Urban civilization has a high impact on the environment and human health. The pollution level of indoor air can be 2–5 times higher than the outdoor air pollution, and sometimes it reaches up to 100 times or more in natural/mechanical ventilated buildings. Even though people spend about 90% of their time indoors, the importance of indoor air quality is less noticed. Cleaning indoor air using plants is an affordable and more environmentally friendly means to purify polluted air. Furthermore, studies show that indoor plants can be used to regulate building temperature, decrease noise levels, and alleviate social stress. Sources of indoor air pollutants and their impact on human health are briefly discussed in our paper. The available literature on phytoremediation, including experimental works for removing volatile organic compound (VOC) and particulate matter from the indoor air and associated challenges and opportunities, are reviewed. The potential role of green walls and potted plants for improving indoor air quality is examined. A list of plant species suitable for indoor air phytoremediation is proposed. Our review paper will help in making informed decisions about integrating plants into the interior building design.
- environmental technology
- biofiltration
- phytoremediation
- indoor air pollution
- VOC
1. Introduction
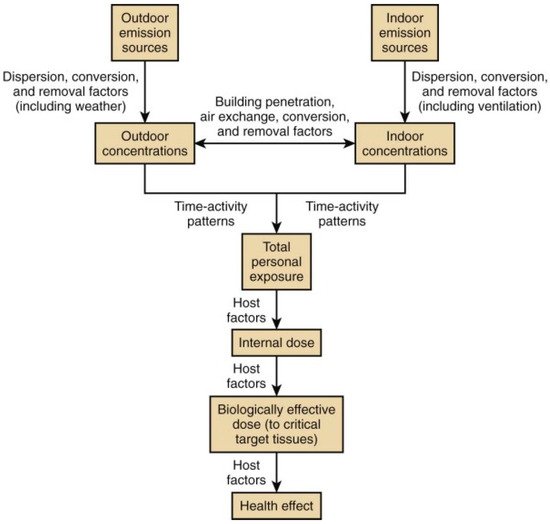
2. Indoor Air Pollution: Sources and Their Health Effects
2.1. Inorganic Pollutants
2.2. Organic Pollutants
VOCs
| Category Description | Acronym | Boiling Point Range, °C |
|---|---|---|
| Very volatile (gaseous) organic compounds | VVOCs | <0 to 50 |
| Volatile organic compounds | VOCs | 50 to 240 |
| Semi-volatile organic compounds | SVOCs | 240 to 380 |
| Organic compounds associated with particulate matter: Particle-bound organic compounds | POCs | >380 |
| Class | Name | Maximum Exposure Guidelines | |
|---|---|---|---|
| Organic pollutant | Carbon monoxide | 100 mg/m3 | 15 min |
| 60 mg/m3 | 30 min | ||
| 30 mg/m3 | 1 h | ||
| 10 mg/m3 | 8 h | ||
| Organic pollutant | Formaldehyde | 0.1 mg/m3 | 30 min |
| Organic pollutant | Tetrichloroethylene (TCE) | 0.25 mg/m3 | Annual |
| Organic pollutant | Tetrachloroethylene (PCE) | 100 ppm | 3 h |
| Organic pollutant | Toluene | 0.26 mg/m3 | 1 week |
| Inorganic pollutant | Asbestos | 500 F*/m3 b | -- |
| Radioactive pollutant | Radon | >1 Becquerel/m3 c | -- |
| Classical pollutant | Nitrogen dioxide | 200 μg/m3 | 1 h |
| 40 μg/m3 | Annual | ||
| 53 ppb a | Annual | ||
| Ozone | 120 μg/m3 | 8 h | |
| 0.08 ppm a | 1 h | ||
3. Indoor Air Pollution Control Techniques
4. The Role of Plants for Indoor Pollutant Removal
4.1. Green Walls
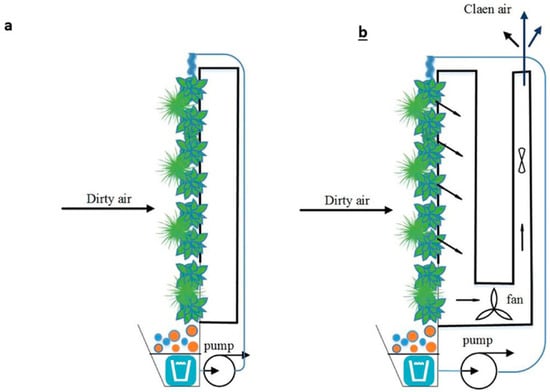
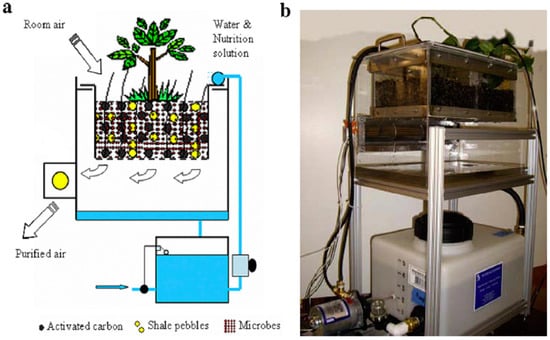
Botanical and Biofiltration System
4.2. Mechanisms of Air Pollutant Removal by Plants
4.3. Volatile Organic Compounds (VOCs) Removal from Indoor Air by Plants
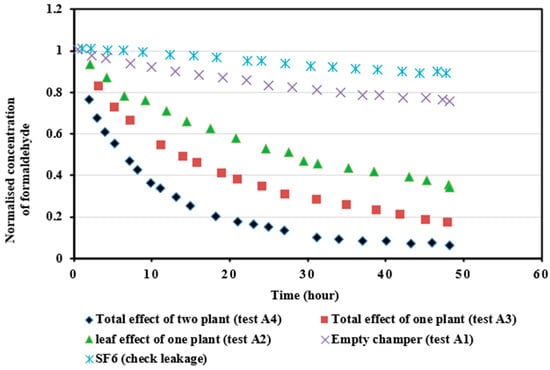
4.3.1. Formaldehyde
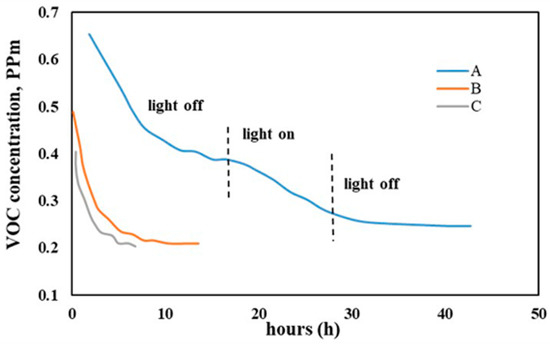
4.3.2. Benzene, Toluene and TVOC Compounds
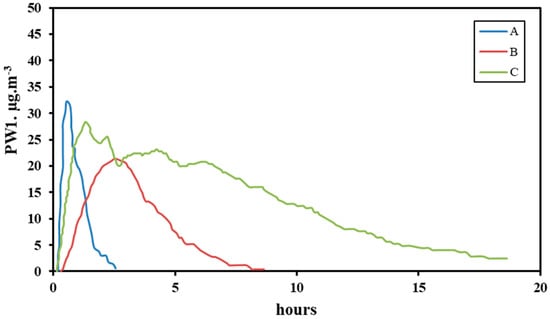
4.4. Particulate Matter Removal from Indoor Air by Plants
4.5. The Choice of Plant
| Pollutant | Potted Plant Species (Remedy) | Results | Ref. |
|---|---|---|---|
| O3 | Peace Lily (Spathiphyllum), Ficus species (Ficus Decora Burgundy), Calathia (Calathia Species), Dieffenbachia (Dieffenbachia Species), Golden Pothos (Epipremnum aureum) | The Golden Pothos had the highest ozone deposition velocity values among plants, and the lowest value was for Peace Lily | [160][159] |
| Toluene and xylene | Schefflera actinophylla and Ficus benghalensis) | Removal of toluene and xylene was 13.3 and 7.0 μg·m−3·m−2 leaf area over a 24-h period in S. actinophylla and was 13.0 and 7.3 μg·m−3·m−2 leaf area in F. benghalensis. It also showed that the root zone has a vital role in toluene and xylene removal. | [148][147] |
