Carbonic anhydrases are of fundamental importance for dynamics of both intracellular and extracellular pH in tumors.
- proton antenna
- transport metabolon
- hypoxia
- cancer cell metabolism
- pH regulation
1. Introduction
Intra- and extracellular pH regulation is a pivotal function of all cells and tissues. Net outward transport of H+ is a prerequisite for normal physiological function, since a number of intracellular processes, such as metabolism and energy supply, produce acid. In tumor tissues, distorted pH regulation results in extracellular acidification and the formation of a hostile environment in which cancer cells can outcompete healthy local host cells. Cancer cells employ a variety of H+/HCO3−-coupled transporters in combination with intra- and extracellular carbonic anhydrase (CA) isoforms, to alter intra- and extracellular pH to values that promote tumor progression. Many of the transporters could closely associate to CAs, to form a protein complex coined “transport metabolon”. While transport metabolons built with HCO3−-coupled transporters require CA catalytic activity, transport metabolons with monocarboxylate transporters (MCTs) operate independently from CA catalytic function.
2. Carbonic Anhydrases in Cancer Cells
Out of the 15 human Carbonic Anhydrases (CA) isoforms, CAIX and CAXII have received most attention in cancer tissue, both as diagnostic markers and potential drug targets. CAIX and CAXII can be highly overexpressed in many tumors and are, therefore, often regarded as tumor-associated carbonic anhydrases [1][2]. While CAXII is highly expressed in many healthy tissues, including kidney, prostate, pancreas, intestine and lymphocytes, expression of CAIX in healthy tissue is believed to be restricted largely to stomach and gut epithelial tissues [3][4][5][6]. Due to this limited expression in healthy cells and the strong upregulation in many aggressive tumors, the CAIX isoform is the preferred isoform for pharmacological intervention. Expression of CAIX is controlled by the hypoxia-inducible factor HIF1α. Therefore, the enzyme is often upregulated in hypoxic tumor regions [7]. Under normoxia HIF1α is constantly hydroxylated at conserved proline residues and marked for ubiquination and proteosomal degradation by the von Hippel–Lindau tumor suppressor (pVHL). Hypoxia stabilizes HIF1α which could form a complex with HIF1β in the nucleus.
The HIF1 complex binds to the hypoxia responsive element (HRE) in the CA9 promotor to induce gene transcription and increase the expression level of CAIX [7][8]. Under chronic and mild hypoxia, CAIX expression can also be regulated by components of the mitogen-activated protein kinase (MAPK) pathway [9][10]. Furthermore, expression of CAIX can be induced by inactivation mutations of the von Hippel–Lindau tumor suppressor (VHL) gene, which leads to constitutive activation of HIF [11][12]. Expression of CAIX is often associated with chemoresistance and an overall poor prognosis in most cancers [13][14][15][16]. In contrast to CAIX, overexpression of CAXII has been linked to both good and bad tumor prognosis. In colorectal and kidney cancer as well as in oral squamous carcinoma, CAXII was found to correlate with poor prognosis [4][17]. In breast, lung and cervical cancer, however, CAXII was shown to correlate with good outcome [18][19][20]. Even though CAIX and CAXII are considered to be the most important CA isoforms in development of tumors, other CA isoform may also play a role in cancer progression. CA isoform I, for example, contributes to microcalcification, tumorigenesis and migration of breast cancer cells [21]. Like CAIX and CAXII, expression of CAII is upregulated in a variety of cancers. However, in the majority of the investigated tumors, a down-regulation of CAII is associated with poor prognosis [22][23][24]. For a comprehensive review about CA isoforms in cancer see [25].
