also often involves local resection of the necrotic bone lesion. Many patients with MRONJ have successful outcomes after treatment, meaning that the local osteonecrosis is stopped, the infection is cleared, and the mucosa heals and once again covers the bone. The treatment the person receives depends on the severity of osteonecrosis of the jaw.
Conservative
Indicated in patients who have evidence of exposed bone but no evidence of infection. It may not necessarily eliminate all the lesions, but it may provide patients with a long term relief. This approach involves a combination of antiseptic mouthwashes and analgesics and the use of teriparatide.[52] However, note that the teriparatide treatment should not be used in cancer patients, or patients with a history of skeletal radiation or active bone metastases. Splints may be used to protect sites of exposed necrotic bone.
Non-surgical
Indicated for people with exposed bone with symptoms of infection. This treatment modality may also be utilised for patients with other co-morbidities which precludes invasive surgical methods. This approach requires antimicrobial mouthwashes, systemic antibiotics and antifungal medication and analgesics.[53]
Surgery
Surgical intervention is indicated in patients with symptomatic exposed bone with fistula formation and one or more of the following: exposed and necrotic bone extending beyond the alveolar bone resulting in pathological fracture; extra-oral fistula; oral antral communication or osteolysis extending from the inferior border of the mandible or the sinus floor. Surgical management involves necrotic bone resection, removal of loose sequestra of necrotic bone and reconstructive surgery. The objective of surgical management is to eliminate areas of exposed bone to prevent the risk of further inflammation and infection. The amount of surgical debridement required remains controversial.
Other
Initial management
This involves patients who are about to start, or very recently have started, taking the drugs of concern. There is a small portion of observational studies which promote the idea of preventative dental treatment to decrease oral complications in patients taking these drugs. These preventative measures may require a change in the patients’ oral hygiene technique and lifestyle factors such as smoking and alcohol consumption. There is also a benefit in prescribing high fluoridated toothpaste if the patient is of high caries risk. Before prescribing of any kind or when noticing a patient is on the treatment already, it is encouraged to tell the patient of the risk of developing MRONJ, although this risk is small. This is followed by personalised advice given to the patient, involving: a healthy diet, excellent oral hygiene, stop smoking, limited alcohol consumption and regular dental appointments. If a patient has a complex medical history and is of particular high risk it is advised before any treatment to commence, communication with a specialist with regards to the clinical assessment and treatment plan. It is also advised for individuals who take bisphosphonates to never allow the tablet to dissolve in the mouth as this causes damage to the oral mucosa. The patient must follow the instructions given with the tablets.
Continuing management
This involves patients who have a regime which actively incorporates the drugs of concern and also for the patients whom undergone initial management. In terms of dental treatment all must be done as normal, accompanied by personalised advice to the patient. If there is a need for an extraction or any procedure which implicates bone a discussion with the patient about the risks and benefits must occur. Due to bacterial resistance and possible side effects of antibiotic therapy, they are only prescribed if there is a necessity for them. There is minimal evidence to say the use of prophylactic antibiotics will reduce MRONJ.[8] For some, it is possible to have a drug holiday during which bisphosphonates are discontinued if the benefit of discontinuing the drug outweighs the risks. If it is possible to have a drug holiday, it is recommended that treatment be carried out during that period. Some patients however have been taking the drug for a prolonged period of time and so the bisphosphonate levels have accumulated in the body. In this case, a drug holiday would be of no benefit.[56] Medical management of MRONJ is most commonly performed for patients who have less severe cases or those whom have contraindicating health conditions. The antimicrobials therapies commonly used are topical, oral and intravenous.
Topical antimicrobials
A commonly used medicament, chlorhexidine gluconate 0.12% is bacteriostatic and bacteriocidal making an effective agent against MRONJ. Advantages of this topical gel is the low cost, ease of use, availability and patient acceptance. The disadvantages of this are the low compliance, patient acceptance, dental staining and risk of opportunistic bacterial resistance. For some patients, it is possible to have a drug holiday during which bisphosphonates are discontinued if the benefit of discontinuing the drug outweighs the risks. If it is possible to have a drug holiday, it is recommended that treatment be carried out during that period. Some patients however have been taking the drug for a prolonged period of time and so the bisphosphonate levels have accumulated in the body. In this case, a drug holiday would be of no benefit.