| Toluene | Hedera helix | The removal rate is 66.5 μg/m2/h for toluene that is effective to remove it. | [161][160] |
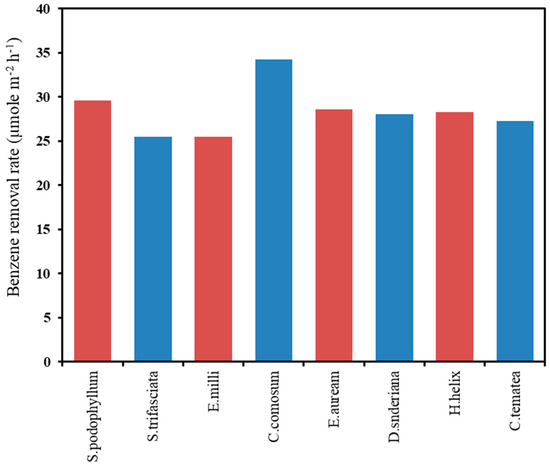
| Benzene | Syngonium podophyllum, Sansevieria trifasciata, Euphorbia milii, Chlorophytum comosum, Epipremnum aureum, Dracaena sanderiana, Hedera helix, Clitoria ternatea | C. comosum was the highest efficient plant for removing benzene during the 96 h. | [8] |
| Trichloroethylene, tetrachloroethylene 1,2-dichloroethane benzene, toluene m, p-xylene | ficus; golden pothos; spider fern; Christmas cactus | Leaf concentrations change with air concentrations, the speed of air. It shows the potential of leaves for removing VOCs in indoor air. | [23] |
| PM | Spider plants (Chlorophytum comosum L.) | The result show accumulation of PM at a high level on surface of leaf | [126][125] |
| Toluene ethylbenzene | Aloe vera, Sansevieria masoniana, Sansevieria trifasciata, Sansevieria hyacinthoides, Sansevieria ehrenbergii, Kalanchoe blossfeldiana, Dracaenaderemensis, Codiaeum variegatum, Chlorophytum comosum, Dracaena sanderiana, Cordyline fruticosa, Aglaonema commutatum | S. trifasciata had the highest value for removing toluene, C. comosum. for removal of ethylbenzene, S. trifasciata and S. hyacinthoides had a high value in the absorption of toluene and ethylbenzene. | [54] |
| Benzene Trichloroethylne Formaldehyde | Chamaedorea seifritzii, Aglaonema modestum, Hedera helix, Ficus benjamina, Gerbera jamesonii, Dracaena deremensis, Dracaena marginata, Dracaena massangeana, Sansevieria laurentii, Spathiphyllum, Chrysanthemum morifolium, Dracaena deremensis | These plants require low light and low metabolic rates. These plants are a suitable selection to decrease sick building syndrome containing many new, energy-efficient buildings. The plant root-soil zone showed high efficiency for removal of VOCs | [136][135] |
| Formaldehyde | Golden Pothos | Dynamic airflow through the root bed and microbes were essential for removing high efficiency; moisture of bed root has a vital role in removing VOCs. | [133][132] |
| Benzenen-hexane | Janet Craig S. Sweet Chico |
The highest value for removing TVOCs (75%) by potted-plants is when indoor average TVOC concentrations are higher than 100 ppb. | [153][152] |
| Benzene | Indoor ornamental plants representing 73 species and cultivars (35 families and 60 genera) | Thirteen species removed between 0.1–9.99% of benzene in contaminated air, 17 species removed 10–20%, and 17 species removed 20–40%. Three species removed 60–80% of benzene in the experimental air. | [142][141] |
| Benzene | Spathiphyllum, Howea forsteriana, Dracaena marginata, Epipremnum aureum, Spathiphyllum, | These potted-plants decrease VOCs, even if the level of VOCs has very low. | [149][148] |
| Schefflera, Dracaena | |||
| Toluene toxicity, 14C-toluene uptake | Soybean (Glycine max) | 14C concentrations in Leaf tissue increased during the light phases and decreased during 12-hr dark phases. | [151][150] |
| Benzene CO2, CO | Zamioculcas, Aglaonema Dracaena |
CO2 concentration increases 10% in offices in the air-conditioned building. The CO level reduces with or without air-conditioning. Higher value removing of benzene appearance by these plants. | [150][149] |
| CO2, acetone methyl acetate Formaldehyde PM | Green wall | The active green wall has high efficiency to increase indoor air quality by absorbing VOC and PM but has not been highly effective for carbon dioxide adsorption. The green wall increases the relative humidity, which is a suitable selection to use in a dry environment. | [137][136] |
| CO2 | Aglaonema commutatum Schott, Aspidistra elatior Blume, Castanospermum australe A.CunnexHoo., Chamaedorea elegans Wild., Dracaena deremensis Engl., Dypsis lutescens, Beentje and J.Dransf., Ficus benjamina L. Howea forsteriana Becc. | These plants can be used in high-intensity light, but if the light intensity is higher than the optimal value, but will become photoinhibited, and possibly be etched off chlorophyll | [15] |