Carbonic anhydrases are of fundamental importance for dynamics of both intracellular and extracellular pH in tumors. Thereby, a central role is attributed to CAIX, which was suggested to function as a “pH-stat”, which sets tumor pHe to a tightly controlled acidic value. Tumors display considerable metabolic heterogeneity and produce a considerable fraction of their energy not only by glycolysis, but also from oxidation in the TCA (for review see [26][27][28]). Therefore, CO2 is a significant source of metabolic acid production also in cancer cells [29]. However, the mere release of metabolic acids alone does not suffice to fully describe the low pHe values found in solid tumors. First evidence for a role of CAIX in pHe control was provided by Svastova and colleagues [30], who showed that ectopic expression of CAIX in hypoxic MDCK canine kidney epithelial cells in culture leads to an acidification of the extracellular medium. Furthermore, they could show that inhibition of CAIX catalytic activity as well as overexpression of a catalytically inactive CAIX mutant reduced extracellular acidification in hypoxic HeLa cells [30]. A later study by Switach et al [31] showed that expression of CAIX in spheroids of HCT116 human colon carcinoma cells results in a higher pHi (6.6 with CAIX vs. 6.3 without CAIX) and a more acidic pHe (6.6 vs. 6.9). The results were confirmed by a study in HCT116 tumor xenografts, which showed that expression of exofacial CAIX results in a slight extracellular acidification (6.71 vs. 6.86) without changing pHi [32]. The ability of CAIX to set pHe precisely to these values might arise from the enzyme’ unique catalytic kinetics. Measurements of CAIX catalytic activity with gas-analysis mass spectrometry revealed that at a pH of 7.4, the enzyme’s rate constant for hydration was faster than for dehydration [33]. At pH values below 6.8, however, the rate of dehydration exceeded the hydration rate. For pH 6.8, the rate constants for hydration and dehydration were essentially the same [33]. CAIX displays an apparent pK of 6.84 and is inhibited at lower pH values [34]. Therefore, a low pHe could limit further H+-production by CAIX in the extracellular space. In other words, at pH values below 6.8, CAIX favors the dehydration reaction, while at pH values above 6.8 the hydration reaction is preferred [30]. Taken together, these observations indicate that CAIX functions as a pH-stat that sets tumor pHe to an acidic value of around 6.8 [30][31][32][33][34]. This more acidic pHe value can be regarded as an evolutionary strategy of cancer cells (“niche engineering”) to create an environment that promotes tumor growth and tumor invasion. In addition, hydrolysis of cell-derived CO2 by CAIX, allows the parallel diffusion of CO2, HCO3− and H+ to the blood capillaries, thereby speeding up CO2 venting from the cell [31] (Figure 1).