Antibiotics
The use of these are based on the clinical evaluation of the condition and if pathogenic bacteria presence is indicated. This is generally a 2-week course for a person with a persistent presentation of the disease or a 4-6 week course for more severe cases. Penicillin is the first line of choice, although if this is contraindicated commonly used antimicrobials are: clindamycin, fluoroquinolones and/or metronidazole. Intravenous antibiotics may be used when specific infections are resist oral therapies. Although this method has perceived greater penetration of tissue there is little evidence of being a substantial greater efficacy when compared to other methods of management.[57]
8. Epidemiology
The likelihood of this condition developing varies widely from less than 1/10,000 to 1/100, as many other factors need to be considered, such as the type, dose and frequency of intake of drug, how long it has been taken for, and why it has been taken.[58] In patients taking drugs for cancer, the likelihood of MRONJ development varies from 0 - 12%. This again, varies with the type of cancer, although prostate cancer and multiple myeloma are reported to be at a higher risk.[8] In patients taking oral drugs for osteoporosis, the likelihood of MRONJ development varies from 0 - 0.2%.[7]
1. Introduction
Bisphosphonates are stable analogues of inorganic pyrophosphate (PPi) which bind to hydroxyapatite crystals at sites of active bone remodeling. They impair intracellular signaling in osteoclasts interfering with osteoclast-mediated bone resorption and therefore are considered one of the most effective antiresorptive drugs (ARDs). Despite the great benefits of bisphosphonates in management of bone metastasis and osteoporosis, a rare but serious side effect, osteonecrosis of the jaw, was reported in 2003. Since then, the number of reported cases has increased dramatically to the point that a causal link has been established between osteonecrosis of the jaw and bisphosphonates intake and the condition was then named bisphosphonate-related osteonecrosis of the jaw (BRONJ). Maxillofacial surgeons observed this complication and thus, a position paper was developed by the American Association of Maxillofacial Surgery (AAOMS) in 2007 to set strategies for treatment and prevention of BRONJ [1][2].
In 2010, osteonecrosis of the jaw was reported in association with the new antiresorptive, denosumab, a monoclonal antibody against the receptor activator of nuclear factor-κB ligand (RANKL) [3]. The same complication was also observed after administration of antiangiogenic medications and tyrosine kinase inhibitors, however there is still little scientific evidence to confirm the association between these medications and osteonecrosis of the jaw [4]. Based on the increasing number of medications that cause osteonecrosis of the jaw, AAOMS proposed the name medication-related osteonecrosis of the jaw (MRONJ) in its last position paper in 2014 in which MRONJ was defined as exposed bone in the jaws or the maxillofacial region that persisted for a minimum period of two months in a patient who has a history of current or previous ARDs or antiangiogenic agents in absence of radiotherapy or metastasis to the jaw [5]. However, recently, the definition of MRONJ has been updated being not only due to the intake of bisphosphonate drugs (current or past), but also further pharmacological therapies such as other antiresorptive agents, or drugs with anti-angiogenic activity. It is also important to perform a thorough physical examination and medical history, together with targeted radiologic examinations. Furthermore, it is important to take into the account not only the presence of exposed necrotic bone but consider also other clinical signs and first/second-level imaging and consider that pain may not always be present in MRONJ cases, especially in the early stages as well as considering that some cases of MRONJ can arise from the presence of dental–periodontal diseases or spontaneously, without any relation to invasive dental procedures [6].
Despite the enormous research efforts made in relation to MRONJ, its pathogenesis is still not fully elucidated. Many hypotheses have been postulated, such as suppression of bone remodeling, inhibition of angiogenesis, constant microtraumas and local infection. However, none of them can fully explain the exact mechanism of MRONJ development and, generally, the process seems to be multifactorial [5]. Clinical observations support ARDs type, dose and frequency as absolute risk factors for MRONJ [5]. Nevertheless, MRONJ does not occur in all patients under ARDs and it is clear that other factors are involved in its onset. Indeed, a considerable and growing body of evidence has accumulated over the last few years and suggests a substantial role of local infection of the jaw bones in initiation of MRONJ. Management of MRONJ can be quite complex and challenging and it is totally agreed that prevention is the best way to face MRONJ, especially in cancer patients under long-term antiresorptive therapy. For this reason, it is crucial to develop a clear understanding of the exact pathophysiology of MRONJ to aid in implementing preventive measures before and after ARDs administration.
2. Summary
Many mechanisms have been suggested, including over-suppression of bone remodelling, anti-angiogenic properties or direct tissue toxicity of antiresorptive drugs, hyper-occlusal forces and local infection. All of the proposed mechanisms might play a part; however, local infection of the jawbone seems to play a major role in the pathogenesis of MRONJ [7][8]. Recently, other etiopathogenetic theories have been suggested such as the effect of ARDs on mesenchymal precursors of components of the periodontium and alveolar bone [9]. Indeed, no other bone in the human body is as frequently affected by infections compared to the jawbones: more than 50% of young adults suffer from moderate to severe periodontitis. The risk increases with aging to reach 75% of the population aged 65 years or more [10]. It is well-known that osteoclasts play a key role in the physiologic response to bone infections. Not only the phagocytosis’ capability of cell detritus but also the ability to resorb small necrotic bone fragments make osteoclasts an irreplaceable player in this battle.