| CO2 | Peace lily, weeping fig, areca palm |
The rate of photosynthesis change with the variation of CO2 concentration in light indoor. The leaf area is effective to decrease CO2. for 3 plants, with the area of the leaf up to 15,000 cm2, the CO2 concentration decreases (just leaf area is effective in the reduction of CO2. | [162][161] |
| CO2 | Sweet Chico, Hahnii, Chamaedorea elegans, Dracaena marginata, Florida Beauty, Lemon Lime, Janet Craig, Ctenanthe, oppenheimiana, Ficus repens, Hedera helix, Epipremnum, aureus, Philodendron, scandens, Dizygotheca, elegantissima | Woody plants species accumulate dry mass (and carbon) better than smaller, herbaceous species. | [163][162] |
References
- Kumar, P.; Hama, S.; Omidvarborna, H.; Sharma, A.; Sahani, J.; Abhijith, K.; Debele, S.E.; Zavala-Reyes, J.C.; Barwise, Y.; Tiwari, A. Temporary reduction in fine particulate matter due to ‘anthropogenic emissions switch-off’ during COVID-19 lockdown in Indian cities. Sustain. Cities Soc. 2020, 62, 102382.
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186.
- Aller, M. Environmental Laws and Regulations. In Library of Congress Cataloging; CRC Press LLC: Boca Raton, FL, USA, 1999.
- Pandey, A.K.; Pandey, M.; Tripathi, B. Air Pollution Tolerance Index of climber plant species to develop Vertical Greenery Systems in a polluted tropical city. Landsc. Urban. Plan. 2015, 144, 119–127.
- Sanjuán-Herráez, D.; Rodríguez-Carrasco, Y.; Juan-Peiró, L.; Pastor, A.; De la Guardia, M. Determination of indoor air quality of a phytosanitary plant. Anal. Chim. Acta 2011, 694, 67–74.
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X.; Gadea, E.; Garrote, P. Impact of formaldehyde and VOCs fromwaste treatment plants upon the ambient air nearby an urban area. Sci. Total Environ. 2016, 568, 369–380.
- Marć, M. Problems and challenges associated with estimating the emissions of organic compounds from indoor materials. Trac Trends Anal. Chem. 2017, 97, 297–308.
- Sriprapat, W.; Thiravetyan, P. Efficacy of ornamental plants for benzene removal from contaminated air and water: Effect of plant associated bacteria. Int. Biodeterior. Biodegrad. 2016, 113, 262–268.
- Schwela, P.D. Indoor Air; Kotzias, D., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2005; Volume 3, pp. 475–489.
- Schwela, D. Pollution, Indoor Air A2—Wexler, Philip. In Encyclopedia of Toxicology, 3rd ed.; Oxford University Press: Oxford, UK, 2014; pp. 1003–1017.
- Kolokotsa, D.; Santamouris, M. Review of the indoor environmental quality and energy consumption studies for low income households in Europe. Sci. Total Environ. 2015, 536, 316–330.
- EPA. Why Indoor Air Quality Is Important to Schools. Available online: (accessed on 5 March 2021).
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2an Indoor Pollutant? Direct Effects of Low-to-Moderate CO2Concentrations on Human Decision-Making Performance. Environ. Health Perspect. 2012, 120, 1671–1677.
- Pereira, M.; Tribess, A.; Buonanno, G.; Stabile, L.; Scungio, M.; Baffo, I. Particle and Carbon Dioxide Concentration Levels in a Surgical Room Conditioned with a Window/Wall Air-Conditioning System. Int. J. Environ. Res. Public Health 2020, 17, 1180.
- Torpy, F.; Irga, P.; Burchett, M. Profiling indoor plants for the amelioration of high CO2 concentrations. Urban For. Urban Green. 2014, 13, 227–233.
- Australia, E. BTEX Personal Exposure Monitoring in Four Australian Cities; Technical Report No. 6; Common-Wealth of Australia: Sydney, Australia, 2003; pp. 1–62.
- Vasile, V.; Petran, H.; Dima, A.; Petcu, C. Indoor Air Quality—A Key Element of the Energy Performance of the Buildings. Energy Procedia 2016, 96, 277–284.
- Cheng, L.; Li, B.; Cheng, Q.; Baldwin, A.N.; Shang, Y. Investigations of indoor air quality of large department store buildings in China based on field measurements. Build. Environ. 2017, 118, 128–143.