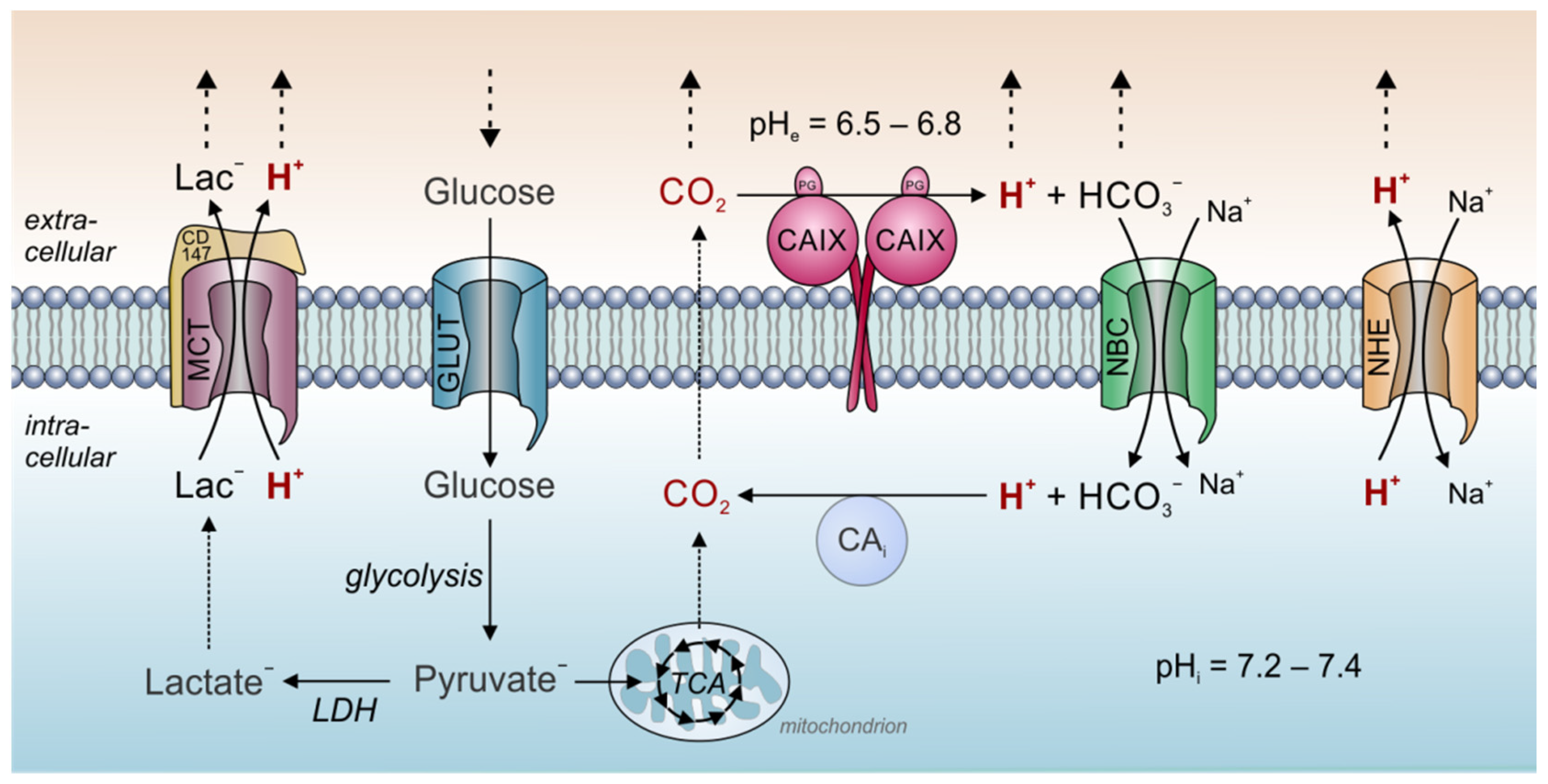
Figure 1. Tumor pH regulation by carbonic anhydrase and acid/base transporters. In tumor cells, metabolic acids are produced primarily by glycolysis and subsequent hydrolysis of ATP (lactate− + H+), and mitochondrial respiration (CO2). At the outer face of the cell membrane, CO2 is hydrated by CAIX to form HCO3− and H+. This allows the parallel diffusion of all three ion species through the extracellular space accelerating CO2 removal to the blood capillary. Furthermore, the hydration of CO2 by CAIX sets extracellular pH to a more acidic value. A fraction of the HCO3− is reimported into the cell by Na+/HCO3− cotransporters (NBC). In the cytosol, HCO3− reacts with H+ to form new CO2, which can leave the cell by diffusion. Thereby, NBC supports the venting of H+ from the cell and contributes to cytosolic pH regulation. Protons are also removed from the cell by Na+/H+ exchangers (NHE) and in cotransport with lactate by monocarboxylate transporters (MCT). In the figure, solid arrows symbolize catalytic reactions or ion transport. Dotted arrows symbolize ion diffusion.
Besides controlling acidity of the extracellular environment, CAs have also been attributed a central function in the regulation of intracellular pH. Heterologous expression of exofacial CAIX in spheroids of RT112 bladder carcinoma cells induces a near uniform pHi, while spheroids of WT RT112 cells not expressing CAIX, or spheroids in which CAIX was pharmacological inhibited with acetazolamide, exhibited an acidic core [35]. The study concluded that CAIX coordinates the spatial pHi spectrum by facilitating CO2 diffusion in the extracellular space. Interestingly, catalytic activity of intracellular CA seems to be of minor importance as compared to extracellular CA catalytic activity. Indeed, it was demonstrated that in cancer cells with high intracellular CA activity, fluctuations in the extracellular CO2 concentration (which can occur in poorly vascularized tumors) evoked faster and larger pHi oscillations [36]. These pHi oscillations increased Ca2+ oscillations, as well as inhibited the mTORC1 pathway, which is a common driver for tumor progression. These findings might explain why low expression of intracellular CAII is associated with good prognosis in various cancer types [22][23][24].