Although antiresorptive drugs in particular denosumab and bisphosphonates possess completely different mechanisms of action and pharmacokinetics, both drugs have the same target cell, namely osteoclasts.
It is well-known and proven in different studies that the osteoclast activity is significantly reduced under antiresorptive treatment [11]. Thus, simultaneous presence of local infection and MRONJ is not a coincidence but rather indicates a significant correlation between the two.
The molar area of the mandible is known to be frequent site of dental and periodontal infections. Furthermore, the disease process of MRONJ in dentate patients usually starts by infected tooth bearing areas of alveolar process or areas of dento–alveolar surgeries without adequate preventive measures. In edentulous patients, pressure sores might increase the need for remodeling beyond the capacities of patients treated with antiresorptive drugs. Clinical presentation of MRONJ is similar to osteomyelitis and basically all clinical signs of MRONJ are well-known signs of infection [12][13].
The risk factors, namely diabetes mellitus, smoking, poor oral hygiene, steroid intake, immunosuppression, may contribute to an increased risk of infection. Bacterial colonization of MRONJ tissue samples by different bacterial species also underlines the plausible link to local infection as a triggering event [14][15].
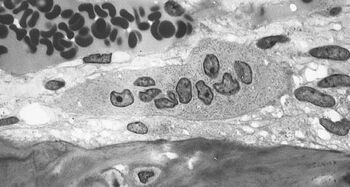
Similarly, MRONJ was reported other than the jaw, in the ear canal, which is also characterized by bacterial colonization and only a very thin epithelial layer covering the bone. This underscores the role of infection in the pathogenesis of MRONJ. Imaging and animal studies have also shown the central role of the infection [16][17][18]. Likewise, prevention of MRONJ aims to prevent infection and thus to eliminate the potential key factor of MRONJ pathogenesis. The decisive role of infection is also evidenced by the drop in incidence of MRONJ in cancer patients who managed to improve their dental hygiene, which thereby prevented potential inflammation and infection [19][20]. Of note, the current treatment of MRONJ is based on controlling existing infection to avoid rapid osteonecrosis progression.
Another aspect is the blood supply of infected bone. There are also other conditions that require stronger remodeling activities and vessel ingrowth especially following surgical procedures.
It is also no coincidence that other drugs which might interfere with host defense and wound healing, especially antiangiogenic drugs, have been recently connected to the development of MRONJ. These drugs (e.g., bevacizumab, Sunitinib, mTOR inhibitors) may act as additional risk factors/co factors for the development of MRONJ in patients receiving antiresorptive drugs [21]. The growing number of studies and models has strengthened the claim that assigns infection as the key trigger in the pathogenesis of MRONJ.
No agreement has been reached in the treatment of MRONJ. Some recommendations focus on the administration of antibiotics, oral antibacterial mouth rinse or surgical debridement or a segmental mandibulectomy and partial maxillectomy with mandibular reconstruction with the fibula flap and covering the exposed areas with tissue flaps. Hyperbaric oxygen (HBO) therapy, fluorescence-guided bone resection, and low-intensity laser therapy have also been studied as therapeutic tools. Other treatment modalities that increase bone wound healing using growth factors had been studied. More recently, teriparatide (N-terminal 34-amino acid recombinant human para-thyroid hormone) has been reported for the medical treatment of MRONJ. Pentoxifylline and a-tocopherol in addition to antimicrobial therapy has been shown to decrease the area of bone exposure and symptoms in MRONJ patients. The use of ozone in combination with antibiotics and surgery for patients with exposed bone lesions has also been the subject of a clinical investigation [22].
The intention of this paper is to stress and reinforce the role of infection in the pathogenesis of MRONJ by collecting pieces of evidence that have been published since the introduction of our hypothesis almost 10 years ago [7]. However, that does by no means disprove any other theory. The infection theory might in fact tie all of the other potentially involved factors and co-factors such as remodeling suppression, inhibition of angiogenesis, inhibition of immunocompetent cells and soft tissue toxicity together. Thus, as detailed above, the infection theory can conclusively explain many of the clinical and radiological features of MRONJ as well as the reasons behind all of the relevant prophylactic and therapeutic measurements.
References
- Von Moos, R.; Costa, L.; Gonzalez-Suarez, E.; Terpos, E.; Niepel, D.; Body, J.J. Management of bone health in solid tumours: From bisphosphonates to a monoclonal antibody. Cancer Treat. Rev. 2019, 76, 57–67.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws AAoOaMS. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376.