- Kazemi, A.; Ghorbanpour, M. Introduction to Environmental Challenges in All Over the World. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 25–48.
- Luengas, A.T.; Hort, C.; Platel, V.; Elias, A.; Barona, A.; Moynault, L. Removal of traces of toluene and p-xylene in indoor air using biofiltration and a hybrid system (biofiltration + adsorption). Environ. Sci. Pollut. Res. 2017, 24, 10674–10684.
- Katsoyiannis, A.; Bogdal, C. Interactions between indoor and outdoor air pollution—Trends and scientific challenges. Environ. Pollut. 2012, 169, 150–151.
- Veetil, D.S.P. Air Pollution: Sources and Effects in Urban Areas and How it Affect the Investment and Economy. Envirocities Emagazine 2012, 3, 5.
- Wetzel, T.A.; Doucette, W.J. Plant leaves as indoor air passive samplers for volatile organic compounds (VOCs). Chemosphere 2015, 122, 32–37.
- Śmiełowska, M.; Marć, M.; Zabiegała, B. Indoor air quality in public utility environments—A review. Environ. Sci. Pollut. Res. 2017, 24, 11166–11176.
- Segalin, B.; Kumar, P.; Micadei, K.; Fornaro, A.; Gonçalves, F.L. Size–Segregated particulate matter inside residences of elderly in the Metropolitan Area of São Paulo, Brazil. Atmos. Environ. 2017, 148, 139–151.
- PM, V. Processing and Characterizations: State-of-the-Art and New Challenges. Nanostruct. Polym. Membr. 2016, 1, 1.
- Thatcher, A.; Milner, K. Is a green building really better for building occupants? A longitudinal evaluation. Build. Environ. 2016, 108, 194–206.
- Ugranli, T.; Gungormus, E.; Sofuoglu, A.; Sofuoglu, S. Indoor Air Quality in Chemical Laboratories. In Elsevier Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 859–878.
- Arif, M.; Katafygiotou, M.; Mazroei, A.; Kaushik, A.; Elsarrag, E. Impact of indoor environmental quality on occupant well-being and comfort: A review of the literature. Int. J. Sustain. Built Environ. 2016, 5, 1–11.
- Balmes, J.R.; Eisner, M.D. Indoor and outdoor air pollution. In Murray & Nadel’s Textbook of Respiratory Medicine E-Book; Elsevier: Amsterdam, The Netherlands, 2016; p. 2208.
- Chandrappa, R.; Kulshrestha, U.C. Sustainable Air Pollution Management Theory and Practice; Hamburg, U.F., Rulkens, W.H., Salomons, W., Eds.; Springer: Cham, Switzerland, 2016.
- Goyal, R.; Khare, M.; Kumar, P. Indoor air quality: Current status, missing links and future road map for India. J. Civ. Environ. Eng. 2012, 2, 2–4.
- Nazaroff, W.W. Exploring the consequences of climate change for indoor air quality. Environ. Res. Lett. 2013, 8, 015022.
- Leung, D.Y.C. Outdoor-indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015, 2.
- Kiurski, J.S.; Marić, B.B.; Aksentijević, S.M.; Oros, I.B.; Kecic, V.S.; Kovačević, I.M. Indoor air quality investigation from screen printing industry. Renew. Sustain. Energy Rev.. 2013, 28, 224–231.
- Jovanović, M.; Vučićević, B.; Turanjanin, V.; Živković, M.; Spasojević, V. Investigation of indoor and outdoor air quality of the classrooms at a school in Serbia. Energy Environ. Sci. 2014, 77, 42–48.
- Dėdelė, A.; Miškinytė, A. Seasonal variation of indoor and outdoor air quality of nitrogen dioxide in homes with gas and electric stoves. Environ. Sci. Pollut. Res. 2016, 23, 17784–17792.
- Cibella, F.; Cuttitta, G.; Della Maggiore, R.; Ruggieri, S.; Panunzi, S.; De Gaetano, A.; Bucchieri, S.; Drago, G.; Melis, M.R.; La Grutta, S.; et al. Effect of indoor nitrogen dioxide on lung function in urban environment. Environ. Res. 2015, 138, 8–16.
- Shen, H.; Tsai, C.M.; Yuan, C.S.; Jen, Y.H.; Ie, I.R. How incense and joss paper burning during the worship activities influences ambient mercury concentrations in indoor and outdoor environments of an Asian temple. Chemosphere 2017, 167, 530–540.
- Loupa, G.; Polyzou, C.; Zarogianni, A.M.; Ouzounis, K.; Rapsomanikis, S. Indoor and outdoor elemental mercury: A comparison of three different cases. Environ. Monit. Assess. 2017, 189.
- Darling, E.; Morrison, G.C.; Corsi, R.L. Passive removal materials for indoor ozone control. Build. Environ. 2016, 106, 33–44.
- Fadeyi, M.O. Ozone in indoor environments: Research progress in the past 15 years. Sustain. Cities Soc. 2015, 18, 78–94.
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606–6630.
- Irga, P.; Paull, N.; Abdo, P.; Torpy, F. An assessment of the atmospheric particle removal efficiency of an in-room botanical biofilter system. Build. Environ. 2017, 115, 281–290.
- Buczyńska, A.J.; Krata, A.; Van Grieken, R.; Brown, A.; Polezer, G.; De Wael, K.; Potgieter-Vermaak, S. Composition of PM2.5 and PM1 on high and low pollution event days and its relation to indoor air quality in a home for the elderly. Sci. Total Environ. 2014, 490, 134–143.
- Defra The Air Quality Strategy for England, Scotland, Wales and Northern Ireland; Department for Environment, Food and Rural Affairs, The Stationery Office: London, UK, 2007.
- Abd El Aziz, N.G.; Mahgoub, M.H.; Azza, M.M.M.; Farahat, M.M.; Abouziena, H.F. Potentiality of Ornamental Plants and Woody Trees as Phytoremidators of Pollutants in the Air: A Review. Int. J. Chemtech Res. 2015, 8, 468–482.