3. Conclusions
Mechanisms of pH regulation belong to a number of unique adaptations of cancer cells to allow tumor tissue to grow and migrate in spite of unfavorable conditions such as hypoxia and the production of large amounts of acid. In order to avoid severe acidosis, expression of CA isoform IX in tumor tissue, in addition to other intra- and extracellular CA isoforms, supports proton translocation. In particular, the formation of transport metabolons with acid/base and metabolite transporters, whereby CAs operate as proton antennae, can promote survival and growth of tumors. Such transport metabolons may well serve as specific targets for therapeutic interventions to derange proton transport and pH regulation in cancer cells.
References
- Parks, S.K.; Chiche, J.; Pouysségur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 2013, 13, 611–623.
- Ivanov, S.; Liao, S.Y.; Ivanova, A.; Danilkovitch-Miagkova, A.; Tarasova, N.; Weirich, G.; Merrill, M.J.; Proescholdt, M.A.; Oldfield, E.H.; Lee, J.; et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 2001, 158, 905–919.
- Pastorek, J.; Pastorekova, S.; Callebaut, I.; Mornon, J.P.; Zelník, V.; Opavský, R.; Zat’ovicová, M.; Liao, S.; Portetelle, D.; Stanbridge, E.J. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 1994, 9, 2877–2888.
- Parkkila, S.; Parkkila, A.K.; Saarnio, J.; Kivela, J.; Karttunen, T.J.; Kaunisto, K.; Waheed, A.; Sly, W.S.; Tureci, O.; Virtanen, I.; et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J. Histochem. Cytochem. 2000, 48, 1601–1608.
- Karhumaa, P.; Kaunisto, K.; Parkkila, S.; Waheed, A.; Pastorekova, S.; Pastorek, J.; Sly, W.S.; Rajaniemi, H. Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol. Hum. Reprod. 2001, 7, 611–616.
- Liao, S.Y.; Lerman, M.I.; Stanbridge, E.J. Expression of transmembrane carbonic anhydrases, CAIX and CAXII, in human development. BMC Dev. Biol. 2009, 9, 1–16.
- Kaluz, S.; Kaluzová, M.; Liao, S.-Y.; Lerman, M.; Stanbridge, E.J. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: A one transcription factor (HIF-1) show? Biochim. Biophys. Acta 2009, 1795, 162–172.
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 2007, cm8.
- Kaluz, S.; Kaluzová, M.; Chrastina, A.; Olive, P.L.; Pastorekova, S.; Pastorek, J.; Lerman, M.I.; Stanbridge, E.J. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1α stabilization: A role for phosphatidylinositol 3′-kinase. Cancer Res. 2002, 62, 4469–4477.
- Kopacek, J.; Barathova, M.; Dequiedt, F.; Sepelakova, J.; Kettmann, R.; Pastorek, J.; Pastorekova, S. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim. Biophys. Acta Gene Struct. Exp. 2005, 1729, 41–49.
- Krieg, M.; Haas, R.; Brauch, H.; Acker, T.; Flamme, I.; Plate, K.H. Up-regulation of hypoxia-inducible factors HIF-1α and HIF-2α under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 2000, 19, 5435–5443.
- Shen, C.; Kaelin, W.G. The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 2013, 23, 18–25.
- Liao, S.Y.; Brewer, C.; Závada, J.; Pastorek, J.; Pastorekova, S.; Manetta, A.; Berman, M.L.; DiSaia, P.J.; Stanbridge, E.J. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinomas. Am. J. Pathol. 1994, 145, 598–609.
- Giatromanolaki, A.; Koukourakis, M.I.; Sivridis, E.; Pastorek, J.; Wykoff, C.C.; Gatter, K.C.; Harris, A.L. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001, 61, 7992–7998.
- Loncaster, J.; Harris, A.L.; Davidson, S.; Logue, J.; Hunter, R.; Wycoff, C.; Pastorek, J.; Ratcliffe, P.; Stratford, I.; West, C. CAIX expression, a potential new intrinsic marker of hypoxia: Correlations with tumour oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001, 61, 6394–6399.
- Smith, A.D.; Truong, M.; Bristow, R.; Yip, P.; Milosevic, M.F.; Joshua, A.M. The utility of serum CA9 for prognostication in prostate cancer. Anticancer Res. 2016, 36, 4489–4492.
- Chien, M.H.; Ying, T.H.; Hsieh, Y.H.; Lin, C.H.; Shih, C.H.; Wei, L.H.; Yang, S.F. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncol. 2012, 48, 417–423.