- Aghaloo, T.L.; Felsenfeld, A.L.; Tetradis, S. Osteonecrosis of the jaw in a patient on Denosumab. J. Oral Maxillofac. Surg. 2010, 68, 959–963.
- Otto, S.; Pautke, C.; van den Wyngaert, T.; Niepel, D.; Schiodt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187.
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956.
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998.
- Kumar, S.K.; Gorur, A.; Schaudinn, C.; Shuler, C.F.; Costerton, J.W.; Sedghizadeh, P.P. The role of microbial biofilms in osteonecrosis of the jaw associated with bisphosphonate therapy. Curr. Osteoporos. Rep. 2010, 8, 40–48.
- Otto, S.; Hafner, S.; Mast, G.; Tischer, T.; Volkmer, E.; Schieker, M.; Sturzenbaum, S.R.; von Tresckow, E.; Kolk, A.; Ehrenfeld, M.; et al. Bisphosphonate-related osteonecrosis of the jaw: Is pH the missing part in the pathogenesis puzzle? J. Oral Maxillofac. Surg. 2010, 68, 1158–1161.
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-dependent effects of zoledronic acid on human periodontal ligament stem cells: An in vitro pilot study. Cell Transplant. 2020, 29, 963689720948497.
- Jordan, R.A.; Bodechtel, C.; Hertrampf, K.; Hoffmann, T.; Kocher, T.; Nitschke, I.; Schiffner, U.; Stark, H.; Zimmer, S.; Micheelis, W. The fifth German oral health study (funfte Deutsche mundgesundheitsstudie, DMS V)—Rationale, design, and methods. BMC Oral Health 2014, 14, 161.
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759.
- Mast, G.; Otto, S.; Mucke, T.; Schreyer, C.; Bissinger, O.; Kolk, A.; Wolff, K.D.; Ehrenfeld, M.; Sturzenbaum, S.R.; Pautke, C. Incidence of maxillary sinusitis and oro-antral fistulae in bisphosphonate-related osteonecrosis of the jaw. J. Craniomaxillofac. Surg. 2012, 40, 568–571.
- Aljohani, S.; Gaudin, R.; Weiser, J.; Troltzsch, M.; Ehrenfeld, M.; Kaeppler, G.; Smeets, R.; Otto, S. Osteonecrosis of the jaw in patients treated with denosumab: A multicenter case series. J. Craniomaxillofac. Surg. 2018, 46, 1515–1525.
- Panya, S.; Fliefel, R.; Probst, F.; Troltzsch, M.; Ehrenfeld, M.; Schubert, S.; Otto, S. Role of microbiological culture and polymerase chain reaction (PCR) of actinomyces in medication-related osteonecrosis of the jaw (MRONJ). J. Craniomaxillofac. Surg. 2017, 45, 357–363.
- De Ceulaer, J.; Tacconelli, E.; Vandecasteele, S.J. Actinomyces osteomyelitis in bisphosphonate-related osteonecrosis of the jaw (BRONJ): The missing link? Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33.
- Otto, S.; Pautke, C.; Jurado, O.M.; Nehrbass, D.; Stoddart, M.J.; Ehrenfeld, M.; Zeiter, S. Further development of the MRONJ minipig large animal model. J. Craniomaxillofac. Surg. 2017, 45, 1503–1514.
- Nowicki, B.; Nehrbass, D.; Arens, D.; Stadelmann, V.A.; Zeiter, S.; Otto, S.; Kircher, P.; Stoddart, M.J. Medication-related osteonecrosis of the jaw in a minipig model: Parameters for developing a macroscopic, radiological, and microscopic grading scheme. J. Craniomaxillofac. Surg. 2019, 47, 1162–1169.
- Aguirre, J.I.; Akhter, M.P.; Kimmel, D.B.; Pingel, J.E.; Williams, A.; Jorgensen, M.; Kesavalu, L.; Wronski, T.J. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J. Bone Miner. Res. 2012, 27, 2130–2143.
- Ripamonti, C.I.; Maniezzo, M.; Campa, T.; Fagnoni, E.; Brunelli, C.; Saibene, G.; Bareggi, C.; Ascani, L.; Cislaghi, E. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann. Oncol. 2009, 20, 137–145.
- Oteri, G.; Bramanti, E.; Nigrone, V.; Cicciu, M. Decayed, missing, and filled teeth index and periodontal health in osteoporotic patients affected by BRONJ: An observational study. J. Osteoporos. 2013, 2013, 231289.
- Fusco, V.; Santini, D.; Armento, G.; Tonini, G.; Campisi, G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert Opin. Drug Saf. 2016, 15, 925–935.
- Fliefel, R.; Troltzsch, M.; Kuhnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585.