- Othman, M.; Latif, M.T.; Mohamed, A.F. The PM10 compositions, sources and health risks assessment in mechanically ventilated office buildings in an urban environment. Air Qual. Atmos. Health 2015, 9, 597–612.
- Bozlaker, A.; Peccia, J.; Chellam, S. Indoor/Outdoor Relationships and Anthropogenic Elemental Signatures in Airborne PM2.5 at a High School: Impacts of Petroleum Refining Emissions on Lanthanoid Enrichment. Environ. Sci. Technol. 2017, 51, 4851–4859.
- Mohammadyan, M.; Ghoochani, M.; Kloog, I.; Abdul-Wahab, S.A.; Yetilmezsoy, K.; Heibati, B.; Pollitt, K.J.G. Assessment of indoor and outdoor particulate air pollution at an urban background site in Iran. Environ. Monit. Assess. 2017, 189, 29.
- Kaur, A.; Misra, A. Impact of Indoor Surface Materials and Environment on Perceived Air Quality. J. Environ. Hum. 2014, 2014, 25–35.
- Mentese, S.; Mirici, N.A.; Otkun, M.T.; Bakar, C.; Palaz, E.; Tasdibi, D.; Cevizci, S.; Cotuker, O. Association between respiratory health and indoor air pollution exposure in Canakkale, Turkey. Build. Environ. 2015, 93, 72–83.
- De Gennaro, G.; Dambruoso, P.R.; Loiotile, A.D.; Di Gilio, A.; Giungato, P.; Tutino, M.; Marzocca, A.; Mazzone, A.; Palmisani, J.; Porcelli, F. Indoor air quality in schools. Environ. Chem. Lett. 2014, 12, 467–482.
- Sriprapat, W.; Suksabye, P.; Areephak, S.; Klantup, P.; Waraha, A.; Sawattan, A.; Thiravetyan, P. Uptake of toluene and ethylbenzene by plants: Removal of volatile indoor air contaminants. Ecotoxicol. Environ. Saf. 2014, 102, 147–151.
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Recent trend in risk assessment of formaldehyde exposures from indoor air. Arch. Toxicol. 2012, 87, 73–98.
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Re-evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Arch. Toxicol. 2016, 91, 35–61.
- Destaillats, H.; Maddalena, R.L.; Singer, B.C.; Hodgson, A.T.; McKone, T.E. Indoor pollutants emitted by office equipment: A review of reported data and information needs. Environ. Energy Technol. Div. 2007, 42, 1371–1388.
- ATSDR. Naphthalene, 1-Methylnapthalene, 2-Methylnapthalene; ATSDR: Atlanta, GA, USA, 2011.
- ATSDR. Trichloroethylene (TCE); ATSDR: Atlanta, GA, USA, 2011.
- Bahr, D.E.; Aldrich, T.E.; Seidu, D.; Brion, G.M.; Tollerud, D.J.; Muldoon, S.; Reinhart, N.; Youseefagha, A.; McKinney, P.; Hughes, T.; et al. Occupational exposure to trichloroethylene and cancer risk for workers at the Paducah Gaseous Diffusion Plant. Int. J. Occup. Med. Environ. Health 2011, 24, 67–77.
- Environmental Health and Medicine Education. Tetrachloroethylene Toxicity, What Are the Physiological Effects of Tetrachloroethylene Exposure? In Agency for Toxic Substances and Disease Registry; ATSDR: Atlanta, GA, USA, 2008.
- Ayoko, G.A.; Wang, H. Volatile Organic Compounds. In Indoor Environments; Pluschke, P., Schleibinger, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–107.
- World Health Organization. Indoor Air Quality: Organic Pollutants. Report on a WHO Meeting, Berlin, Germany, 23–27 August 1987; EURO Reports and Studies 111; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 1989.
- WHO. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000.
- Besis, A.; Samara, C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments—A review on occurrence and human exposure. Environ. Pollut. 2012, 169, 217–229.
- Darnerud, P.O.; Eriksen, G.S.; Jóhannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001, 109, 49–68.
- Hooper, K.; McDonald, T.A. The PBDEs: An emerging environmental challenge and another reason for breast-milk monitoring programs. Environ. Health Perspect. 2000, 108, 387–392.
- Gaspar, F.W.; Chevrier, J.; Bornman, R.; Crause, M.; Obida, M.; Barr, D.B.; Bradman, A.; Bouwman, H.; Eskenazi, B. Corrigendum to Undisturbed dust as a metric of long-term indoor insecticide exposure: Residential DDT contamination from indoor residual spraying and its association with serum levels in the VHEMBE cohort. Environ. Int. 2016, 94, 778–783.
- Sánchez, A.M.; Nuevo, M.J. Actions for remediation in cases with large concentration of radon indoor. J. Radioanal. Nucl. Chem. 2016, 311, 1219–1225.
- Al-Zoughool, M.; Krewski, D. Health effects of radon: A review of the literature. Int. J. Radiat. Biol. 2009, 85, 57–69.
- Jenkins, P.L.; Phillips, T.J.; Mulberg, E.J.; Hui, S.P. Activity patterns of Californians: Use of and proximity to indoor pollutant sources. Atmos. Environ. Part A Gen. Top. 1992, 26, 2141–2148.
- Bruce, N.; Perez-Padilla, R.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull. World Health Organ. 2000, 78, 1078–1092.
- Ezzati, M. Indoor Air Pollution/Developing Countries; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 547–553.
- Smith, K.R.; Mehta, S.; Maeusezahl-Feuz, M. Indoor air pollution from household solid fuel use. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 1435–1493.
- Smith, K.R. Biofuels, Air Pollution, and Health: A Global Review; Plenum Press: New York, NY, USA, 1987.