- Yoo, C.W.; Nam, B.H.; Kim, J.Y.; Shin, H.J.; Lim, H.; Lee, S.; Lee, S.K.; Lim, M.C.; Song, Y.J. Carbonic anhydrase XII expression is associated with histologic grade of cervical cancer and superior radiotherapy outcome. Radiat. Oncol. 2010, 5, 1–10.
- Ilie, M.I.; Hofman, V.; Ortholan, C.; Ammadi, R.E.; Bonnetaud, C.; Havet, K.; Venissac, N.; Mouroux, J.; Mazure, N.M.; Pouysségur, J.; et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int. J. Cancer 2011, 128, 1614–1623.
- Kobayashi, M.; Matsumoto, T.; Ryuge, S.; Yanagita, K.; Nagashio, R.; Kawakami, Y.; Goshima, N.; Jiang, S.X.; Saegusa, M.; Iyoda, A.; et al. CAXII is a sero-diagnostic marker for lung cancer. PLoS ONE 2012, 7, e33952.
- Zheng, Y.; Xu, B.; Zhao, Y.; Gu, H.; Li, C.; Wang, Y.; Chang, X. CA1 contributes to microcalcification and tumourigenesis in breast cancer. BMC Cancer 2015, 15, 1–15.
- Parkkila, S.; Lasota, J.; Fletcher, J.A.; Ou, W.B.; Kivelä, A.J.; Nuorva, K.; Parkkila, A.K.; Ollikainen, J.; Sly, W.S.; Waheed, A.; et al. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod. Pathol. 2010, 23, 743–750.
- Sheng, W.; Dong, M.; Zhou, J.; Li, X.; Dong, Q. Down regulation of CAII is associated with tumor differentiation and poor prognosis in patients with pancreatic cancer. J. Surg. Oncol. 2013, 107, 536–543.
- Zhou, R.; Huang, W.; Yao, Y.; Wang, Y.; Li, Z.; Shao, B.; Zhong, J.; Tang, M.; Liang, S.; Zhao, X.; et al. CA II, a potential biomarker by proteomic analysis, exerts significant inhibitory effect on the growth of colorectal cancer cells. Int. J. Oncol. 2013, 43, 611–621.
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8, 19.
- Gentric, G.; Mieulet, V.; Mechta-Grigoriou, F. Heterogeneity in Cancer Metabolism: New Concepts in an Old Field. Antioxid. Redox Signal. 2017, 26, 462–485.
- Strickaert, A.; Saiselet, M.; Dom, G.; De Deken, X.; Dumont, J.E.; Feron, O.; Sonveaux, P.; Maenhaut, C. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene 2017, 36, 2637–2642.
- Kim, J.; DeBerardinis, R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019, 30, 434–446.
- Lee, S.H.; Griffiths, J.R. How and why are cancers acidic? Carbonic anhydrase ix and the homeostatic control of tumour extracellular pH. Cancers 2020, 12, 1616.
- Švastová, E.; Hulíková, A.; Rafajová, M.; Zat’Ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445.
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310.
- Lee, S.-H.; McIntyre, D.; Honess, D.; Hulikova, A.; Pacheco-Torres, J.; Cerdán, S.; Swietach, P.; Harris, A.L.; Griffiths, J.R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br. J. Cancer 2018, 119, 622–630.
- Li, Y.; Tu, C.; Wang, H.; Silverman, D.N.; Frost, S.C. Catalysis and pH control by membrane-associated carbonic anhydrase IX in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2011, 286, 15789–15796.
- McIntyre, A.; Patiar, S.; Wigfield, S.; Li, J.L.; Ledaki, I.; Turley, H.; Leek, R.; Snell, C.; Gatter, K.; Sly, W.S.; et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin. Cancer Res. 2012, 18, 3100–3111.
- Swietach, P.; Wigfield, S.; Cobden, P.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 2008, 283, 20473–20483.
- Hulikova, A.; Aveyard, N.; Harris, A.L.; Vaughan-Jones, R.D.; Swietach, P. Intracellular carbonic anhydrase activity sensitizes cancer cell pH signaling to dynamic changes in CO2 partial pressure. J. Biol. Chem. 2014, 289, 25418–25430.