- Moya, T.A.; Dobbelsteen, A.V.D.; Ottelé, M.; Bluyssen, P.M. A review of green systems within the indoor environment. Indoor Built Environ. 2018, 28, 298–309.
- Irga, P.J.; Pettit, T.; Irga, R.F.; Paull, N.J.; Douglas, A.N.; Torpy, F.R. Does plant species selection in functional active green walls influence VOC phytoremediation efficiency? Environ. Sci. Pollut. Res. 2019, 26, 1–8.
- Kaunelienė, V.; Prasauskas, T.; Krugly, E.; Stasiulaitienė, I.; Čiužas, D.; Šeduikytė, L.; Martuzevičius, D. Indoor air quality in low energy residential buildings in Lithuania. Build. Environ. 2016, 108, 63–72.
- Rounaghi, G.; Kakhki, R.M.; Eshghi, H. Efficient transport of lead (II) cations in natural water using a liquid membrane system with dicyclohexano-18-crown-6 as carrier. Arab. J. Chem. 2017, 10, S339–S346.
- Liu, G.; Xiao, M.; Zhang, X.; Gal, C.; Chen, X.; Liu, L.; Pan, S.; Wu, J.; Tang, L.; Clements-Croome, D. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 2017, 32, 375–396.
- Jimenez-Relinque, E.; Castellote, M. Influence of the inlet air in efficiency of photocatalytic devices for mineralization of VOCs in air-conditioning installations. Environ. Sci. Pollut. Res. 2014, 21, 11198–11207.
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. Reviews in Environmental Science and Bio/Technology. Rev. Environ. Sci. Biotechnol. 2015, 14, 499–522.
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410.
- Agarwal, P.; Sarkar, M.; Chakraborty, B.; Banerjee, T. Phytoremediation of Air Pollutants: Prospects and Challenges. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–241.
- Brilli, F.; Fares, S.; Ghirardo, A.; de Visser, P.; Calatayud, V.; Muñoz, A.; Annesi-Maesano, I.; Sebastiani, F.; Alivernini, A.; Varriale, V.; et al. Plants for Sustainable Improvement of Indoor Air Quality. Trends Plant. Sci. 2018, 23, 507–512.
- Cummings, B.E.; Waring, M.S. Potted plants do not improve indoor air quality: A review and analysis of reported VOC removal efficiencies. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 253–261.
- Han, K.-T.; Ruan, L.-W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 16019–16051.
- Abhijith, K.; Kumar, P.; Gallagher, J.; McNabola, A.; Baldauf, R.; Pilla, F.; Broderick, B.; Di Sabatino, S.; Pulvirenti, B. Air pollution abatement performances of green infrastructure in open road and built-up street canyon environments—A review. Atmos. Environ. 2017, 162, 71–86.
- Wannomai, T.; Kemacheevakul, P.; Thiravetyan, P. Removal of Trimethylamine from Indoor Air Using Potted Plants under Light and Dark Conditions. Aerosol Air Qual. Res. 2019, 19, 1105–1113.
- Aydogan, A.; Cerone, R. Review of the effects of plants on indoor environments. Indoor Built Environ. 2020.
- Tiwari, A.; Kumar, P.; Kalaiarasan, G.; Ottosen, T.-B. The impacts of existing and hypothetical green infrastructure scenarios on urban heat island formation. Environ. Pollut. 2021, 274, 115898.
- Weyens, N.; Thijs, S.; Popek, R.; Witters, N.; Przybysz, A.; Espenshade, J.; Gawronska, H.; Vangronsveld, J.; Gawronski, S.W. The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. Mol. Sci. 2015, 16, 25576–25604.
- Cuce, E. Thermal regulation impact of green walls: An experimental and numerical investigation. Appl. Energy 2017, 194, 247–254.
- Maslauskas, T. Green Walls—The Vertical Planting Systems; VIA University College: Horsens, Denmark, 2015; p. 43.
- Pastore, L.; Corrao, R.; Heiselberg, P.K. The effects of vegetation on indoor thermal comfort: The application of a multi-scale simulation methodology on a residential neighborhood renovation case study. Energy Build. 2017, 146, 1–11.
- Ravindu, S.; Rameezdeen, R.; Zuo, J.; Zhou, Z.; Chandratilake, R. Indoor environment quality of green buildings: Case study of an LEED platinum certified factory in a warm humid tropical climate. Build. Environ. 2015, 84, 105–113.
- Safikhani, T.; Abdullah, A.M.; Ossen, D.R.; Baharvand, M. A review of energy characteristic of vertical greenery systems. Renew. Sustain. Energy Rev. 2014, 40, 450–462.
- Gunawardena, K.; Steemers, K. Living walls in indoor environments. Build. Environ. 2019, 148, 478–487.
- Green Roofs for Healthy Cities: Introduction to Green Walls, Introduction to Green Walls Technology Benefits & Design. 2008. Available online: (accessed on 1 April 2021).
- Modirrousta, S.; Mohammadi, Z. Necessity and Methods of Designing Green Buildings in Cities and its Effect on Energy Efficiency. Eur. Online J. Nat. Soc. Sci. Proc. 2015, 4, 304–314.
- Tomson, M.; Kumar, P.; Barwise, Y.; Perez, P.; Forehead, H.; French, K.; Morawska, L.; Watts, J.F. Green infrastructure for air quality improvement in street canyons. Environ. Int. 2021, 146, 106288.
- Wolverton, B.C.B.; NASA. Improving Indoor Air Quality with Plant-Based Systems; NASA: Washington, DC, USA, 2009.
- Darlington, A.B.; Dat, J.F.; Dixon, M.A. The Biofiltration of Indoor Air: Air Flux and Temperature Influences the Removal of Toluene, Ethylbenzene, and Xylene. Environ. Sci. Technol. 2000, 35, 240–246.
- Darlington, A.B. Room Air Cleansing Using Hydroponic Plants. U.S. Patent No. 6,727,09, 27 April 2004.
- Llewellyn, D.; Darlington, A.; van Ras, N.; Kraakman, B.; Dixon, M. A hybridized membrane-botanical biofilter for improving air quality in occupied spaces. In Proceedings of the 37th COSPAR Scientific Assembly, Montreal, QC, Canada, 13–20 July 2008; Volume 37, p. 1813.
- Prodanovic, V.; Wang, A.; Deletic, A. Assessing water retention and correlation to climate conditions of five plant species in greywater treating green walls. Water Res. 2019, 167, 115092.
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco, A.; Egea, G. Influence of an active living wall on indoor temperature and humidity conditions. Ecol. Eng. 2016, 90, 120–124.
- Sadeghian, M.M. A Review on Green Wall Classification and Function. Sci. Technol. 2016, 2, 47–51.
- Pettit, T.; Irga, P.; Torpy, F. Functional green wall development for increasing air pollutant phytoremediation: Substrate development with coconut coir and activated carbon. J. Hazard. Mater. 2018, 360, 594–603.
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Rev. Environ. Sci. Bio/Technol. 2018, 17, 395–415.
- Sowa, J.; Hendiger, J.; Maziejuk, M.; Sikora, T.; Osuchowski, L.; Kamińska, H. Potted Plants as Active and Passive Biofilters Improving Indoor Air Quality. Iop Conf. Ser. Earth Environ. Sci. 2019, 290, 012150.
- Abdo, P.; Huynh, B.P.; Irga, P.J.; Torpy, F.R. Evaluation of air flow through an active green wall biofilter. Urban For. Urban Green. 2019, 41, 75–84.
- Hernandez, M.; Peden, D.B. Air Pollution: Indoor and Outdoor. In Middleton’s Allergy: Principles and Practice; Adkinson, N.F., Jr., Bochner, B.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 482–496.
- Pettit, T.; Bettes, M.; Chapman, A.R.; Hoch, L.M.; James, N.D.; Irga, P.J.; Torpy, F.R.; Plants and Environmental Quality Research Group. The botanical biofiltration of VOCs with active airflow: Is removal efficiency related to chemical properties? Atmos. Environ. 2019, 214, 116839.
- Fleck, R.; Pettit, T.J.; Douglas, A.N.; Irga, P.J.; Torpy, F.R. 15—Botanical biofiltration for reducing indoor air pollution. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Pacheco-Torgal, F., Ivanov, V., Tsang, D.C.W., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 305–327.
- Curtis, L.; Stuart, M. Enhancing CHBE Indoor Air Quality: Biowall Technology; Student Report; University of British Columbia Social Ecological Economic Development Studies (SEEDS): Vancouver, BC, Canada, 2010.
- Dickinson, N.M.; Baker, A.J.; Doronila, A.; Laidlaw, S.; Reeves, R.D. Phytoremediation of inorganics: Realism and synergies. Int. J. Phytoremediat. 2009, 11, 97–114.
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827.
- Chaney, R.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284.
- Ensley, B. Rationale for use of phytoremediation. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley & Sons: New York, NY, USA, 2000; pp. 3–11.
- Prasad, M.N.V.; Freitas, H.M.D.O. Metal hyperaccumulation in plants—Biodiversity prospecting for phytoremediation technology. Electron. J. Biotechnol. 2003, 6, 285–321.
- Schnoor, J.; Light, L.A.; McCutcheon, S.C.; Wolfe, N.L.; Carreia, L.H. Phytoremediation of organic and nutrient contaminants. Environ. Sci. Technol. 1995, 29, 318A–323A.
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2007, 7, 47–59.
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from indoor air by ornamental potted plants: A pilot study using a palm species under the controlled environment. Chemosphere 2018, 197, 375–381.
- Gawrońska, H.; Bakera, B. Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual. Atmos. Health 2014, 8, 265–272.
- Soreanu, G.; Dixon, M.; Darlington, A. Botanical biofiltration of indoor gaseous pollutants—A mini-review. Chem. Eng. J. 2013, 229, 585–594.
- Aydogan, A.; Montoya, L.D. Formaldehyde removal by common indoor plant species and various growing media. Atmos. Environ. 2011, 45, 2675–2682.
- Su, Y.; Liang, Y. Foliar uptake and translocation of formaldehyde with Bracket plants (Chlorophytum comosum). J. Hazard. Mater. 2015, 291, 120–128.
- Lee, J.H. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol. Bioprocess. Eng. 2013, 18, 431–439.
- Kvesitadze, E.; Sadunishvili, T.; Kvesitadze, G. Mechanisms of organic contaminants uptake and degradation in plants. World Acad. Sci. Eng. Technol. 2009, 55, 458–468.
- Pettit, T.; Irga, P.J.; Torpy, F.R. The in situ pilot-scale phytoremediation of airborne VOCs and particulate matter with an active green wall. Air Qual. Atmos. Health 2018, 12, 33–44.
- Wang, Z.; Pei, J.; Zhang, J.S. Experimental investigation of the formaldehyde removal mechanismsin a dynamic botanical filtration system for indoor air purification. Hazard. Mater. 2014, 280, 235–243.
- Wang, Y.; Groot, F.B.; Wörtche, H. Effect of ecosystem services provided by urban green infrastructure on indoor environment: A literature review. Build. Environ. 2014, 77, 88–100.
- Llewellyn, D.; Dixon, M. Can Plants Really Improve Indoor Air Quality? Reference Module in Life Sciences. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 331–337.
- Wolverton, B.C.; Johnson, A.; Bounds, K. Interior Landscape Plants for Indoor Air Pollution Abatement; NASA Stennis Space Centre: Hancock, MS, USA, 1989.
- Bondarevs, A.; Huss, P.; Gong, S.; Weister, O.; Liljedahl, R. Green Walls Utilizing Internet of Things. Sens. Transduct. 2015, 192, 16–21.
- Treesubsuntorn, C.; Thiravetyan, P. Botanical biofilter for indoor toluene removal and reduction of carbon dioxide emission under low light intensity by using mixed C3 and CAM plants. J. Clean. Prod. 2018, 194, 94–100.
- Wang, Z.; Zhang, J.S. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build. Environ. 2011, 46, 758–768.
- Xu, Z.; Qin, N.; Wang, J.; Tong, H. Formaldehyde biofiltration as affected by spider plant. Bioresour. Technol. 2010, 101, 6930–6934.
- Weidner, M.; Da Silvia, J.A.T. Potential and Limitations of Ornamental Plants for Indoor-Air Purification; Silva, J.A.T.D., Ed.; Floriculture, Ornamental and Plant Biotechnology Advances and Topical Issues; Global Science Books: London, UK, 2006; pp. 54–63.
- Liu, Y.J.; Mu, Y.J.; Zhu, Y.G.; Ding, H.; Arens, N.C. Which ornamental plant species effectively remove benzene from indoor air? Atmos. Environ. 2007, 41, 650–654.
- Gong, Y.; Zhou, T.; Wang, P.; Lin, Y.; Zheng, R.; Zhao, Y.; Xu, B. Fundamentals of Ornamental Plants in Removing Benzene in Indoor Air. Atmosphere 2019, 10, 221.
- Yoo, M.H.; Kwon, Y.J.; Son, K.-C.; Kays, S.J. Efficacy of Indoor Plants for the Removal of Single and Mixed Volatile Organic Pollutants and Physiological Effects of the Volatiles on the Plants. J. Am. Soc. Hortic. Sci. 2006, 131, 452–458.
- Yang, D.S.; Pennisi, S.V.; Son, K.-C.; Kays, S.J. Screening Indoor Plants for Volatile Organic Pollutant Removal Efficiency. HortScience 2009, 44, 1377–1381.
- Treesubsuntorn, C.; Thiravetyan, P. Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmos. Environ. 2012, 57, 317–321.
- Sriprapat, W.; Thiravetyan, P. Phytoremediation of BTEX from Indoor Air by Zamioculcas zamiifolia. Water Air Soil Pollut. 2013, 224, 1–9.
- Kim, K.J.; Kim, H.J.; Khalekuzzaman, M.; Yoo, E.H.; Jung, H.H.; Jang, H.S. Removal ratio of gaseous toluene and xylene transported from air to root zone via the stem by indoor plants. Environ. Sci. Pollut. Res. 2016, 23, 6149–6158.
- Orwell, R.L.; Wood, R.L.; Tarran, J.; Torpy, F.; Burchett, M.D. Removal of Benzene by the Indoor Plant/Substrate Microcosm and Implications for Air Quality. Water Air Soil Pollut. 2004, 157, 193–207.
- Tarran, J.; Torpy, F.; Burchett, M. Use of living pot-plants to cleanse indoor air—Research review. In Proceedings of the 6th International Conferece on Indoor Air Quality, Ventilation & Energy Conservation, Sustainable Built Environment, Sendai, Japan, 28–31 October 2007; pp. 249–256.
- Jen, M.S.; Hoylman, A.M.; Edwards, N.T.; Walton, B.T. Experimental method to measure gaseous uptake of 14C-toluene by foliage. Environ. Exp. Bot. 1995, 35, 389–398.
- Caron, A.; Redon, N.; Thevenet, F.; Hanoune, B.; Coddeville, P. Performances and limitations of electronic gas sensors to investigate an indoor air quality event. Build. Environ. 2016, 107, 19–28.
- Wood, R.A.; Burchett, M.D.; Alquezar, R.; Orwell, R.L.; Tarran, J.; Torpy, F. The Potted-Plant Microcosm Substantially Reduces Indoor Air VOC Pollution: I. Office Field-Study. Water Air Soil Pollut. 2006, 175, 163–180.
- Gupta, G.P.; Kulshrestha, U. Biomonitoring and Remediation by Plants. Plant. Responses Air Pollut. 2016, 119–132.
- Li, Z.; Wen, Q.; Zhang, R. Sources, health effects and control strategies of indoor fine particulate matter (PM 2.5): A review. Sci. Total Environ. 2017, 586, 610–622.
- Shaneyfelt, K.M.; Anderson, A.R.; Kumar, P.; Hunt, W.F. Air quality considerations for stormwater green street design. Environ. Pollut. 2017, 231, 768–778.
- Tomašević, M.; Anicic, M. Trace element content in urban tree leaves and SEM-EDAX characterization of deposited particles. Facta Univ. Ser. Phys. Chem. Technol. 2010, 8, 1–13.
- Ram, S.S.; Majumder, S.; Chaudhuri, P.; Chanda, S.; Santra, S.C.; Chakraborty, A.; Sudarshan, M. A Review on Air Pollution Monitoring and Management Using Plants with Special Reference to Foliar Dust Adsorption and Physiological Stress Responses. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2489–2522.
- Barwise, Y.; Kumar, P. Designing vegetation barriers for urban air pollution abatement: A practical review for appropriate plant species selection. NPJ Clim. Atmos. Sci. 2020, 3, 1–19.
- Abbass, O.A.; Sailor, D.J.; Gall, E.T. Effectiveness of indoor plants for passive removal of indoor ozone. Build. Environ. 2017, 119, 62–70.
- Cruz, M.D.; Müller, R.; Svensmark, B.; Pedersen, J.S.; Christensen, J.H. Assessment of volatile organic compound removal by indoor plants—A novel experimental setup. Environ. Sci. Pollut. Res. 2014, 21, 7838–7846.
- Oh, G.; Jung, G.J.; Seo, M.H.; Im, Y.B. Experimental study on variations of CO2 concentration in the presence of in door plants and respiration of experimental animals. Environ. Biotechnol. 2011, 52, 321–329.
- Pennisi, S.V.; van Iersel, M.W. Quantification of carbon assimilation of plants in simulated and in situ interiorscapes. HortScience 2012, 47, 468–476.